

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50095105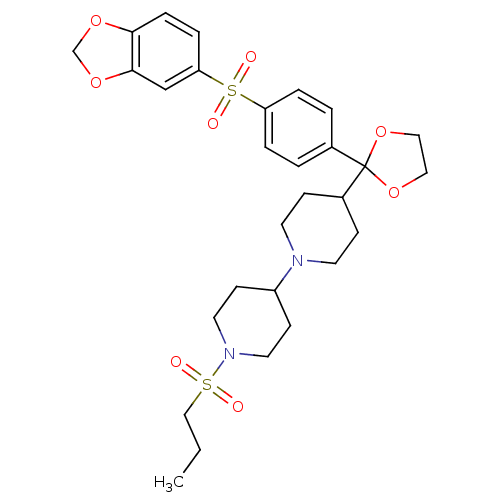 (4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103771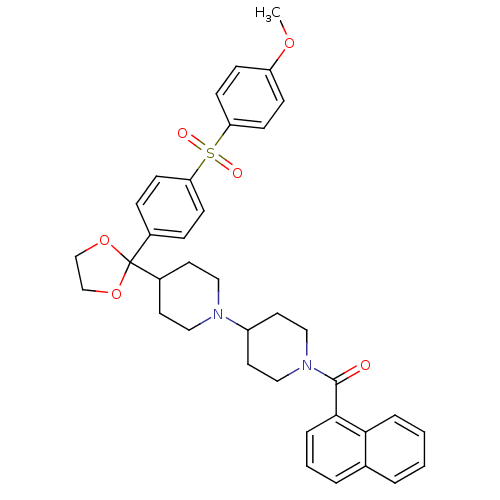 ((4-{2-[4-(4-Methoxy-benzenesulfonyl)-phenyl]-[1,3]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103774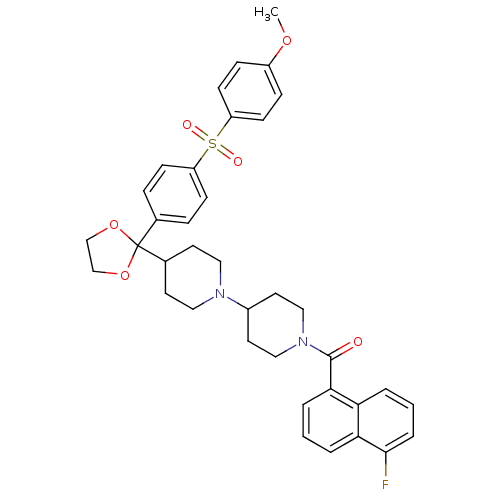 ((5-Fluoro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103772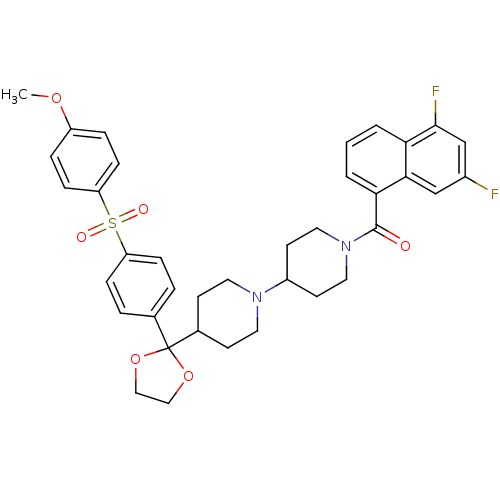 ((5,7-Difluoro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103767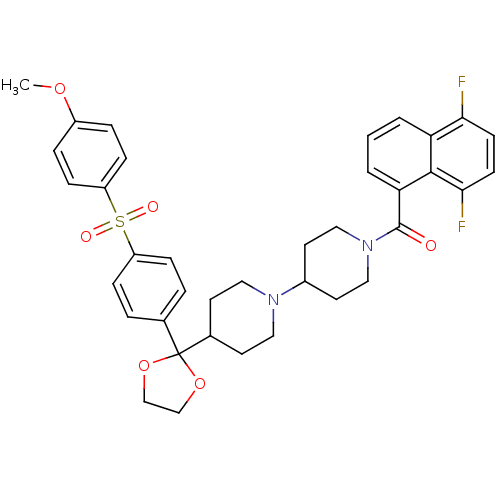 ((5,8-Difluoro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103768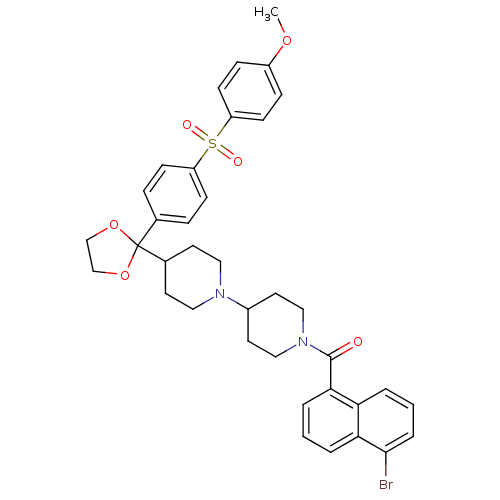 ((5-Bromo-naphthalen-1-yl)-(4-{2-[4-(4-methoxy-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103773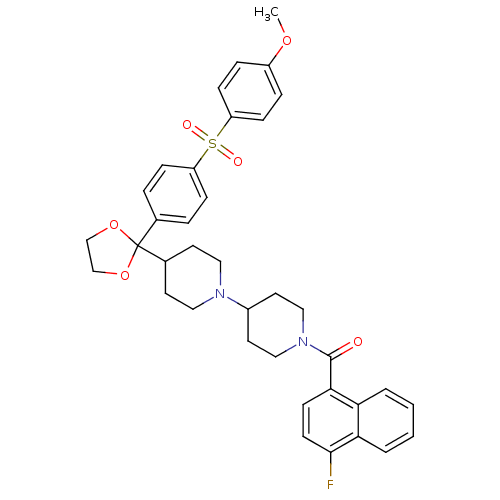 ((4-Fluoro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50065428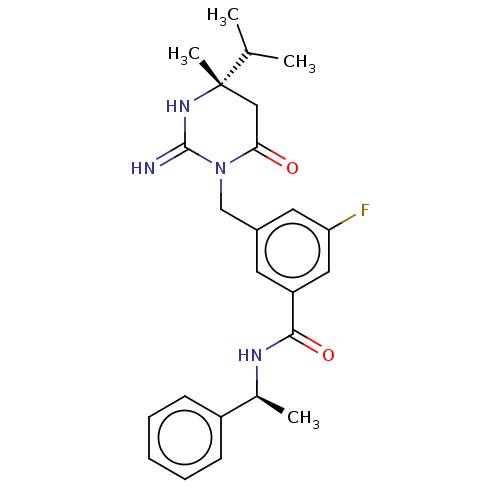 (CHEMBL3401350) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | Bioorg Med Chem Lett 25: 1592-6 (2015) Article DOI: 10.1016/j.bmcl.2015.02.003 BindingDB Entry DOI: 10.7270/Q2X63PMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103775 ((6-Fluoro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103769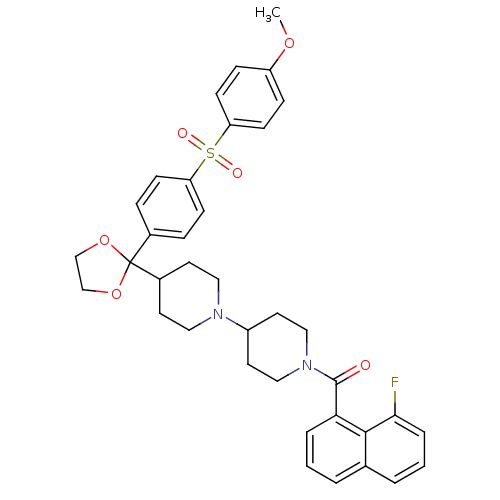 ((8-Fluoro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50104933 (CHEMBL105821 | {4-[(4-Bromo-phenyl)-ethoxyimino-me...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50104940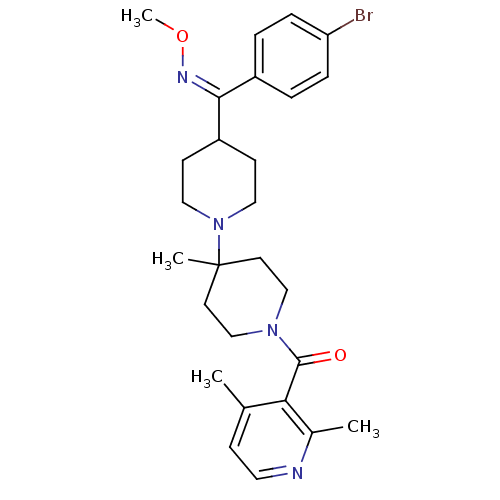 (CHEMBL323172 | {4-[(4-Bromo-phenyl)-methoxyimino-m...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103770 ((7-Fluoro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50104942 ((4-Fluoro-naphthalen-1-yl)-{4-[4-(4-methoxy-benzen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Muscarinic acetylcholine receptor M2 | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103776 ((6-Chloro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115512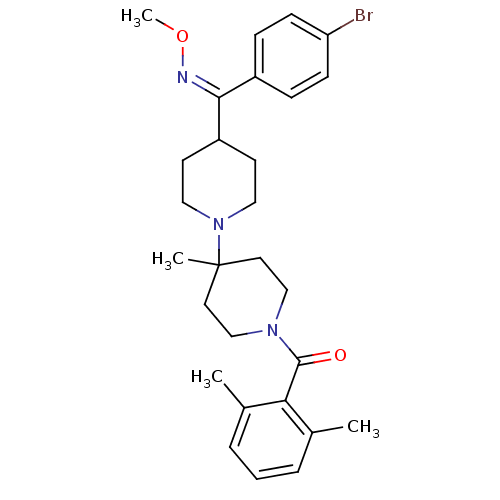 (CHEMBL324671 | {4-[(4-Bromo-phenyl)-methoxyimino-m...