

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50062051 (1-Dimethylcarbamoyl-6-(4-fluoro-phenyl)-3-[4-(2-me...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency against Platelet activating factor (PAF) receptor using [3H]-C18-PAF as radioligand on rabbit platelet membranes | J Med Chem 41: 74-95 (1998) Article DOI: 10.1021/jm970389+ BindingDB Entry DOI: 10.7270/Q2MC8Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50062066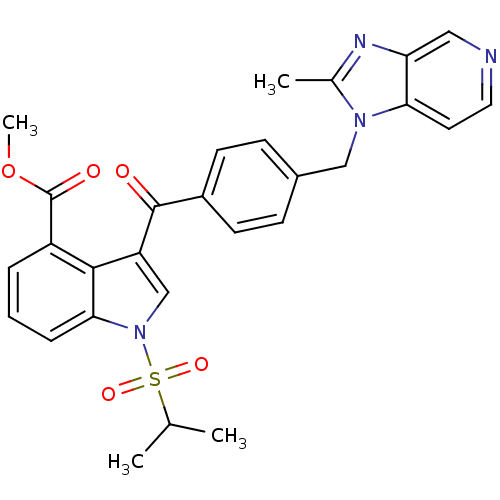 (3-[4-(2-Methyl-imidazo[4,5-c]pyridin-1-ylmethyl)-b...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency against Platelet activating factor (PAF) receptor using [3H]-C18-PAF as radioligand on rabbit platelet membranes | J Med Chem 41: 74-95 (1998) Article DOI: 10.1021/jm970389+ BindingDB Entry DOI: 10.7270/Q2MC8Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50078161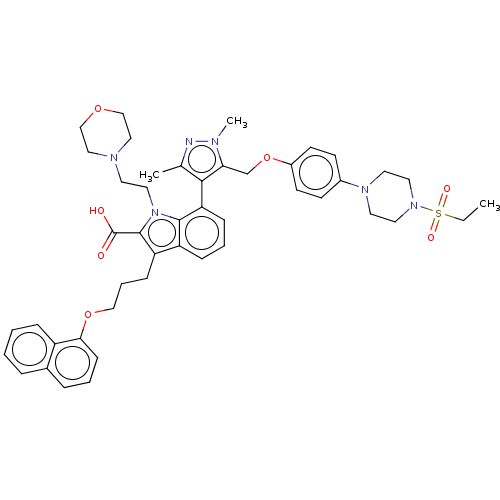 (CHEMBL3417702) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Displacement of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide) from MCL1 (unknown origin) after 1 hr by TR-FRET assay | J Med Chem 58: 2180-94 (2015) Article DOI: 10.1021/jm501258m BindingDB Entry DOI: 10.7270/Q2HX1FC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50078159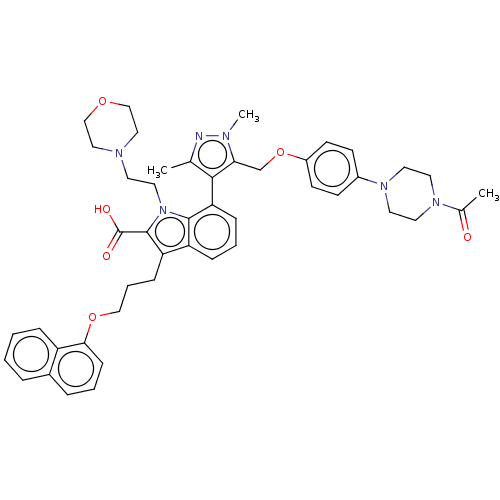 (CHEMBL3417700) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Displacement of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide) from MCL1 (unknown origin) after 1 hr by TR-FRET assay | J Med Chem 58: 2180-94 (2015) Article DOI: 10.1021/jm501258m BindingDB