 Found 286 hits with Last Name = 'mukaiyama' and Initial = 'h'
Found 286 hits with Last Name = 'mukaiyama' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50273137

((3-{[(3-Carbamimidoyl-phenyl)-({4-[1-(1-imino-ethy...)Show SMILES CC(=N)N1CCC(CC1)c1ccc(NC(=O)CN(Cc2cccc(OCC(O)=O)c2)c2cccc(c2)C(N)=N)cc1 Show InChI InChI=1S/C31H36N6O4/c1-21(32)36-14-12-24(13-15-36)23-8-10-26(11-9-23)35-29(38)19-37(27-6-3-5-25(17-27)31(33)34)18-22-4-2-7-28(16-22)41-20-30(39)40/h2-11,16-17,24,32H,12-15,18-20H2,1H3,(H3,33,34)(H,35,38)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50273136

(CHEMBL455932 | CHEMBL463179 | {4-[2-(5-Carbamimido...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(CCNS(=O)(=O)c2cc(ccc2O)C(N)=N)c(OCC(O)=O)c1 Show InChI InChI=1S/C24H25N3O8S2/c1-36(31,32)21-5-3-2-4-18(21)16-7-6-15(20(12-16)35-14-23(29)30)10-11-27-37(33,34)22-13-17(24(25)26)8-9-19(22)28/h2-9,12-13,27-28H,10-11,14H2,1H3,(H3,25,26)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50273299
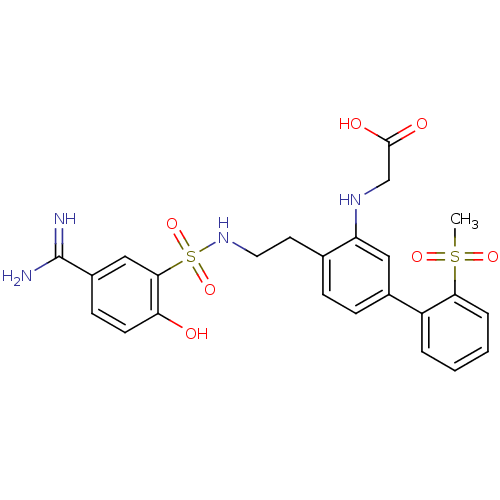
(CHEMBL455933 | {4-[2-(5-Carbamimidoyl-2-hydroxy-be...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(CCNS(=O)(=O)c2cc(ccc2O)C(N)=N)c(NCC(O)=O)c1 Show InChI InChI=1S/C24H26N4O7S2/c1-36(32,33)21-5-3-2-4-18(21)16-7-6-15(19(12-16)27-14-23(30)31)10-11-28-37(34,35)22-13-17(24(25)26)8-9-20(22)29/h2-9,12-13,27-29H,10-11,14H2,1H3,(H3,25,26)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50273297
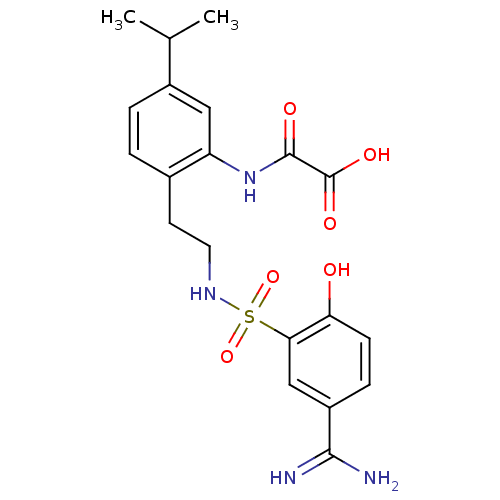
(CHEMBL459136 | N-{2-[2-(5-Carbamimidoyl-2-hydroxy-...)Show SMILES CC(C)c1ccc(CCNS(=O)(=O)c2cc(ccc2O)C(N)=N)c(NC(=O)C(O)=O)c1 Show InChI InChI=1S/C20H24N4O6S/c1-11(2)13-4-3-12(15(9-13)24-19(26)20(27)28)7-8-23-31(29,30)17-10-14(18(21)22)5-6-16(17)25/h3-6,9-11,23,25H,7-8H2,1-2H3,(H3,21,22)(H,24,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50273229
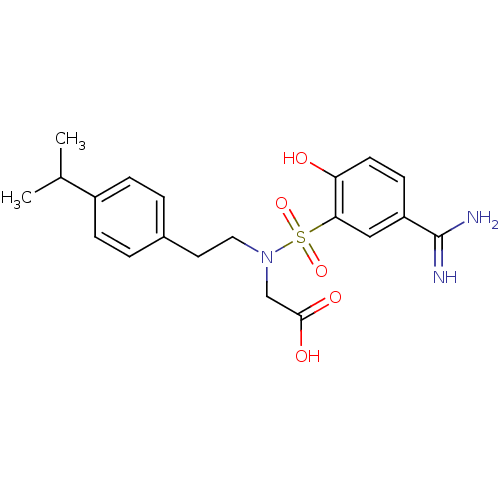
(CHEMBL455916 | {(5-Carbamimidoyl-2-hydroxy-benzene...)Show SMILES CC(C)c1ccc(CCN(CC(O)=O)S(=O)(=O)c2cc(ccc2O)C(N)=N)cc1 Show InChI InChI=1S/C20H25N3O5S/c1-13(2)15-5-3-14(4-6-15)9-10-23(12-19(25)26)29(27,28)18-11-16(20(21)22)7-8-17(18)24/h3-8,11,13,24H,9-10,12H2,1-2H3,(H3,21,22)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50273188

