

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50042235 (2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Endothelin A receptor | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125339 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50042235 (2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50125343 (2'-(3,3-Dimethyl-2-oxo-pyrrolidin-1-ylmethyl)-4'-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human ETA receptor expressed in CHO-K1 cells in the presence of 0.05 nM [125I]-labeled endothelin 1 | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50042235 (2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125362 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-ethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Endothelin A receptor | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50117910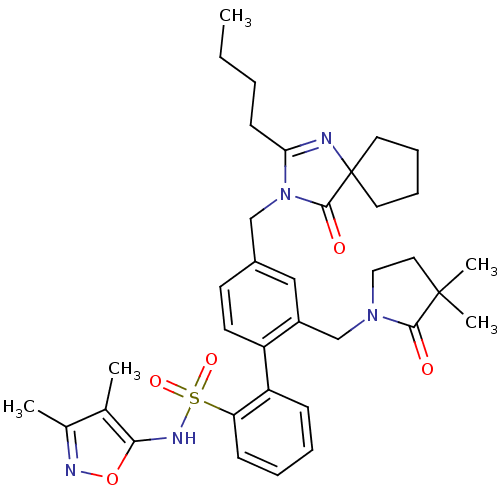 (4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Endothelin A receptor | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125352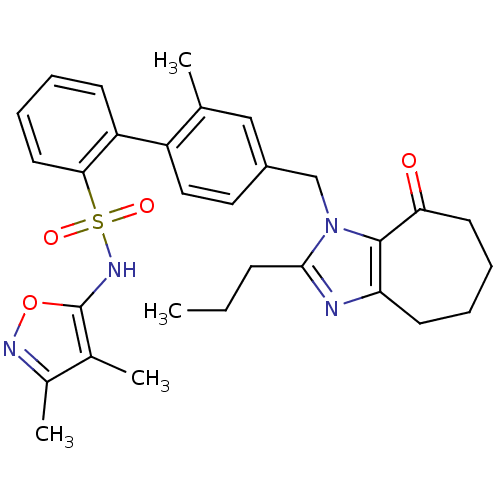 (2'-Methyl-4'-(8-oxo-2-propyl-5,6,7,8-tetrahydro-4H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125359 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50117910 (4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human ETA receptor expressed in CHO-K1 cells in the presence of 0.05 nM [125I]-labeled endothelin 1 | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125356 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-(2-f...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125350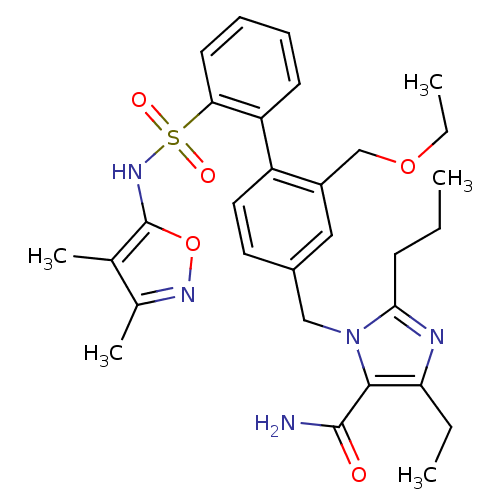 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-etho...