Entry DOI: 10.7270/Q2HX1FC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220432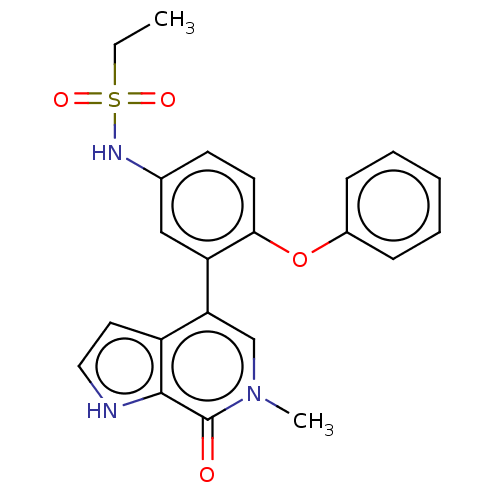 (US9296741, 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.25 | -54.8 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50078162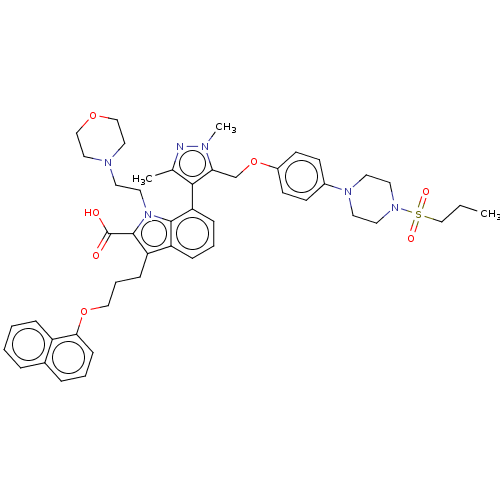 (CHEMBL3417703) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Displacement of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide) from MCL1 (unknown origin) after 1 hr by TR-FRET assay | J Med Chem 58: 2180-94 (2015) Article DOI: 10.1021/jm501258m BindingDB Entry DOI: 10.7270/Q2HX1FC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50286027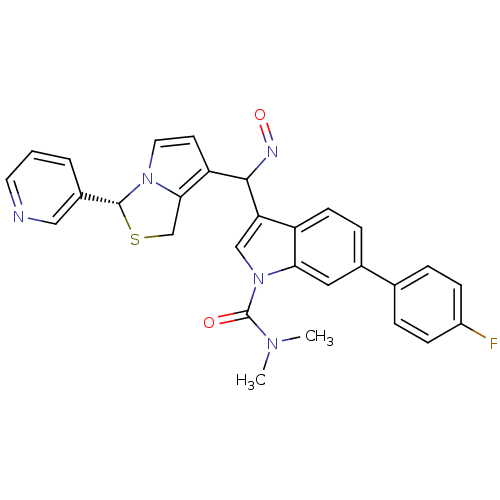 (6-(4-Fluoro-phenyl)-3-[[(Z)-hydroxyimino]-((R)-3-p...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for platelet activating factor receptor using [3H]-PAF in rabbit platelet membranes | Bioorg Med Chem Lett 5: 2913-2918 (1995) Article DOI: 10.1016/0960-894X(95)00511-Q BindingDB Entry DOI: 10.7270/Q2NC6157 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220432 (US9296741, 21) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.390 | -53.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50048485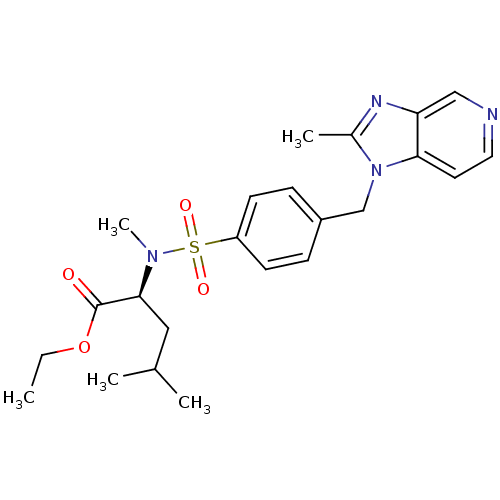 ((S)-4-Methyl-2-{methyl-[4-(2-methyl-imidazo[4,5-c]...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity against PAF receptor in rabbit platelet | J Med Chem 41: 74-95 (1998) Article DOI: 10.1021/jm970389+ BindingDB Entry DOI: 10.7270/Q2MC8Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50048485 ((S)-4-Methyl-2-{methyl-[4-(2-methyl-imidazo[4,5-c]...