(3-[2-(4-tert-Butyl-phenyl)-ethylsulfamoyl]-4-hydro...)Show SMILES CC(C)(C)c1ccc(CCNS(=O)(=O)c2cc(ccc2O)C(N)=N)cc1 Show InChI InChI=1S/C19H25N3O3S/c1-19(2,3)15-7-4-13(5-8-15)10-11-22-26(24,25)17-12-14(18(20)21)6-9-16(17)23/h4-9,12,22-23H,10-11H2,1-3H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50273138
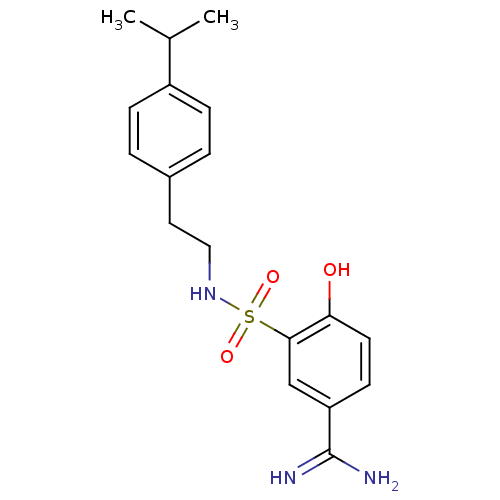
(4-Hydroxy-3-[2-(4-isopropyl-phenyl)-ethylsulfamoyl...)Show SMILES CC(C)c1ccc(CCNS(=O)(=O)c2cc(ccc2O)C(N)=N)cc1 Show InChI InChI=1S/C18H23N3O3S/c1-12(2)14-5-3-13(4-6-14)9-10-21-25(23,24)17-11-15(18(19)20)7-8-16(17)22/h3-8,11-12,21-22H,9-10H2,1-2H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17283

((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)[C@H](Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O |r| Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a in human plasma |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50273262
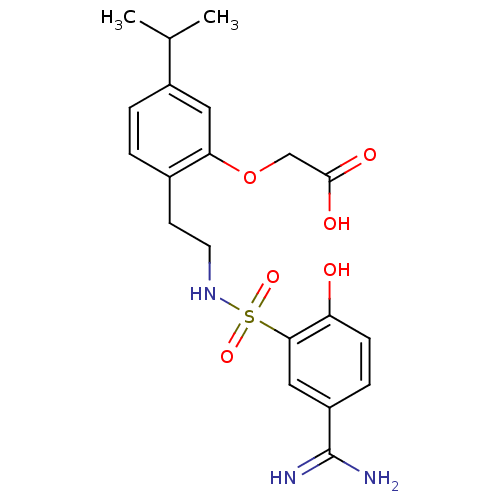
(CHEMBL458067 | {2-[2-(5-Carbamimidoyl-2-hydroxy-be...)Show SMILES CC(C)c1ccc(CCNS(=O)(=O)c2cc(ccc2O)C(N)=N)c(OCC(O)=O)c1 Show InChI InChI=1S/C20H25N3O6S/c1-12(2)14-4-3-13(17(9-14)29-11-19(25)26)7-8-23-30(27,28)18-10-15(20(21)22)5-6-16(18)24/h3-6,9-10,12,23-24H,7-8,11H2,1-2H3,(H3,21,22)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50273227

(4-Hydroxy-3-{[2-(4-isopropyl-phenyl)-ethyl]-methyl...)Show SMILES CC(C)c1ccc(CCN(C)S(=O)(=O)c2cc(ccc2O)C(N)=N)cc1 Show InChI InChI=1S/C19H25N3O3S/c1-13(2)15-6-4-14(5-7-15)10-11-22(3)26(24,25)18-12-16(19(20)21)8-9-17(18)23/h4-9,12-13,23H,10-11H2,1-3H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50273265
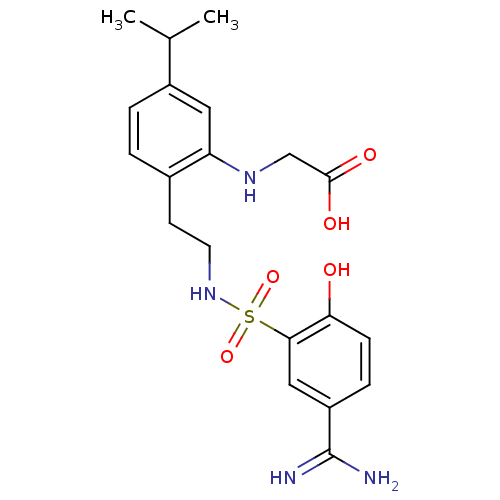
(CHEMBL457633 | {2-[2-(5-Carbamimidoyl-2-hydroxy-be...)Show SMILES CC(C)c1ccc(CCNS(=O)(=O)c2cc(ccc2O)C(N)=N)c(NCC(O)=O)c1 Show InChI InChI=1S/C20H26N4O5S/c1-12(2)14-4-3-13(16(9-14)23-11-19(26)27)7-8-24-30(28,29)18-10-15(20(21)22)5-6-17(18)25/h3-6,9-10,12,23-25H,7-8,11H2,1-2H3,(H3,21,22)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50273187
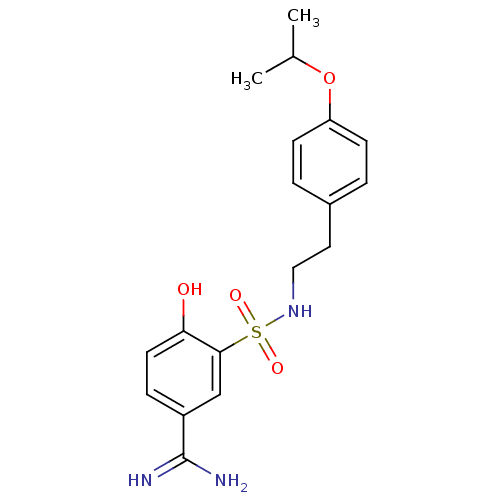
(4-Hydroxy-3-[2-(4-isopropoxy-phenyl)-ethylsulfamoy...)Show SMILES CC(C)Oc1ccc(CCNS(=O)(=O)c2cc(ccc2O)C(N)=N)cc1 Show InChI InChI=1S/C18H23N3O4S/c1-12(2)25-15-6-3-13(4-7-15)9-10-21-26(23,24)17-11-14(18(19)20)5-8-16(17)22/h3-8,11-12,21-22H,9-10H2,1-2H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50273136