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125345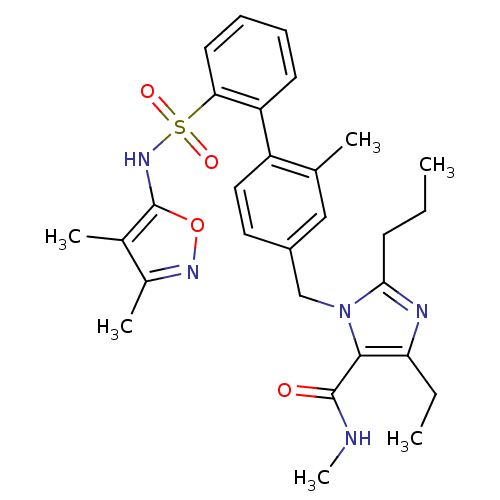 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50125339 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human ETA receptor expressed in CHO-K1 cells in the presence of 0.05 nM [125I]-labeled endothelin 1 | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125340 (2'-Methoxymethyl-4'-(8-oxo-2-propyl-5,6,7,8-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50117911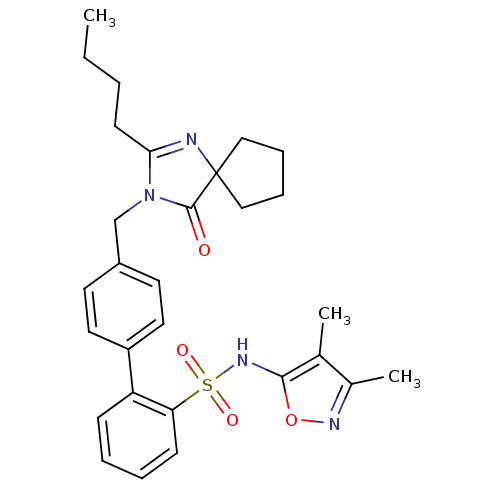 (4'-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125360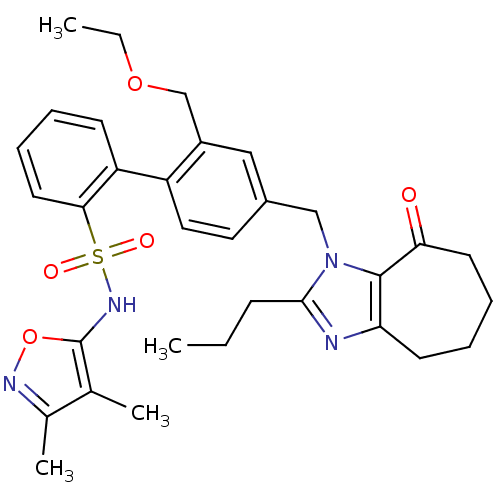 (2'-Ethoxymethyl-4'-(8-oxo-2-propyl-5,6,7,8-tetrahy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50125345 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human ETA receptor expressed in CHO-K1 cells in the presence of 0.05 nM [125I]-labeled endothelin 1 | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50125359 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human ETA receptor expressed in CHO-K1 cells in the presence of 0.05 nM [125I]-labeled endothelin 1 | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50194976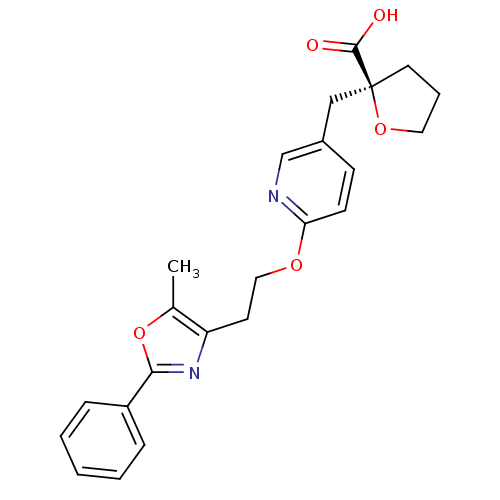 ((S)-2-((6-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Displacement of radiolabeled darglitazone from human PPAR gamma by SPA binding assay | Bioorg Med Chem Lett 16: 6120-3 (2006) Article DOI: 10.1016/j.bmcl.2006.08.110 BindingDB Entry DOI: 10.7270/Q20Z72XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50117910 (4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50117910 (4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50004108 ((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharmaceuticals Curated by PDSP Ki Database | Bioorg Med Chem Lett 18: 188-93 (2008) Article DOI: 10.1016/j.bmcl.2007.10.101 BindingDB Entry DOI: 10.7270/Q2C827W3 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125358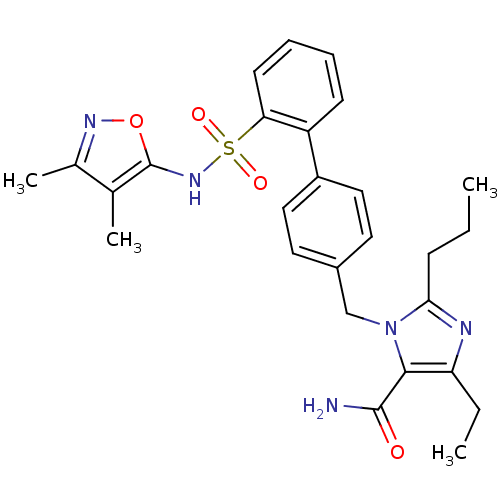 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-biphen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50194957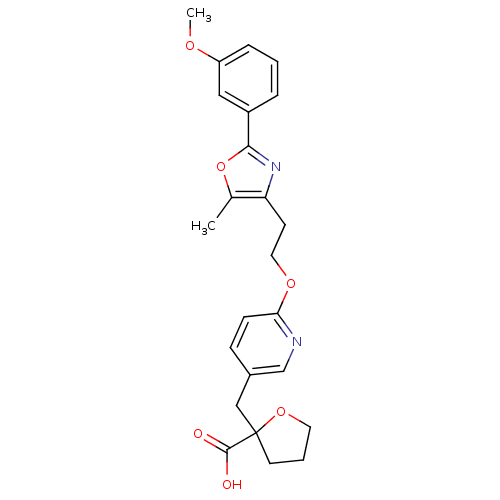 (2-((6-(2-(2-(3-methoxyphenyl)-5-methyloxazol-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Displacement