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115528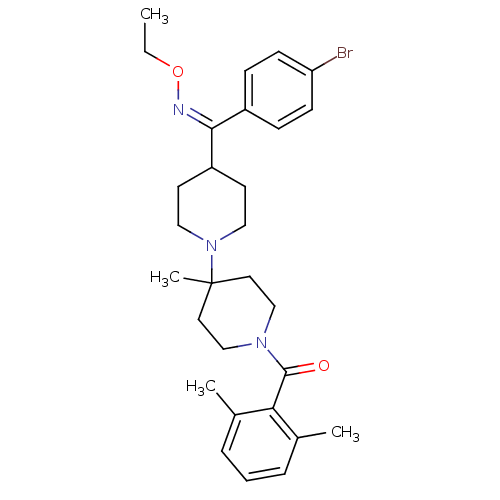 ((Z)-(4-((4-bromophenyl)(ethoxyimino)methyl)-4'-met...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50065395 (CHEMBL3401345) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | Bioorg Med Chem Lett 25: 1592-6 (2015) Article DOI: 10.1016/j.bmcl.2015.02.003 BindingDB Entry DOI: 10.7270/Q2X63PMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50104931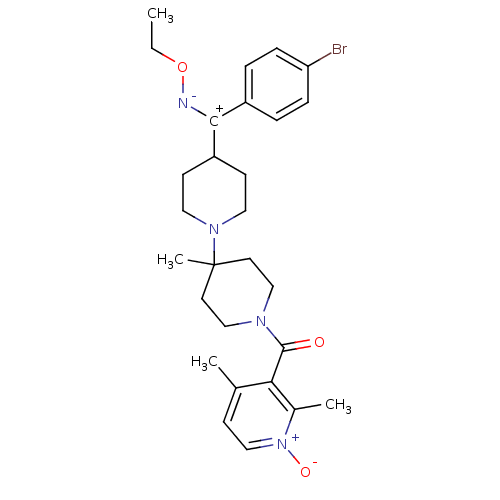 ((4-{(4-Bromo-phenyl)-[(E)-ethoxyimino]-methyl}-4''...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50065426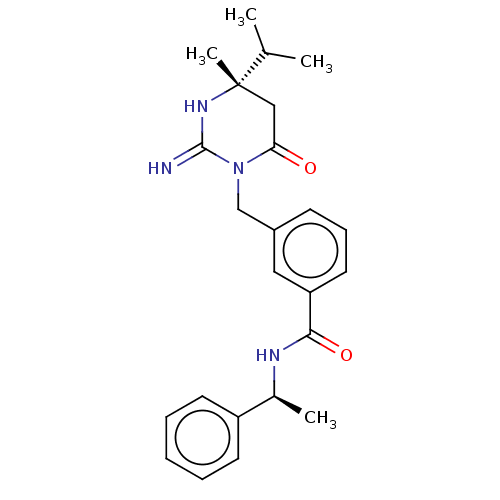 (CHEMBL3401348) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | Bioorg Med Chem Lett 25: 1592-6 (2015) Article DOI: 10.1016/j.bmcl.2015.02.003 BindingDB Entry DOI: 10.7270/Q2X63PMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115511 (CHEMBL321413 | {4-[(4-Bromo-phenyl)-ethoxyimino-me...