(CHEMBL455932 | CHEMBL463179 | {4-[2-(5-Carbamimido...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(CCNS(=O)(=O)c2cc(ccc2O)C(N)=N)c(OCC(O)=O)c1 Show InChI InChI=1S/C24H25N3O8S2/c1-36(31,32)21-5-3-2-4-18(21)16-7-6-15(20(12-16)35-14-23(29)30)10-11-27-37(33,34)22-13-17(24(25)26)8-9-19(22)28/h2-9,12-13,27-28H,10-11,14H2,1H3,(H3,25,26)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50273189
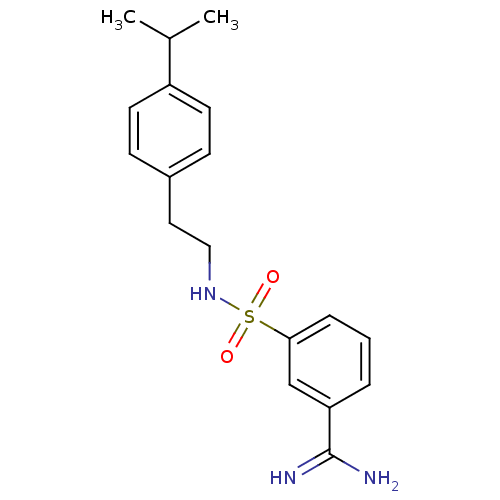
(3-[2-(4-Isopropyl-phenyl)-ethylsulfamoyl]-benzamid...)Show SMILES CC(C)c1ccc(CCNS(=O)(=O)c2cccc(c2)C(N)=N)cc1 Show InChI InChI=1S/C18H23N3O2S/c1-13(2)15-8-6-14(7-9-15)10-11-21-24(22,23)17-5-3-4-16(12-17)18(19)20/h3-9,12-13,21H,10-11H2,1-2H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50273190
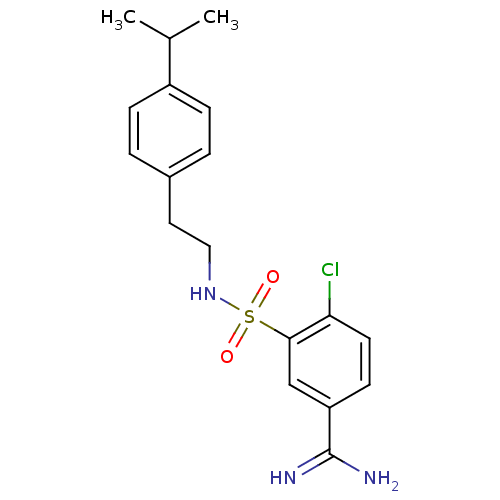
(4-Chloro-3-[2-(4-isopropyl-phenyl)-ethylsulfamoyl]...)Show SMILES CC(C)c1ccc(CCNS(=O)(=O)c2cc(ccc2Cl)C(N)=N)cc1 Show InChI InChI=1S/C18H22ClN3O2S/c1-12(2)14-5-3-13(4-6-14)9-10-22-25(23,24)17-11-15(18(20)21)7-8-16(17)19/h3-8,11-12,22H,9-10H2,1-2H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50273264

(CHEMBL457632 | ethyl 2-(2-(2-(2-hydroxy-5-(N'-hydr...)Show SMILES CCOC(=O)COc1cc(ccc1CCNS(=O)(=O)c1cc(ccc1O)C(=N)NO)C(C)C Show InChI InChI=1S/C22H29N3O7S/c1-4-31-21(27)13-32-19-11-16(14(2)3)6-5-15(19)9-10-24-33(29,30)20-12-17(22(23)25-28)7-8-18(20)26/h5-8,11-12,14,24,26,28H,4,9-10,13H2,1-3H3,(H2,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50273231

(CHEMBL455919 | ethyl 2-(N-(4-isopropylphenethyl)-2...)Show SMILES CCOC(=O)CN(CCc1ccc(cc1)C(C)C)S(=O)(=O)c1cc(ccc1O)C(=N)NO Show InChI InChI=1S/C22H29N3O6S/c1-4-31-21(27)14-25(12-11-16-5-7-17(8-6-16)15(2)3)32(29,30)20-13-18(22(23)24-28)9-10-19(20)26/h5-10,13,15,26,28H,4,11-12,14H2,1-3H3,(H2,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50273230

(CHEMBL509493 | {[2-Hydroxy-5-(N-hydroxycarbamimido...)Show SMILES CC(C)c1ccc(CCN(CC(O)=O)S(=O)(=O)c2cc(ccc2O)C(=N)NO)cc1 Show InChI InChI=1S/C20H25N3O6S/c1-13(2)15-5-3-14(4-6-15)9-10-23(12-19(25)26)30(28,29)18-11-16(20(21)22-27)7-8-17(18)24/h3-8,11,13,24,27H,9-10,12H2,1-2H3,(H2,21,22)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50273228
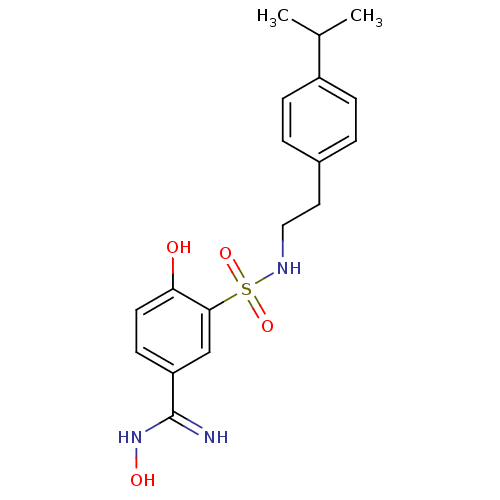
(4,N-Dihydroxy-3-[2-(4-isopropyl-phenyl)-ethylsulfa...)Show SMILES CC(C)c1ccc(CCNS(=O)(=O)c2cc(ccc2O)C(=N)NO)cc1 Show InChI InChI=1S/C18H23N3O4S/c1-12(2)14-5-3-13(4-6-14)9-10-20-26(24,25)17-11-15(18(19)21-23)7-8-16(17)22/h3-8,11-12,20,22-23H,9-10H2,1-2H3,(H2,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50273263
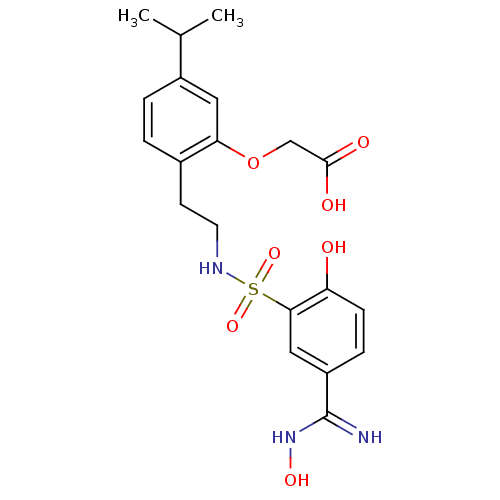
(2-(2-(2-(2-hydroxy-5-(N'-hydroxycarbamimidoyl)phen...)Show SMILES CC(C)c1ccc(CCNS(=O)(=O)c2cc(ccc2O)C(=N)NO)c(OCC(O)=O)c1 Show InChI InChI=1S/C20H25N3O7S/c1-12(2)14-4-3-13(17(9-14)30-11-19(25)26)7-8-22-31(28,29)18-10-15(20(21)23-27)5-6-16(18)24/h3-6,9-10,12,22,24,27H,7-8,11H2,1-2H3,(H2,21,23)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50273138