of radiolabeled darglitazone from human PPAR gamma by SPA binding assay | Bioorg Med Chem Lett 16: 6120-3 (2006) Article DOI: 10.1016/j.bmcl.2006.08.110 BindingDB Entry DOI: 10.7270/Q20Z72XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50125362 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-ethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human ETA receptor expressed in CHO-K1 cells in the presence of 0.05 nM [125I]-labeled endothelin 1 | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50125361 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human ETA receptor expressed in CHO-K1 cells in the presence of 0.05 nM [125I]-labeled endothelin 1 | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125341 (4'-(8-Oxo-2-propyl-5,6,7,8-tetrahydro-4H-cyclohept...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50125356 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-(2-f...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human ETA receptor expressed in CHO-K1 cells in the presence of 0.05 nM [125I]-labeled endothelin 1 | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125361 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50117910 (4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM86831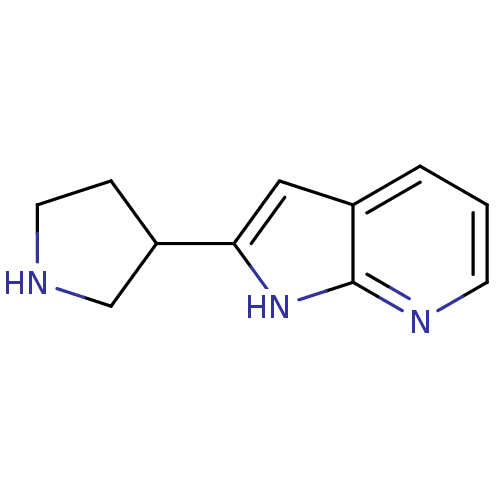 ((+/-)-2-(pyrrolidin-3-yl)-1H-pyrrolo[2,3-b]pyridin...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharmaceuticals Curated by PDSP Ki Database | Bioorg Med Chem Lett 18: 188-93 (2008) Article DOI: 10.1016/j.bmcl.2007.10.101 BindingDB Entry DOI: 10.7270/Q2C827W3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50194955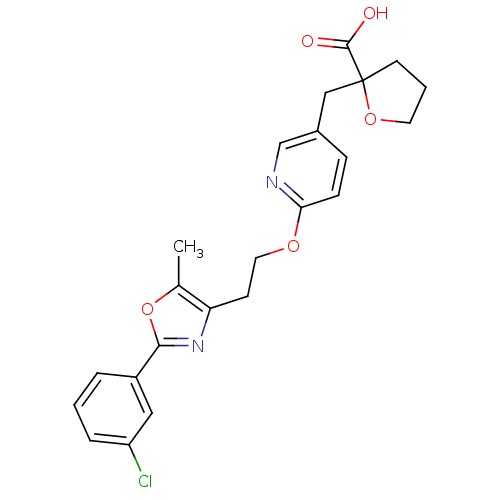 (2-((6-(2-(2-(3-chlorophenyl)-5-methyloxazol-4-yl)e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Displacement of radiolabeled darglitazone from human PPAR gamma by SPA binding assay | Bioorg Med Chem Lett 16: 6120-3 (2006) Article DOI: 10.1016/j.bmcl.2006.08.110 BindingDB Entry DOI: 