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50104936 ((2-Amino-6-chloro-phenyl)-{4-[(4-bromo-phenyl)-met...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115549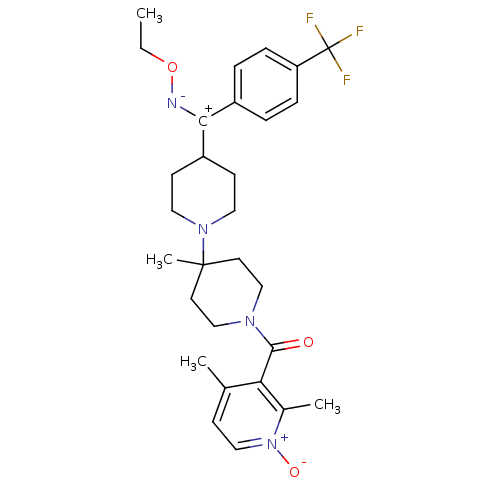 ((2,4-Dimethyl-1-oxy-pyridin-3-yl)-{4-[ethoxyimino-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115550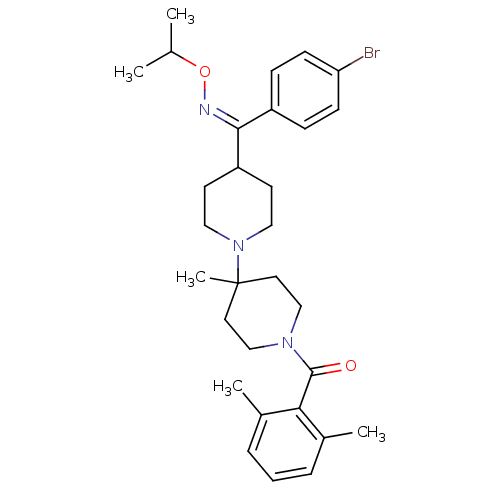 (CHEMBL109008 | {4-[(4-Bromo-phenyl)-isopropoxyimin...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115527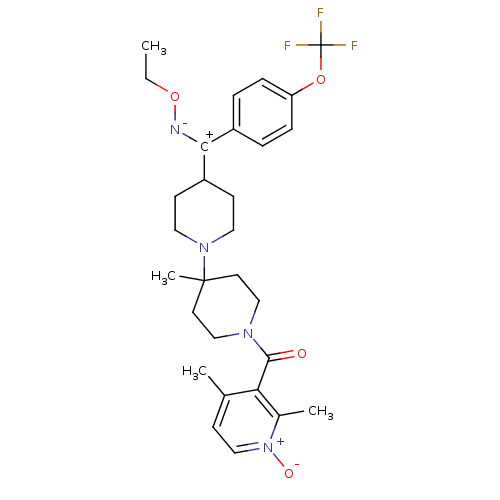 ((2,4-Dimethyl-1-oxy-pyridin-3-yl)-{4-[ethoxyimino-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50104938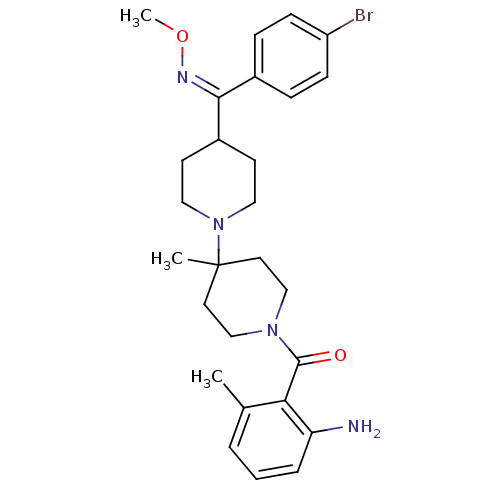 ((2-Amino-6-methyl-phenyl)-{4-[(4-bromo-phenyl)-met...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115531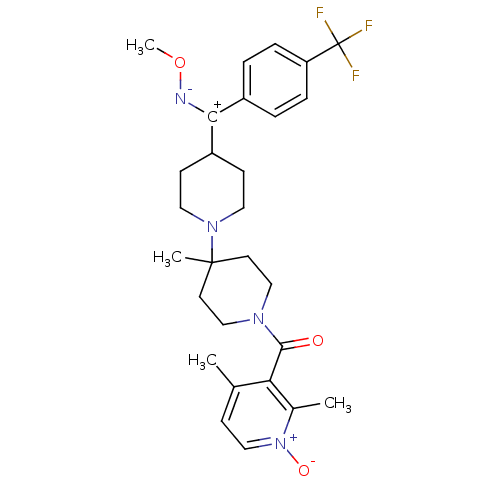 ((2,4-Dimethyl-1-oxy-pyridin-3-yl)-{4-[methoxyimino...