(4-Hydroxy-3-[2-(4-isopropyl-phenyl)-ethylsulfamoyl...)Show SMILES CC(C)c1ccc(CCNS(=O)(=O)c2cc(ccc2O)C(N)=N)cc1 Show InChI InChI=1S/C18H23N3O3S/c1-12(2)14-5-3-13(4-6-14)9-10-21-25(23,24)17-11-15(18(19)20)7-8-16(17)22/h3-8,11-12,21-22H,9-10H2,1-2H3,(H3,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50273187

(4-Hydroxy-3-[2-(4-isopropoxy-phenyl)-ethylsulfamoy...)Show SMILES CC(C)Oc1ccc(CCNS(=O)(=O)c2cc(ccc2O)C(N)=N)cc1 Show InChI InChI=1S/C18H23N3O4S/c1-12(2)25-15-6-3-13(4-7-15)9-10-21-26(23,24)17-11-14(18(19)20)5-8-16(17)22/h3-8,11-12,21-22H,9-10H2,1-2H3,(H3,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50273189

(3-[2-(4-Isopropyl-phenyl)-ethylsulfamoyl]-benzamid...)Show SMILES CC(C)c1ccc(CCNS(=O)(=O)c2cccc(c2)C(N)=N)cc1 Show InChI InChI=1S/C18H23N3O2S/c1-13(2)15-8-6-14(7-9-15)10-11-21-24(22,23)17-5-3-4-16(12-17)18(19)20/h3-9,12-13,21H,10-11H2,1-2H3,(H3,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50273191
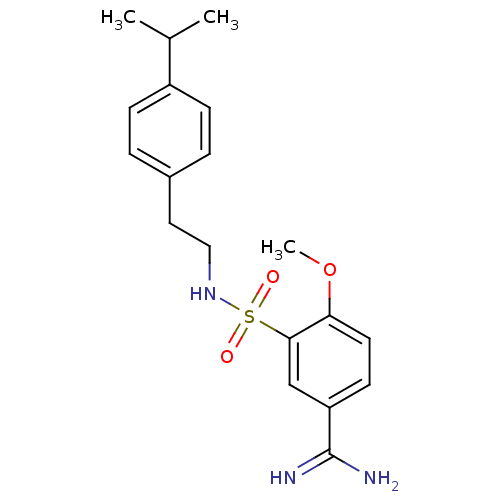
(3-[2-(4-Isopropyl-phenyl)-ethylsulfamoyl]-4-methox...)Show SMILES COc1ccc(cc1S(=O)(=O)NCCc1ccc(cc1)C(C)C)C(N)=N Show InChI InChI=1S/C19H25N3O3S/c1-13(2)15-6-4-14(5-7-15)10-11-22-26(23,24)18-12-16(19(20)21)8-9-17(18)25-3/h4-9,12-13,22H,10-11H2,1-3H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50273137

((3-{[(3-Carbamimidoyl-phenyl)-({4-[1-(1-imino-ethy...)Show SMILES CC(=N)N1CCC(CC1)c1ccc(NC(=O)CN(Cc2cccc(OCC(O)=O)c2)c2cccc(c2)C(N)=N)cc1 Show InChI InChI=1S/C31H36N6O4/c1-21(32)36-14-12-24(13-15-36)23-8-10-26(11-9-23)35-29(38)19-37(27-6-3-5-25(17-27)31(33)34)18-22-4-2-7-28(16-22)41-20-30(39)40/h2-11,16-17,24,32H,12-15,18-20H2,1H3,(H3,33,34)(H,35,38)(H,39,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50273136

(CHEMBL455932 | CHEMBL463179 | {4-[2-(5-Carbamimido...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(CCNS(=O)(=O)c2cc(ccc2O)C(N)=N)c(OCC(O)=O)c1 Show InChI InChI=1S/C24H25N3O8S2/c1-36(31,32)21-5-3-2-4-18(21)16-7-6-15(20(12-16)35-14-23(29)30)10-11-27-37(33,34)22-13-17(24(25)26)8-9-19(22)28/h2-9,12-13,27-28H,10-11,14H2,1H3,(H3,25,26)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin by Dixon-plot method |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM17283

((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)[C@H](Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O |r| Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 18: 4682-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.009
BindingDB Entry DOI: 10.7270/Q208654H |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Canis familiaris) | BDBM25392

(4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;...)Show InChI InChI=1S/C11H17NO3/c1-7(2)12-6-11(15)8-3-4-9(13)10(14)5-8/h3-5,7,11-15H,6H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Agonistic activity towards beta-2 adrenoceptor. IC50, the mean concentration required to produce 50% inhibition of uterine contraction |
J Med Chem 44: 1436-45 (2001)
BindingDB Entry DOI: 10.7270/Q2Q52QVH |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Canis familiaris) | BDBM25392