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity against PAF receptor in rabbit platelet | J Med Chem 41: 74-95 (1998) Article DOI: 10.1021/jm970389+ BindingDB Entry DOI: 10.7270/Q2MC8Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50078160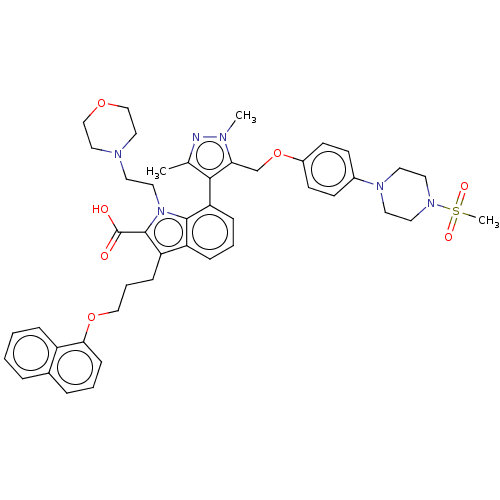 (CHEMBL3417701) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Displacement of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide) from MCL1 (unknown origin) after 1 hr by TR-FRET assay | J Med Chem 58: 2180-94 (2015) Article DOI: 10.1021/jm501258m BindingDB Entry DOI: 10.7270/Q2HX1FC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50078163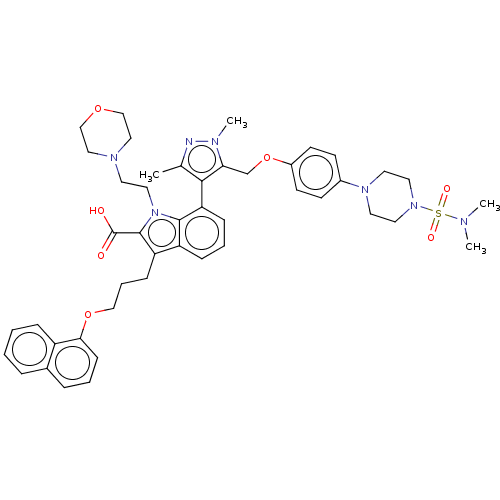 (CHEMBL3417704) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Displacement of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide) from MCL1 (unknown origin) after 1 hr by TR-FRET assay | J Med Chem 58: 2180-94 (2015) Article DOI: 10.1021/jm501258m BindingDB Entry DOI: 10.7270/Q2HX1FC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220626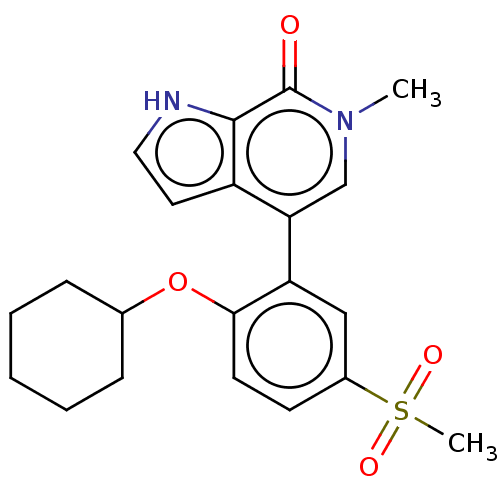 (US9296741, 215) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.460 | -53.3 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM50457489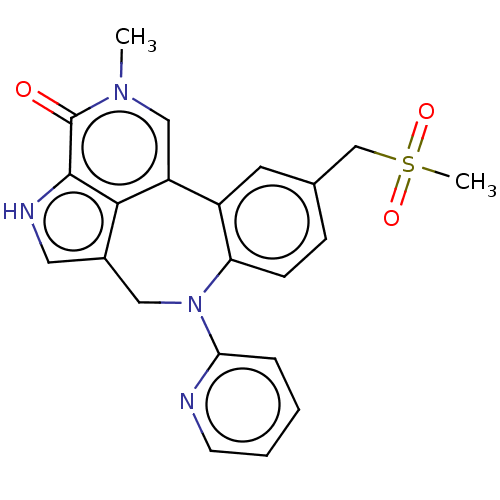 (CHEMBL4208129) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to BRD2 bromodomain 1 to 2 (G73 to A560 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent probe by b... | Bioorg Med Chem Lett 28: 1804-1810 (2018) Article DOI: 