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115548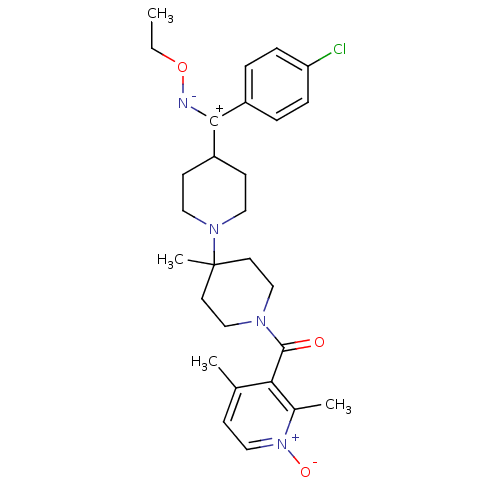 (CHEMBL343826 | CHEMBL419735 | {4-[(4-Chloro-phenyl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50065424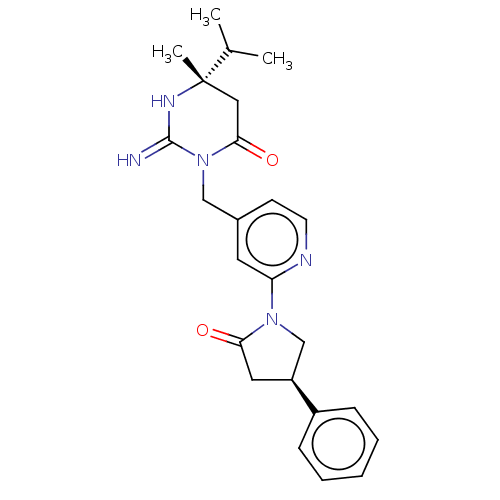 (CHEMBL3401346) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | Bioorg Med Chem Lett 25: 1592-6 (2015) Article DOI: 10.1016/j.bmcl.2015.02.003 BindingDB Entry DOI: 10.7270/Q2X63PMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115517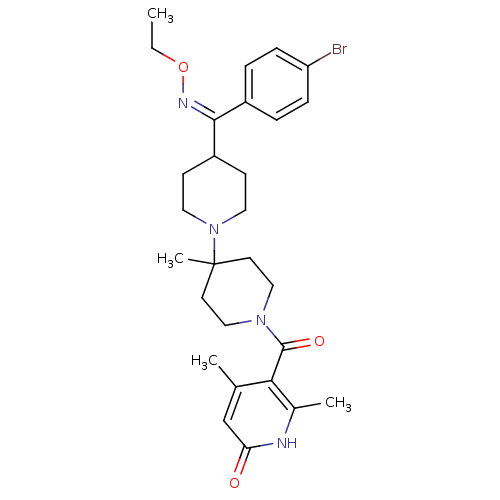 (CHEMBL110757 | {4-[(4-Bromo-phenyl)-ethoxyimino-me...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115522 ((2,4-Dimethyl-1-oxy-pyridin-3-yl)-{4-[methoxyimino...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115542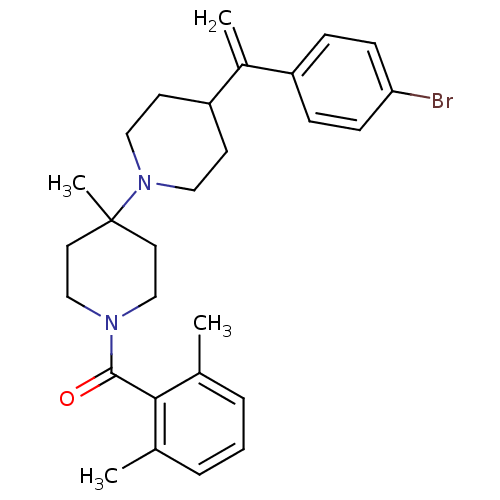 (CHEMBL107154 | {4-[1-(4-Bromo-phenyl)-vinyl]-4'-me...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115529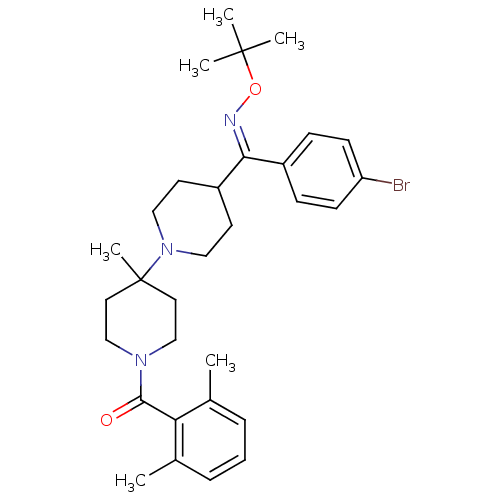 (CHEMBL418997 | {4-[(4-Bromo-phenyl)-tert-butoxyimi...