(4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;...)Show InChI InChI=1S/C11H17NO3/c1-7(2)12-6-11(15)8-3-4-9(13)10(14)5-8/h3-5,7,11-15H,6H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Agonistic activity for Beta-2 adrenergic receptor was assessed by it's inhibitory effect on spontaneous contractions in isolated rat uterus |
J Med Chem 46: 105-12 (2002)
Article DOI: 10.1021/jm020177z
BindingDB Entry DOI: 10.7270/Q2BP03J1 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Rattus norvegicus) | BDBM25392

(4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;...)Show InChI InChI=1S/C11H17NO3/c1-7(2)12-6-11(15)8-3-4-9(13)10(14)5-8/h3-5,7,11-15H,6H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of spontaneous contractions in isolated rat uterus |
J Med Chem 46: 105-12 (2002)
Article DOI: 10.1021/jm020177z
BindingDB Entry DOI: 10.7270/Q2BP03J1 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50375355
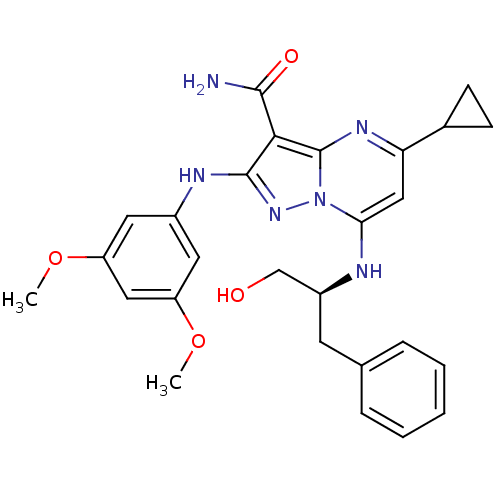
(CHEMBL260861)Show SMILES COc1cc(Nc2nn3c(N[C@H](CO)Cc4ccccc4)cc(nc3c2C(N)=O)C2CC2)cc(OC)c1 Show InChI InChI=1S/C27H30N6O4/c1-36-20-11-18(12-21(13-20)37-2)30-26-24(25(28)35)27-31-22(17-8-9-17)14-23(33(27)32-26)29-19(15-34)10-16-6-4-3-5-7-16/h3-7,11-14,17,19,29,34H,8-10,15H2,1-2H3,(H2,28,35)(H,30,32)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human cSrc |
Bioorg Med Chem 16: 909-21 (2008)
Article DOI: 10.1016/j.bmc.2007.10.068
BindingDB Entry DOI: 10.7270/Q2CF9R01 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50059889

((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...)Show SMILES CN[C@@H]1CC2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20?,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human Syk expressed in Sf9 cells |
Bioorg Med Chem 16: 9247-60 (2008)
Article DOI: 10.1016/j.bmc.2008.09.015
BindingDB Entry DOI: 10.7270/Q2GM887N |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50059889

((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...)Show SMILES CN[C@@H]1CC2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20?,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta2 (unknown origin) |
Bioorg Med Chem 16: 7347-57 (2008)
Article DOI: 10.1016/j.bmc.2008.06.017
BindingDB Entry DOI: 10.7270/Q2HQ3ZP6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249539
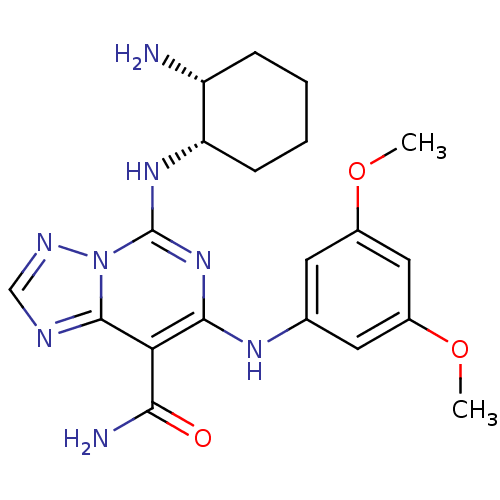
(5-((1S,2R)-2-aminocyclohexylamino)-7-(3,5-dimethox...)Show SMILES COc1cc(Nc2nc(N[C@H]3CCCC[C@H]3N)n3ncnc3c2C(N)=O)cc(OC)c1 |r| Show InChI InChI=1S/C20H26N8O3/c1-30-12-7-11(8-13(9-12)31-2)25-18-16(17(22)29)19-23-10-24-28(19)20(27-18)26-15-6-4-3-5-14(15)21/h7-10,14-15,25H,3-6,21H2,1-2H3,(H2,22,29)(H,26,27)/t14-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human Syk expressed in Sf9 cells |
Bioorg Med Chem 16: 9247-60 (2008)
Article DOI: 10.1016/j.bmc.2008.09.015
BindingDB Entry DOI: 10.7270/Q2GM887N |
More data for this
Ligand-Target Pair | |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM50249539