10.1016/j.bmcl.2018.04.020 BindingDB Entry DOI: 10.7270/Q2542R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50078164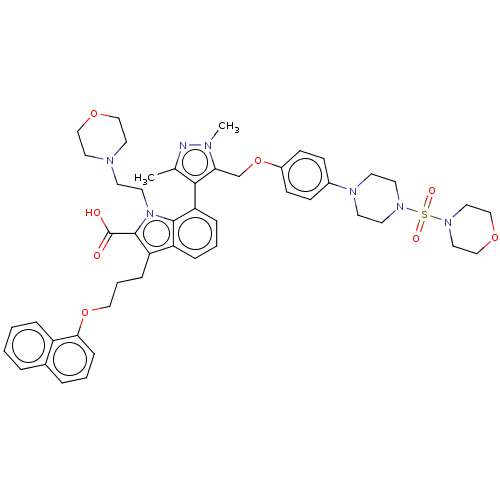 (CHEMBL3417705) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Displacement of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide) from MCL1 (unknown origin) after 1 hr by TR-FRET assay | J Med Chem 58: 2180-94 (2015) Article DOI: 10.1021/jm501258m BindingDB Entry DOI: 10.7270/Q2HX1FC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511849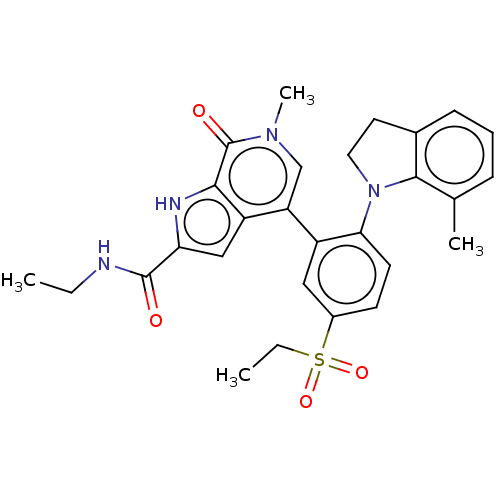 (CHEMBL4465299) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511875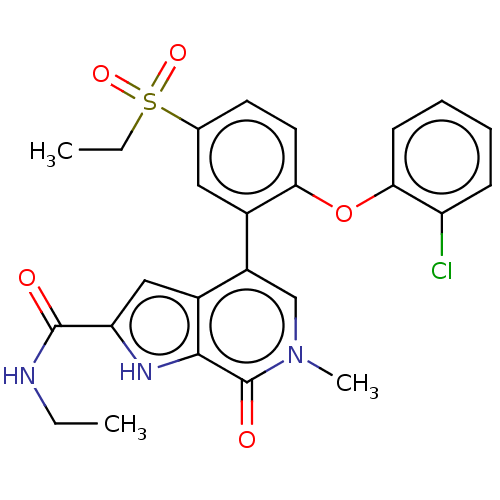 (CHEMBL4548794) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511850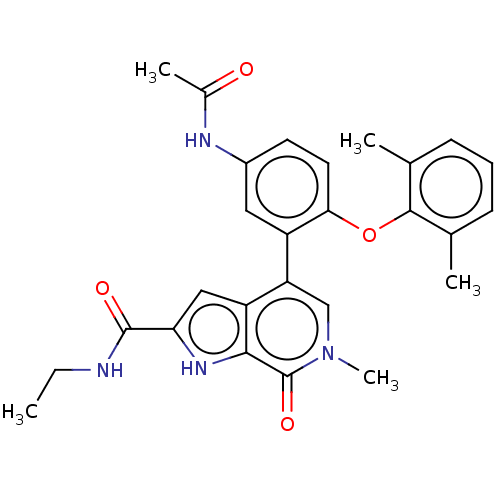 (CHEMBL4435166) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220484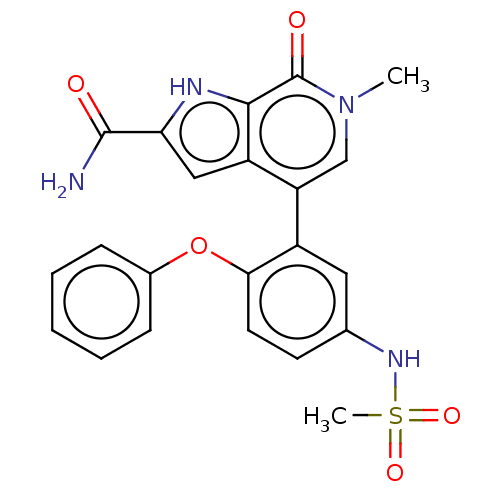 (US9296741, 73) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM439590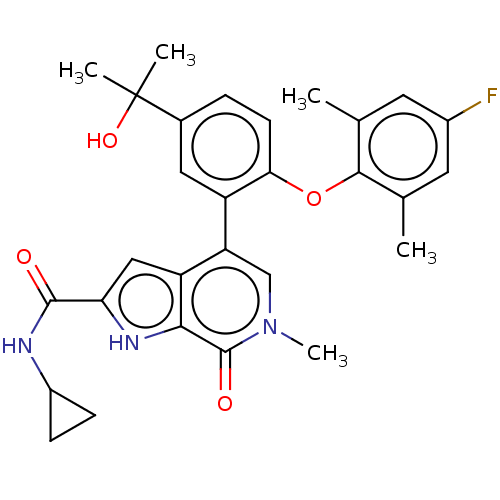 (US10633379, Example 121) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220518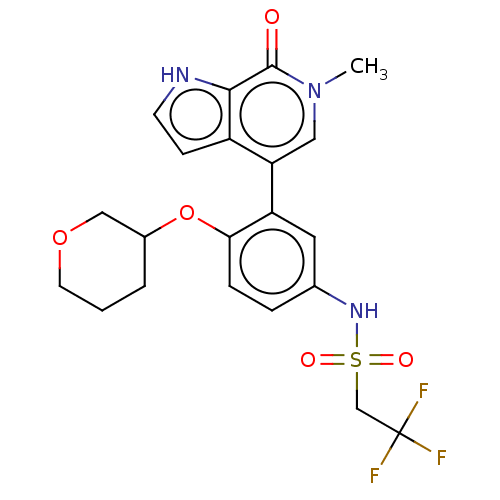 (US9296741, 107) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220452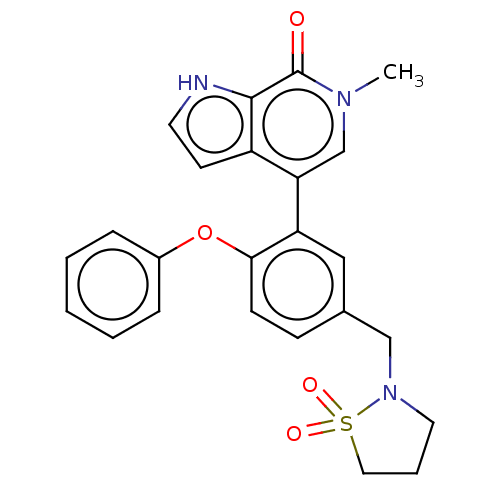 (US9296741, 41) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.510 | -53.0 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50062069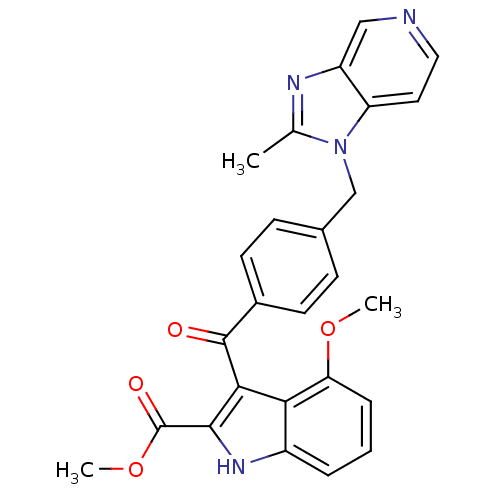 (4-Methoxy-3-[4-(2-methyl-imidazo[4,5-c]pyridin-1-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency against Platelet activating