10.7270/Q20Z72XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM86826 ((S)-5-(5-methoxypyridin-3-yl)-N-methylpent-4-en-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharmaceuticals Curated by PDSP Ki Database | Bioorg Med Chem Lett 18: 188-93 (2008) Article DOI: 10.1016/j.bmcl.2007.10.101 BindingDB Entry DOI: 10.7270/Q2C827W3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125344 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-biphen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50125352 (2'-Methyl-4'-(8-oxo-2-propyl-5,6,7,8-tetrahydro-4H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human ETA receptor expressed in CHO-K1 cells in the presence of 0.05 nM [125I]-labeled endothelin 1 | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125348 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-etho...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50125347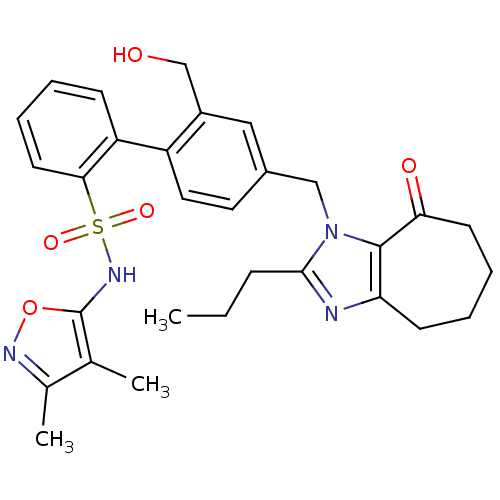 (2'-Hydroxymethyl-4'-(8-oxo-2-propyl-5,6,7,8-tetrah...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human ETA receptor expressed in CHO-K1 cells in the presence of 0.05 nM [125I]-labeled endothelin 1 | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50194968 (3-(6-(4-(3-chlorophenoxy)-2-methylphenethoxy)pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Displacement of radiolabeled darglitazone from human PPAR gamma by SPA binding assay | Bioorg Med Chem Lett 16: 6120-3 (2006) Article DOI: 10.1016/j.bmcl.2006.08.110 BindingDB Entry DOI: 10.7270/Q20Z72XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50194971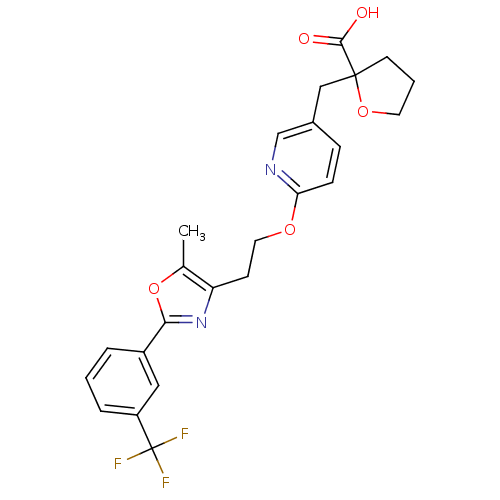 (2-((6-(2-(5-methyl-2-(3-(trifluoromethyl)phenyl)ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Displacement of radiolabeled darglitazone from human PPAR gamma by SPA binding assay | Bioorg Med Chem Lett 16: 6120-3 (2006) Article DOI: 10.1016/j.bmcl.2006.08.110 BindingDB Entry DOI: 10.7270/Q20Z72XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50125354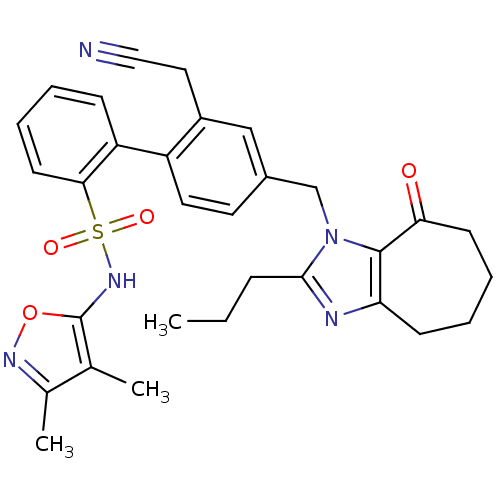 (2'-Cyanomethyl-4'-(8-oxo-2-propyl-5,6,7,8-tetrahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human ETA receptor expressed in CHO-K1 cells in the presence of 0.05 nM [125I]-labeled endothelin 1 | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50194961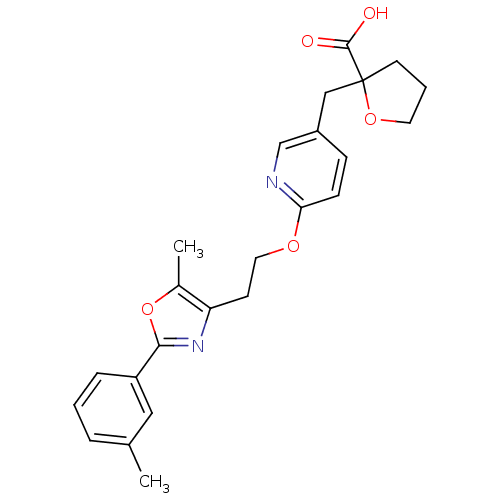 (2-((6-(2-(5-methyl-2-m-tolyloxazol-4-yl)ethoxy)pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Displacement of radiolabeled darglitazone from human PPAR gamma by SPA binding assay | Bioorg Med Chem Lett 16: 6120-3 (2006) Article DOI: 10.1016/j.bmcl.2006.08.110 BindingDB Entry DOI: 10.7270/Q20Z72XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125354 (2'-Cyanomethyl-4'-(8-oxo-2-propyl-5,6,7,8-tetrahyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125355 (2'-Fluoro-4'-(8-oxo-2-propyl-5,6,7,8-tetrahydro-4H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM86831 ((+/-)-2-(pyrrolidin-3-yl)-1H-pyrrolo[2,3-b]pyridin...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharmaceuticals Curated by PDSP Ki Database | Bioorg Med Chem Lett 18: 188-93 (2008) Article DOI: 10.1016/j.bmcl.2007.10.101 BindingDB Entry DOI: 10.7270/Q2C827W3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50125344 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-biphen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human ETA receptor expressed in CHO-K1 cells in the presence of 0.05 nM [125I]-labeled endothelin 1 | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50194950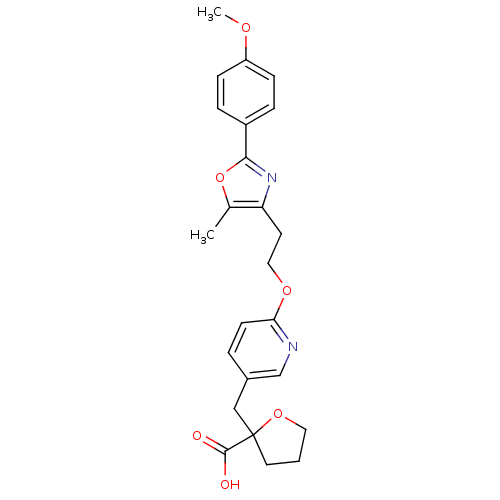 (2-((6-(2-(2-(4-methoxyphenyl)-5-methyloxazol-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Displacement of radiolabeled darglitazone from human PPAR gamma by SPA binding assay | Bioorg Med Chem Lett 16: 6120-3 (2006) Article DOI: 10.1016/j.bmcl.2006.08.110 BindingDB Entry DOI: 10.7270/Q20Z72XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 696 total ) | Next | Last >> |