(5-((1S,2R)-2-aminocyclohexylamino)-7-(3,5-dimethox...)Show SMILES COc1cc(Nc2nc(N[C@H]3CCCC[C@H]3N)n3ncnc3c2C(N)=O)cc(OC)c1 |r| Show InChI InChI=1S/C20H26N8O3/c1-30-12-7-11(8-13(9-12)31-2)25-18-16(17(22)29)19-23-10-24-28(19)20(27-18)26-15-6-4-3-5-14(15)21/h7-10,14-15,25H,3-6,21H2,1-2H3,(H2,22,29)(H,26,27)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human Syk |
Bioorg Med Chem 16: 7347-57 (2008)
Article DOI: 10.1016/j.bmc.2008.06.017
BindingDB Entry DOI: 10.7270/Q2HQ3ZP6 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50375360
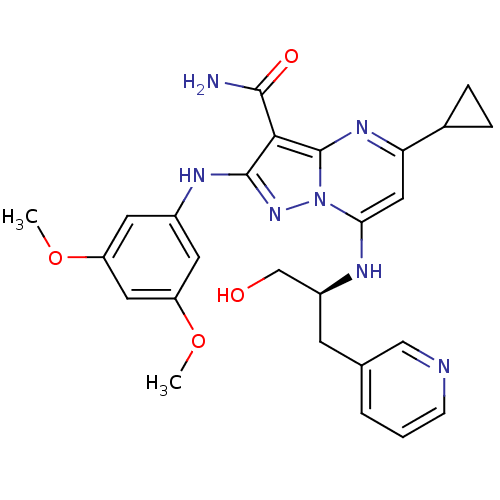
(CHEMBL261188)Show SMILES COc1cc(Nc2nn3c(N[C@H](CO)Cc4cccnc4)cc(nc3c2C(N)=O)C2CC2)cc(OC)c1 Show InChI InChI=1S/C26H29N7O4/c1-36-19-9-17(10-20(11-19)37-2)30-25-23(24(27)35)26-31-21(16-5-6-16)12-22(33(26)32-25)29-18(14-34)8-15-4-3-7-28-13-15/h3-4,7,9-13,16,18,29,34H,5-6,8,14H2,1-2H3,(H2,27,35)(H,30,32)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human cSrc |
Bioorg Med Chem 16: 909-21 (2008)
Article DOI: 10.1016/j.bmc.2007.10.068
BindingDB Entry DOI: 10.7270/Q2CF9R01 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50375361
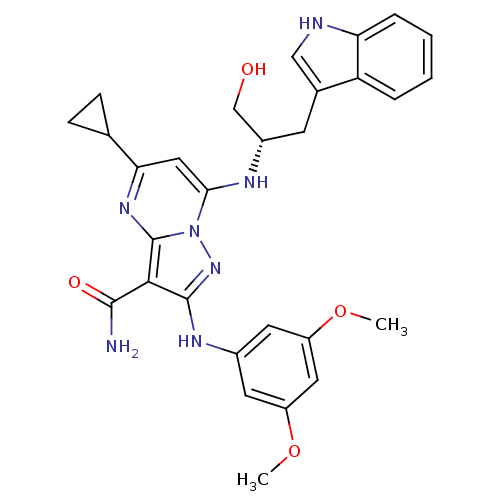
(CHEMBL260157)Show SMILES COc1cc(Nc2nn3c(N[C@H](CO)Cc4c[nH]c5ccccc45)cc(nc3c2C(N)=O)C2CC2)cc(OC)c1 Show InChI InChI=1S/C29H31N7O4/c1-39-20-10-18(11-21(12-20)40-2)33-28-26(27(30)38)29-34-24(16-7-8-16)13-25(36(29)35-28)32-19(15-37)9-17-14-31-23-6-4-3-5-22(17)23/h3-6,10-14,16,19,31-32,37H,7-9,15H2,1-2H3,(H2,30,38)(H,33,35)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human cSrc |
Bioorg Med Chem 16: 909-21 (2008)
Article DOI: 10.1016/j.bmc.2007.10.068
BindingDB Entry DOI: 10.7270/Q2CF9R01 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50274673
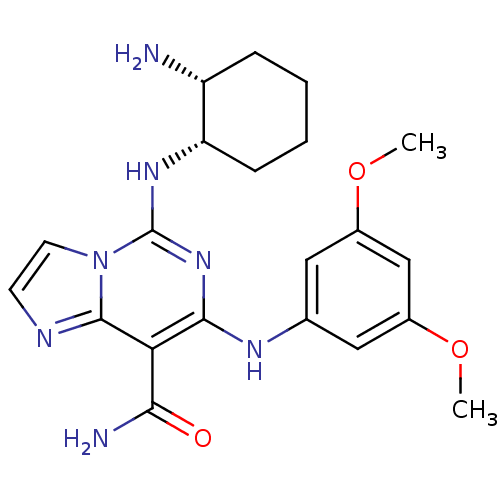
(CHEMBL485204 | cis-5-(2-Aminocyclohexylamino)-7-(3...)Show SMILES COc1cc(Nc2nc(N[C@H]3CCCC[C@H]3N)n3ccnc3c2C(N)=O)cc(OC)c1 |r| Show InChI InChI=1S/C21H27N7O3/c1-30-13-9-12(10-14(11-13)31-2)25-19-17(18(23)29)20-24-7-8-28(20)21(27-19)26-16-6-4-3-5-15(16)22/h7-11,15-16,25H,3-6,22H2,1-2H3,(H2,23,29)(H,26,27)/t15-,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human Syk expressed in Sf9 cells |
Bioorg Med Chem 16: 9247-60 (2008)
Article DOI: 10.1016/j.bmc.2008.09.015
BindingDB Entry DOI: 10.7270/Q2GM887N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50274756

(CHEMBL459146 | cis-5-(2-Aminocyclohexylamino)-7-(3...)Show SMILES Cc1cc(C)cc(Nc2nc(N[C@H]3CCCC[C@H]3N)n3ccnc3c2C(N)=O)c1 |r| Show InChI InChI=1S/C21H27N7O/c1-12-9-13(2)11-14(10-12)25-19-17(18(23)29)20-24-7-8-28(20)21(27-19)26-16-6-4-3-5-15(16)22/h7-11,15-16,25H,3-6,22H2,1-2H3,(H2,23,29)(H,26,27)/t15-,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human Syk expressed in Sf9 cells |
Bioorg Med Chem 16: 9247-60 (2008)
Article DOI: 10.1016/j.bmc.2008.09.015
BindingDB Entry DOI: 10.7270/Q2GM887N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50375355