factor (PAF) receptor using [3H]-C18-PAF as radioligand on rabbit platelet membranes | J Med Chem 41: 74-95 (1998) Article DOI: 10.1021/jm970389+ BindingDB Entry DOI: 10.7270/Q2MC8Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220516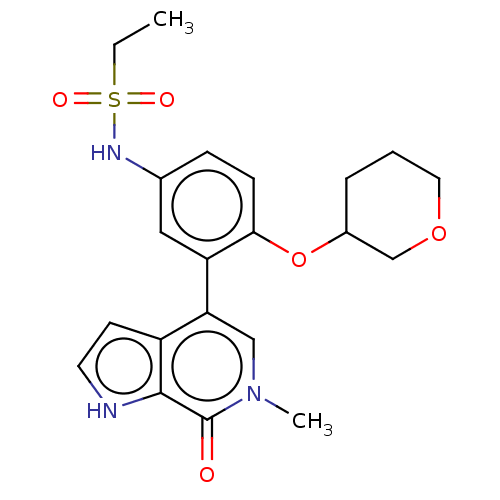 (US9296741, 105) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.520 | -53.0 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220679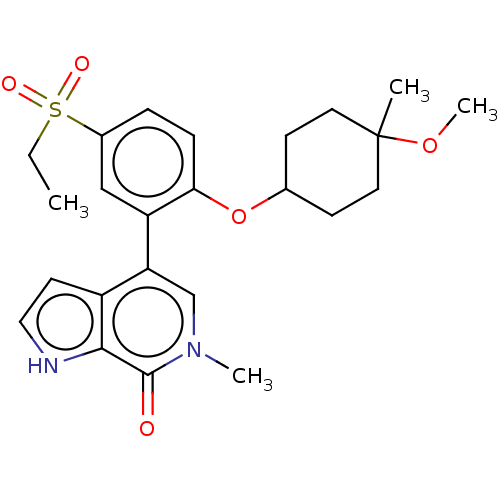 (US9296741, 268) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | -52.8 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM439461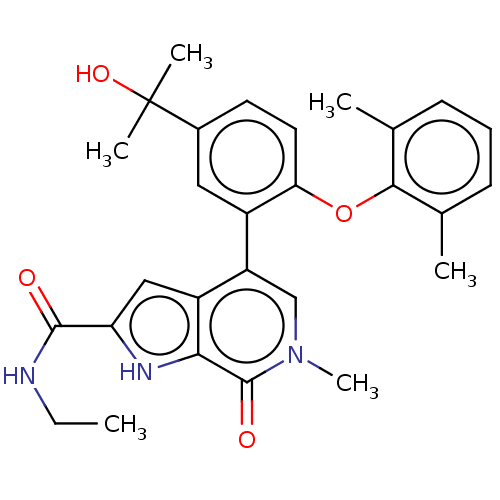 (US10633379, Example 3 | US10633379, Example 68) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511864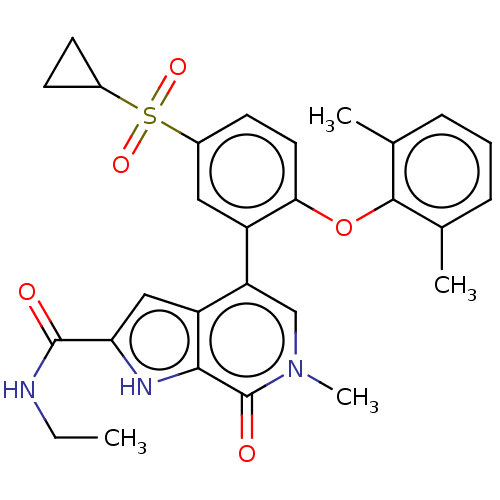 (CHEMBL4564879) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038757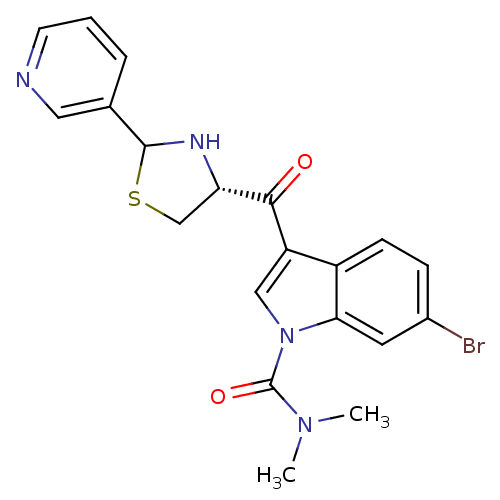 (6-Bromo-3-((R)-2-pyridin-3-yl-thiazolidine-4-carbo...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50457489 (CHEMBL4208129) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to His-tagged BRD4 bromodomain 1 to 2 (K57 to K550 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent... | Bioorg Med Chem Lett 28: 1804-1810 (2018) Article DOI: 10.1016/j.bmcl.2018.04.020 BindingDB Entry DOI: 10.7270/Q2542R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220507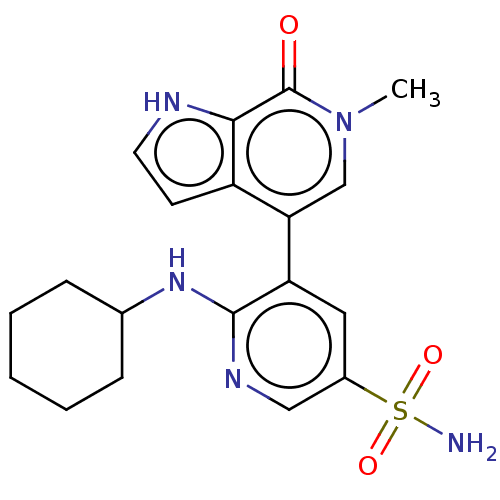 (US9296741, 96) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.630 | -52.5 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50062101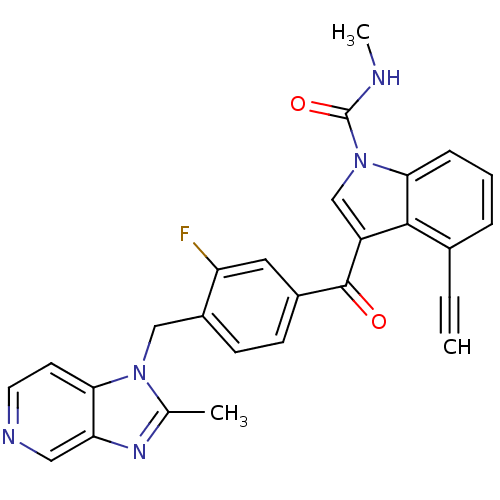 (4-Ethynyl-3-[3-fluoro-4-(2-methyl-imidazo[4,5-c]py...