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115526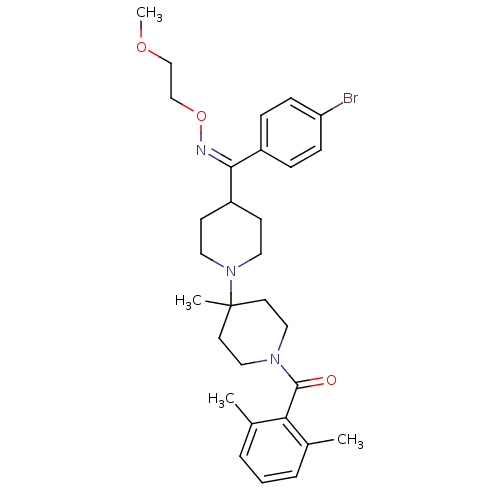 (CHEMBL107363 | {4-[(4-Bromo-phenyl)-(2-methoxy-eth...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50065425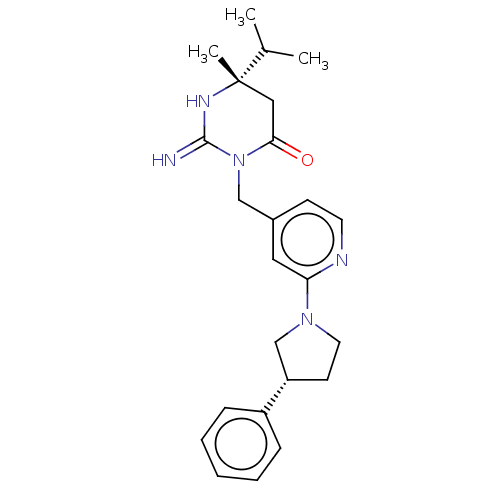 (CHEMBL3401347) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | Bioorg Med Chem Lett 25: 1592-6 (2015) Article DOI: 10.1016/j.bmcl.2015.02.003 BindingDB Entry DOI: 10.7270/Q2X63PMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115538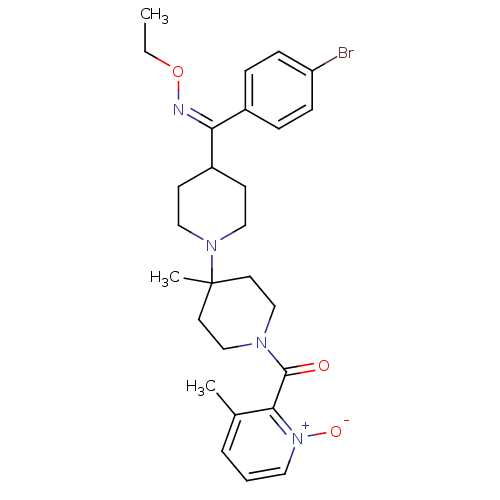 (4-[(Z)-(4-Bromophenyl)(ethoxyimino)methyl]-4'-meth...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50104937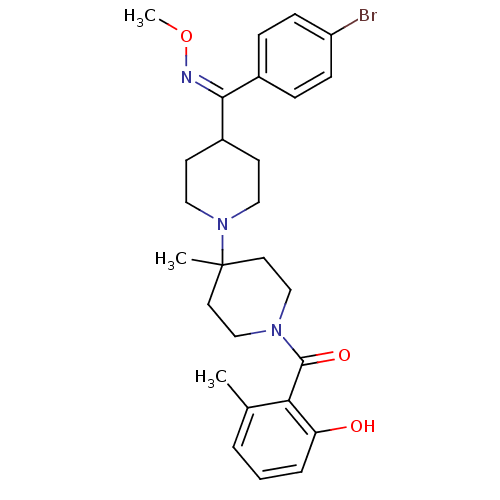 (1'-(2-Hydroxy-6-methylbenzoyl)-4-[(Z)-(4-bromophen...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50065393 (CHEMBL3401344) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | Bioorg Med Chem Lett 25: 1592-6 (2015) Article