(CHEMBL260861)Show SMILES COc1cc(Nc2nn3c(N[C@H](CO)Cc4ccccc4)cc(nc3c2C(N)=O)C2CC2)cc(OC)c1 Show InChI InChI=1S/C27H30N6O4/c1-36-20-11-18(12-21(13-20)37-2)30-26-24(25(28)35)27-31-22(17-8-9-17)14-23(33(27)32-26)29-19(15-34)10-16-6-4-3-5-7-16/h3-7,11-14,17,19,29,34H,8-10,15H2,1-2H3,(H2,28,35)(H,30,32)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem 16: 909-21 (2008)
Article DOI: 10.1016/j.bmc.2007.10.068
BindingDB Entry DOI: 10.7270/Q2CF9R01 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50375366
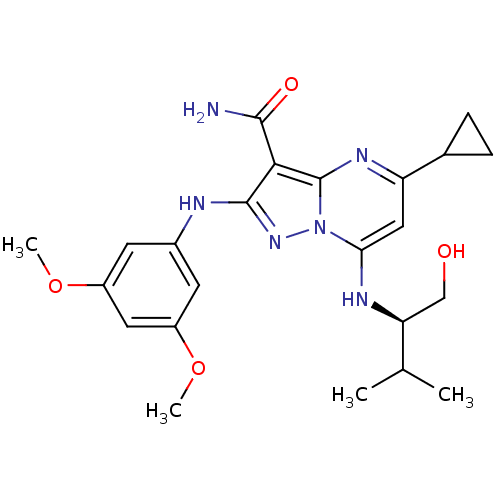
(CHEMBL406418)Show SMILES COc1cc(Nc2nn3c(N[C@@H](CO)C(C)C)cc(nc3c2C(N)=O)C2CC2)cc(OC)c1 Show InChI InChI=1S/C23H30N6O4/c1-12(2)18(11-30)26-19-10-17(13-5-6-13)27-23-20(21(24)31)22(28-29(19)23)25-14-7-15(32-3)9-16(8-14)33-4/h7-10,12-13,18,26,30H,5-6,11H2,1-4H3,(H2,24,31)(H,25,28)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human cSrc |
Bioorg Med Chem 16: 909-21 (2008)
Article DOI: 10.1016/j.bmc.2007.10.068
BindingDB Entry DOI: 10.7270/Q2CF9R01 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50198353
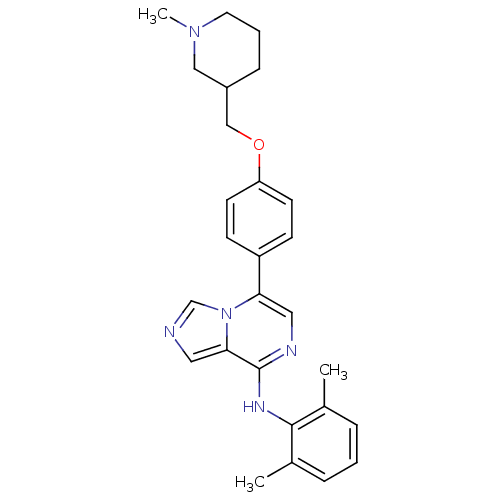
(CHEMBL227753 | CHEMBL534948 | N-(2,6-dimethylpheny...)Show SMILES CN1CCCC(COc2ccc(cc2)-c2cnc(Nc3c(C)cccc3C)c3cncn23)C1 Show InChI InChI=1S/C27H31N5O/c1-19-6-4-7-20(2)26(19)30-27-25-14-28-18-32(25)24(15-29-27)22-9-11-23(12-10-22)33-17-21-8-5-13-31(3)16-21/h4,6-7,9-12,14-15,18,21H,5,8,13,16-17H2,1-3H3,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem 15: 868-85 (2006)
Article DOI: 10.1016/j.bmc.2006.10.041
BindingDB Entry DOI: 10.7270/Q2DZ094H |
More data for this
Ligand-Target Pair | |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM50249253
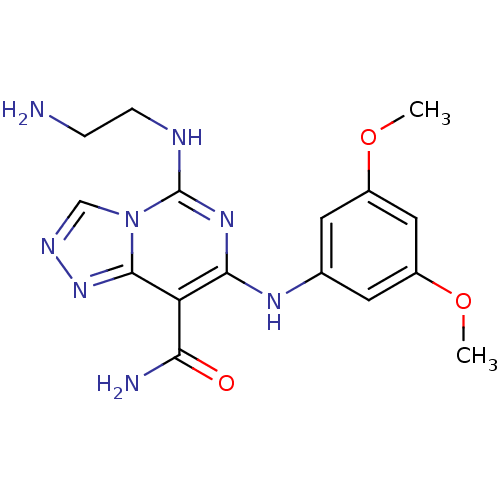
(5-(2-Aminoethylamino)-7-(3,5-dimethoxyphenylamino)...)Show InChI InChI=1S/C16H20N8O3/c1-26-10-5-9(6-11(7-10)27-2)21-14-12(13(18)25)15-23-20-8-24(15)16(22-14)19-4-3-17/h5-8,21H,3-4,17H2,1-2H3,(H2,18,25)(H,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human Syk |
Bioorg Med Chem 16: 7347-57 (2008)
Article DOI: 10.1016/j.bmc.2008.06.017
BindingDB Entry DOI: 10.7270/Q2HQ3ZP6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249254
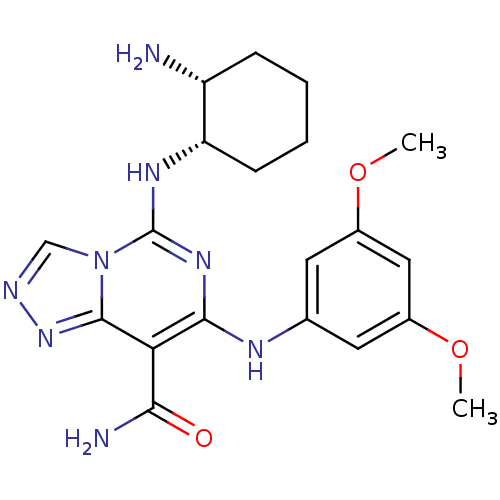
(5-((1S,2R)-2-aminocyclohexylamino)-7-(3,5-dimethox...)Show SMILES COc1cc(Nc2nc(N[C@H]3CCCC[C@H]3N)n3cnnc3c2C(N)=O)cc(OC)c1 |r| Show InChI InChI=1S/C20H26N8O3/c1-30-12-7-11(8-13(9-12)31-2)24-18-16(17(22)29)19-27-23-10-28(19)20(26-18)25-15-6-4-3-5-14(15)21/h7-10,14-15,24H,3-6,21H2,1-2H3,(H2,22,29)(H,25,26)/t14-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human Syk expressed in Sf9 cells |
Bioorg Med Chem 16: 9247-60 (2008)
Article DOI: 10.1016/j.bmc.2008.09.015
BindingDB Entry DOI: 10.7270/Q2GM887N |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50375364
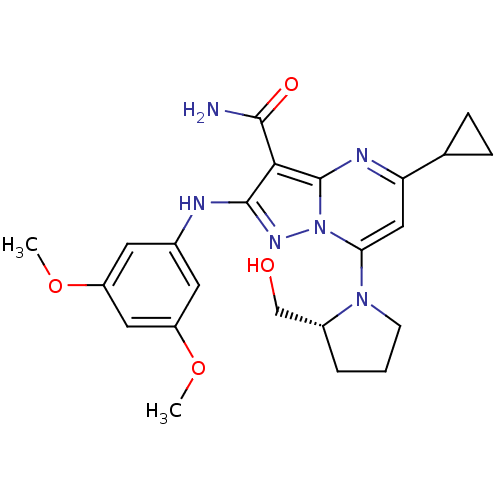
(CHEMBL261743)Show SMILES COc1cc(Nc2nn3c(cc(nc3c2C(N)=O)C2CC2)N2CCC[C@@H]2CO)cc(OC)c1 Show InChI InChI=1S/C23H28N6O4/c1-32-16-8-14(9-17(10-16)33-2)25-22-20(21(24)31)23-26-18(13-5-6-13)11-19(29(23)27-22)28-7-3-4-15(28)12-30/h8-11,13,15,30H,3-7,12H2,1-2H3,(H2,24,31)(H,25,27)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human cSrc |
Bioorg Med Chem 16: 909-21 (2008)
Article DOI: 10.1016/j.bmc.2007.10.068
BindingDB Entry DOI: 10.7270/Q2CF9R01 |
More data for this
Ligand-Target Pair | |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM50249254