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency against Platelet activating factor (PAF) receptor using [3H]-C18-PAF as radioligand on rabbit platelet membranes | J Med Chem 41: 74-95 (1998) Article DOI: 10.1021/jm970389+ BindingDB Entry DOI: 10.7270/Q2MC8Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50062056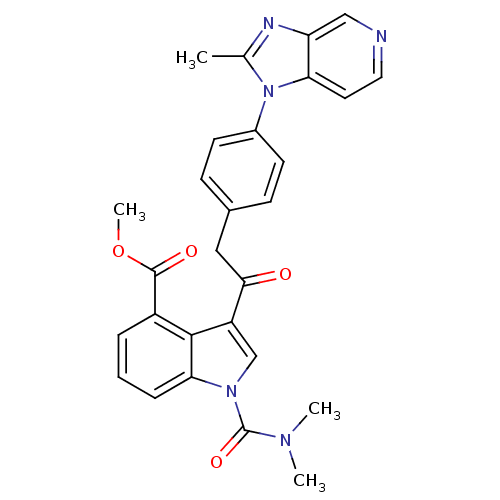 (1-Dimethylcarbamoyl-3-{2-[4-(2-methyl-imidazo[4,5-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency against Platelet activating factor (PAF) receptor using [3H]-C18-PAF as radioligand on rabbit platelet membranes | J Med Chem 41: 74-95 (1998) Article DOI: 10.1021/jm970389+ BindingDB Entry DOI: 10.7270/Q2MC8Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220495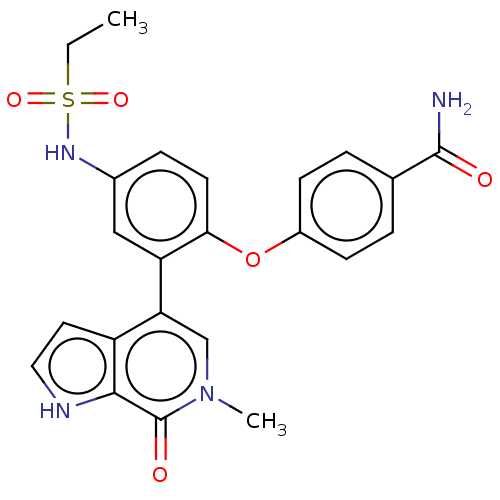 (US9296741, 84) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.650 | -52.4 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50062061 (1-(3,3-Dimethyl-2-oxo-butyl)-3-[4-(2-methyl-imidaz...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency against Platelet activating factor (PAF) receptor using [3H]-C18-PAF as radioligand on rabbit platelet membranes | J Med Chem 41: 74-95 (1998) Article DOI: 10.1021/jm970389+ BindingDB Entry DOI: 10.7270/Q2MC8Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220431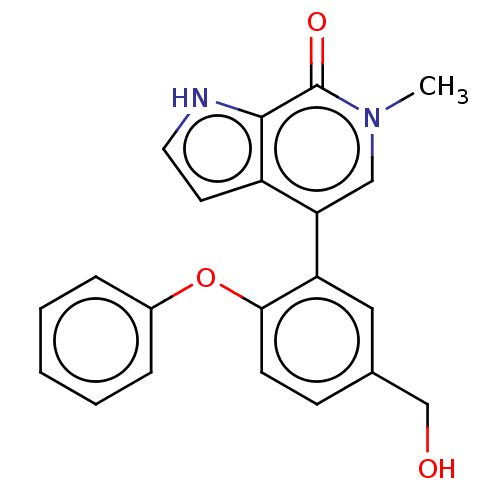 (US10633379, Compound Z | US9296741, 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.680 | -52.3 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50457489 (CHEMBL4208129) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to BRDT bromodomain 1 to 2 (N21to P380 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent probe by br... | Bioorg Med Chem Lett 28: 1804-1810 (2018) Article DOI: 10.1016/j.bmcl.2018.04.020 BindingDB Entry DOI: 10.7270/Q2542R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220614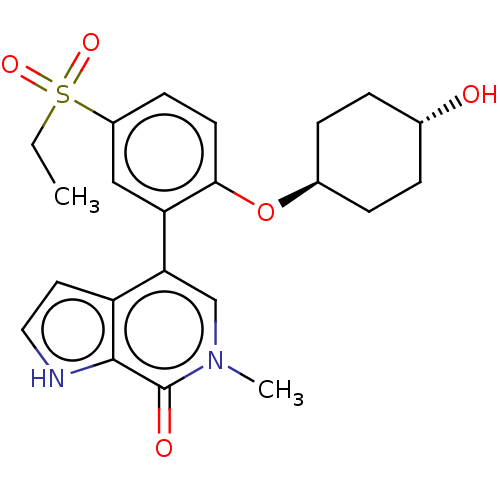 (US9296741, 203) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.690 | -52.3 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511870 (CHEMBL4461291) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50285986 (2-[6-(4-Fluoro-phenyl)-3-(3-pyridin-3-yl-1H-pyrrol...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for antagonistic activity to displace [3H]-PAF from rabbit platelet membrane PAF receptors | Bioorg Med Chem Lett 5: 2903-2908 (1995) Article DOI: 10.1016/0960-894X(95)00509-R BindingDB Entry DOI: 10.7270/Q2WW7HN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511855 (CHEMBL4454597) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220520 (US9296741, 109) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.720 | -52.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50457496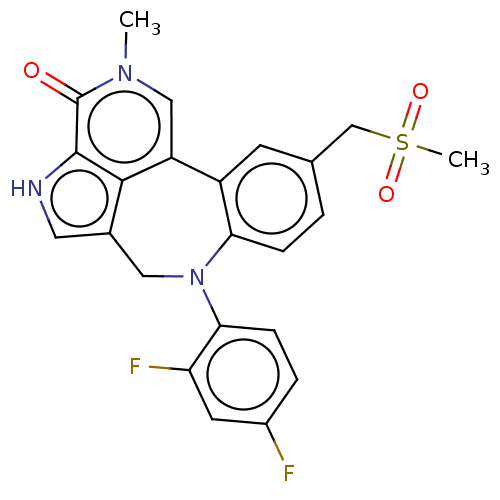 (CHEMBL4217457) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to BRD4 bromodomain 2 (E352 to M457 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent probe by bromo... | Bioorg Med Chem Lett 28: 1804-1810 (2018) Article DOI: 10.1016/j.bmcl.2018.04.020 BindingDB Entry DOI: 10.7270/Q2542R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220477 (US9296741, 66) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.740 | -52.