DOI: 10.1016/j.bmcl.2015.02.003 BindingDB Entry DOI: 10.7270/Q2X63PMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50104941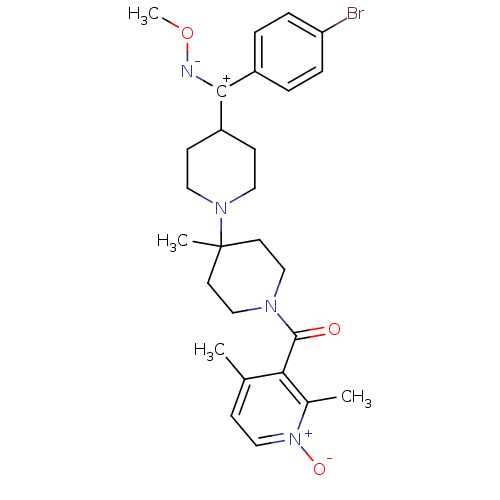 (CHEMBL106098 | CHEMBL138198 | {4-[(4-Bromo-phenyl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115537 ((2,4-Dimethyl-1-oxy-pyridin-3-yl)-{4-[ethoxyimino-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115518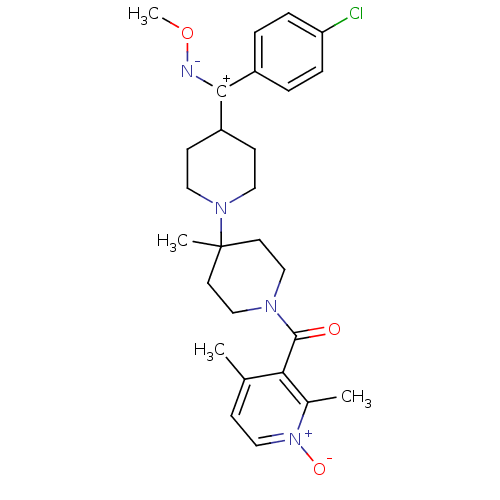 (CHEMBL106099 | CHEMBL138143 | {4-[(4-Chloro-phenyl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50104929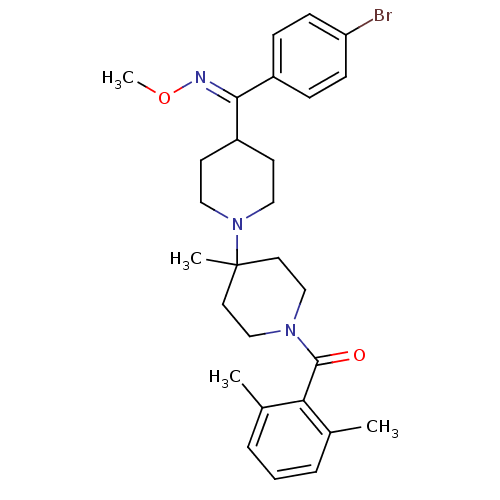 ((4-{(4-Bromo-phenyl)-[(E)-methoxyimino]-methyl}-4'...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115543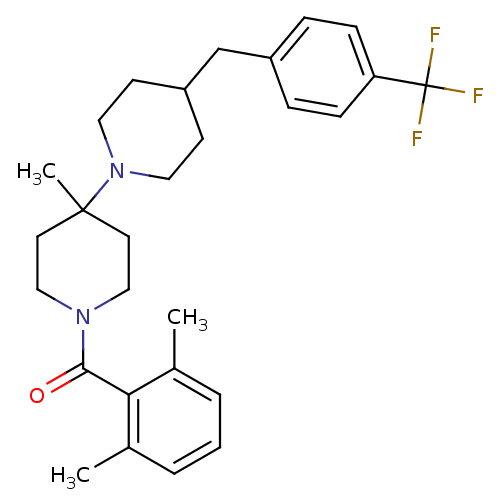 ((2,6-Dimethyl-phenyl)-[4'-methyl-4-(4-trifluoromet...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115519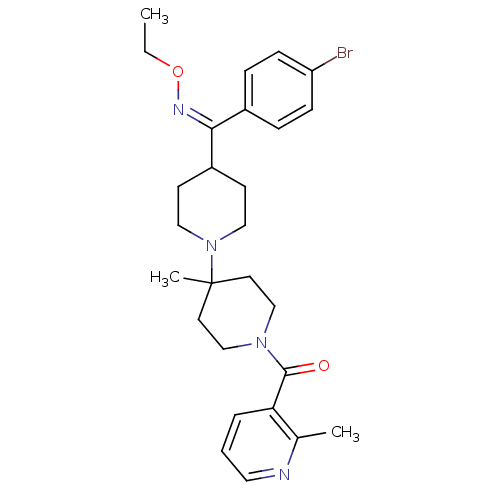 (4-[(Z)-(4-Bromophenyl)(ethoxyimino)methyl]-4'-meth...