(5-((1S,2R)-2-aminocyclohexylamino)-7-(3,5-dimethox...)Show SMILES COc1cc(Nc2nc(N[C@H]3CCCC[C@H]3N)n3cnnc3c2C(N)=O)cc(OC)c1 |r| Show InChI InChI=1S/C20H26N8O3/c1-30-12-7-11(8-13(9-12)31-2)24-18-16(17(22)29)19-27-23-10-28(19)20(26-18)25-15-6-4-3-5-14(15)21/h7-10,14-15,24H,3-6,21H2,1-2H3,(H2,22,29)(H,25,26)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human Syk |
Bioorg Med Chem 16: 7347-57 (2008)
Article DOI: 10.1016/j.bmc.2008.06.017
BindingDB Entry DOI: 10.7270/Q2HQ3ZP6 |
More data for this
Ligand-Target Pair | |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM50059889

((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...)Show SMILES CN[C@@H]1CC2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20?,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human Syk |
Bioorg Med Chem 16: 7347-57 (2008)
Article DOI: 10.1016/j.bmc.2008.06.017
BindingDB Entry DOI: 10.7270/Q2HQ3ZP6 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Rattus norvegicus) | BDBM50002133
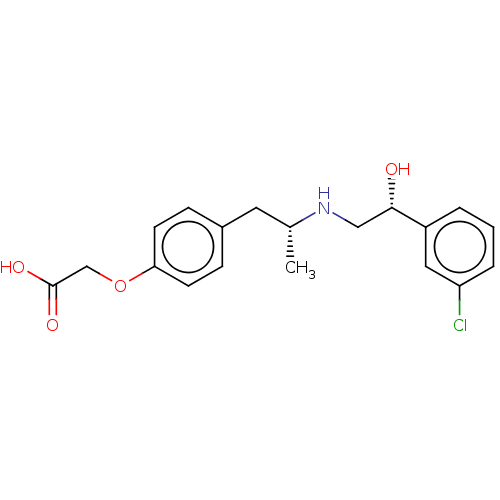
((+/-)-2-(4-((R)-2-((R)-2-(3-chlorophenyl)-2-hydrox...)Show SMILES C[C@H](Cc1ccc(OCC(O)=O)cc1)NC[C@H](O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H22ClNO4/c1-13(21-11-18(22)15-3-2-4-16(20)10-15)9-14-5-7-17(8-6-14)25-12-19(23)24/h2-8,10,13,18,21-22H,9,11-12H2,1H3,(H,23,24)/t13-,18+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of spontaneous contractions in isolated rat uterus |
J Med Chem 46: 105-12 (2002)
Article DOI: 10.1021/jm020177z
BindingDB Entry DOI: 10.7270/Q2BP03J1 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Canis familiaris) | BDBM50002133

((+/-)-2-(4-((R)-2-((R)-2-(3-chlorophenyl)-2-hydrox...)Show SMILES C[C@H](Cc1ccc(OCC(O)=O)cc1)NC[C@H](O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H22ClNO4/c1-13(21-11-18(22)15-3-2-4-16(20)10-15)9-14-5-7-17(8-6-14)25-12-19(23)24/h2-8,10,13,18,21-22H,9,11-12H2,1H3,(H,23,24)/t13-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Agonistic activity for Beta-2 adrenergic receptor was assessed by it's inhibitory effect on spontaneous contractions in isolated rat uterus |
J Med Chem 46: 105-12 (2002)
Article DOI: 10.1021/jm020177z
BindingDB Entry DOI: 10.7270/Q2BP03J1 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Rattus norvegicus) | BDBM50002133

((+/-)-2-(4-((R)-2-((R)-2-(3-chlorophenyl)-2-hydrox...)Show SMILES C[C@H](Cc1ccc(OCC(O)=O)cc1)NC[C@H](O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H22ClNO4/c1-13(21-11-18(22)15-3-2-4-16(20)10-15)9-14-5-7-17(8-6-14)25-12-19(23)24/h2-8,10,13,18,21-22H,9,11-12H2,1H3,(H,23,24)/t13-,18+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.12 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Agonistic activity towards beta-2 adrenoceptor. Mean concentration required to produce 50% inhibition of uterine contraction |
J Med Chem 44: 1436-45 (2001)
BindingDB Entry DOI: 10.7270/Q2Q52QVH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data