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220614 (US9296741, 203) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.75 | -52.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220471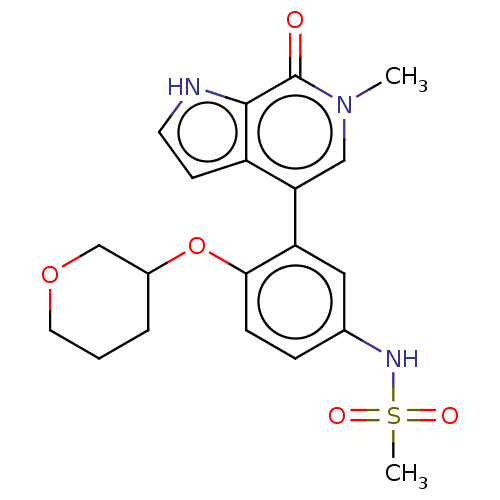 (US9296741, 60) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.75 | -52.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220434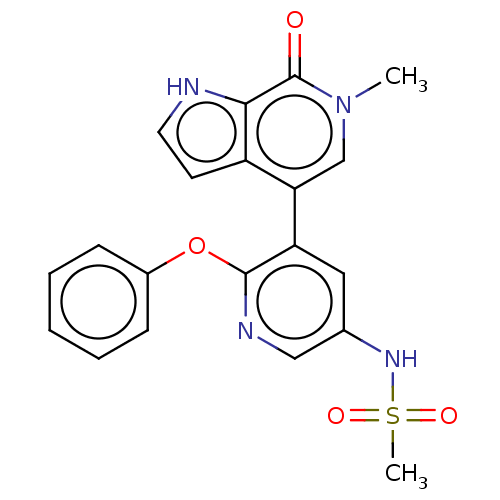 (US9296741, 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.75 | -52.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220679 (US9296741, 268) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.760 | -52.0 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220649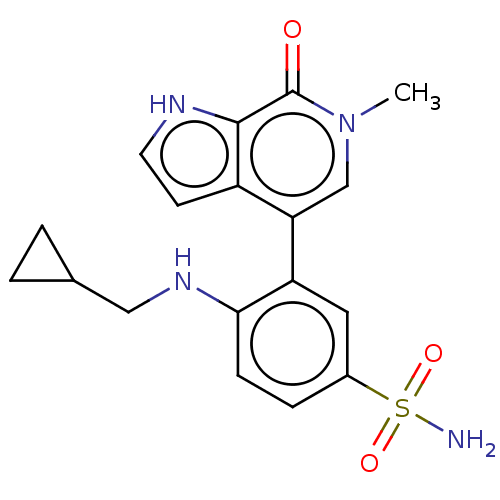 (US9296741, 238) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.780 | -52.0 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50062045 (6-Benzofuran-2-yl-1-dimethylcarbamoyl-3-[4-(2-meth...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency against Platelet activating factor (PAF) receptor using [3H]-C18-PAF as radioligand on rabbit platelet membranes | J Med Chem 41: 74-95 (1998) Article DOI: 10.1021/jm970389+ BindingDB Entry DOI: 10.7270/Q2MC8Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220608 (US9296741, 197) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.790 | -52.0 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2545 total ) | Next | Last >> |