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50104932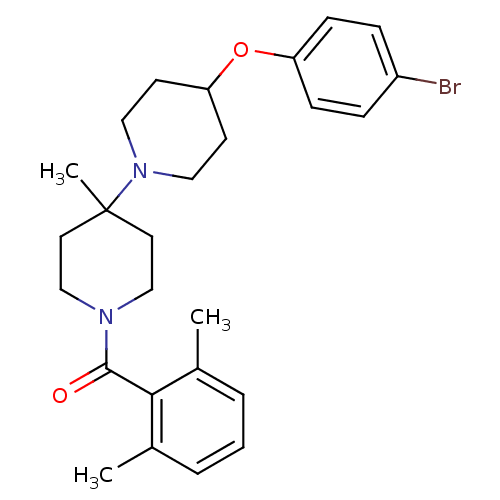 (CHEMBL108569 | [4-(4-Bromo-phenoxy)-4'-methyl-[1,4...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115552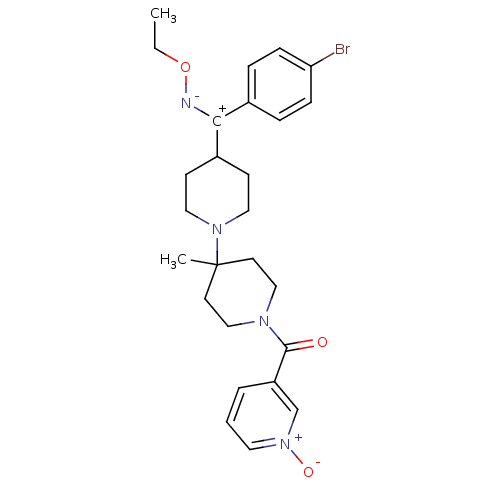 (CHEMBL107018 | CHEMBL140922 | {4-[(4-Bromo-phenyl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115515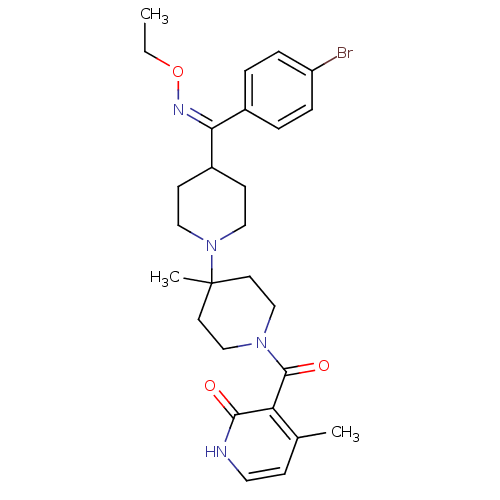 (CHEMBL322267 | {4-[(4-Bromo-phenyl)-ethoxyimino-me...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115536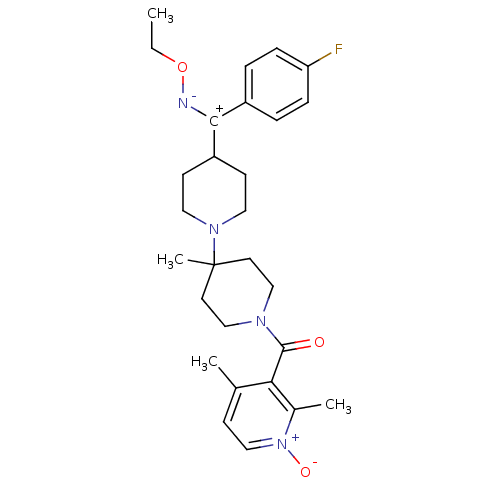 ((2,4-Dimethyl-1-oxy-pyridin-3-yl)-{4-[ethoxyimino-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115551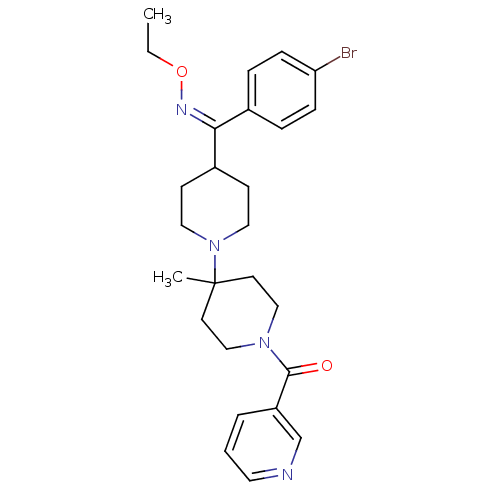 (CHEMBL108959 | {4-[(4-Bromo-phenyl)-ethoxyimino-me...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50115525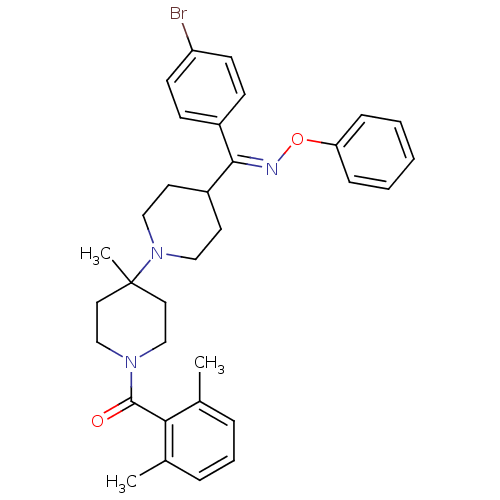 (CHEMBL106573 | {4-[(4-Bromo-phenyl)-phenoxyimino-m...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 698 total ) | Next | Last >> |