

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194790 (US9206167, 11 | US9206167, 12 | USRE48059, Compoun...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194790 (US9206167, 11 | US9206167, 12 | USRE48059, Compoun...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | -57.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194790 (US9206167, 11 | US9206167, 12 | USRE48059, Compoun...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | -57.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194790 (US9206167, 11 | US9206167, 12 | USRE48059, Compoun...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50065562 (CHEMBL96279 | [2-(6-Fluoro-2,2-dimethyl-chroman-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50065578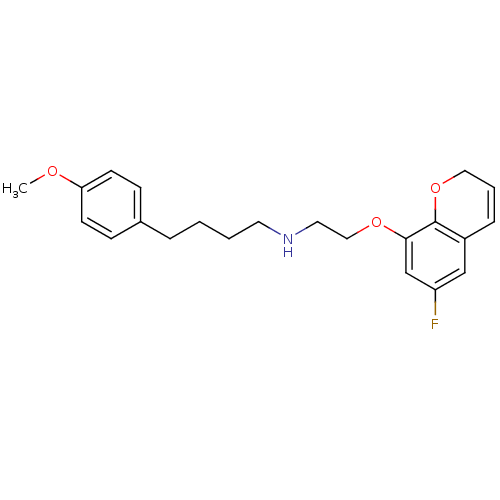 (CHEMBL419147 | [2-(6-Fluoro-2H-chromen-8-yloxy)-et...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.159 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor on rat hippocampus using [3H]-8-OH-DPAT as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50065555 (CHEMBL96578 | [2-(6-Fluoro-2-methyl-chroman-8-ylox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor on rat hippocampus using [3H]-8-OH-DPAT as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194794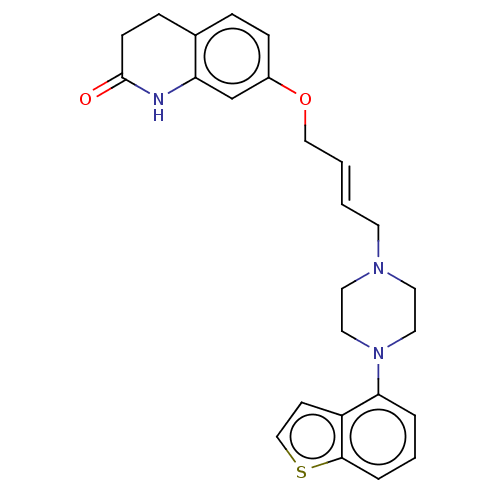 (US9206167, 15 | USRE48059, Compound of Example 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | -55.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194780 (7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194772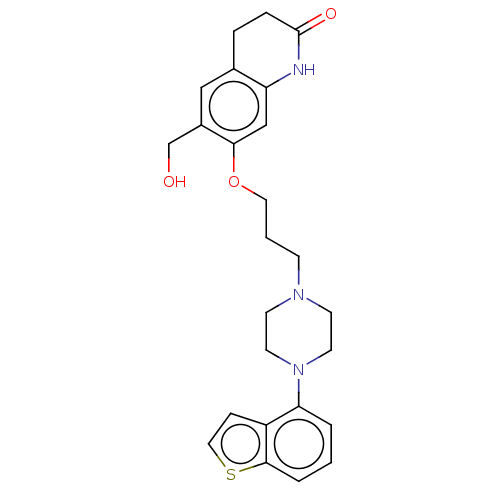 (US9206167, 139) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | -55.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM448338 (USRE48059, Compound of Example 139) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194780 (7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | US Patent | 0.200 | -55.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194794 (US9206167, 15 | USRE48059, Compound of Example 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50057436 (CHEMBL25213 | [2-(6-Fluoro-chroman-8-yloxy)-ethyl]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.221 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor on rat hippocampus using [3H]-8-OH-DPAT as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50057436 (CHEMBL25213 | [2-(6-Fluoro-chroman-8-yloxy)-ethyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.259 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50065573 (CHEMBL96222 | [2-(6-Fluoro-2-methyl-chroman-8-ylox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor on rat hippocampus using [3H]-8-OH-DPAT as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50065578 (CHEMBL419147 | [2-(6-Fluoro-2H-chromen-8-yloxy)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.288 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50065573 (CHEMBL96222 | [2-(6-Fluoro-2-methyl-chroman-8-ylox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.312 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM448330 (USRE48059, Compound of Example 90) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194764 (US9206167, 90) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194789 (US9206167, 10 | USRE48059, Compound of Example 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194789 (US9206167, 10 | USRE48059, Compound of Example 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Concentration of the compound which inhibit [3H]-AVP binding to human Vasopressin V2 receptor coded HeLa cells by 50% | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50065562 (CHEMBL96279 | [2-(6-Fluoro-2,2-dimethyl-chroman-8-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.435 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor on rat hippocampus using [3H]-8-OH-DPAT as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194783 (US9206167, 4 | USRE48059, Compound of Example 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194786 (US9206167, 7 | USRE48059, Compound of Example 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194782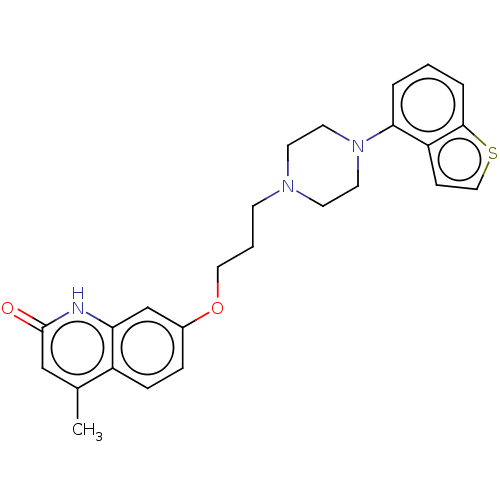 (US9206167, 3 | USRE48059, Compound of Example 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194783 (US9206167, 4 | USRE48059, Compound of Example 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194786 (US9206167, 7 | USRE48059, Compound of Example 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194782 (US9206167, 3 | USRE48059, Compound of Example 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated against Dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand; ND = Not determined | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM35667 (AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194784 (US9206167, 5 | USRE48059, Compound of Example 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM194787 (US9206167, 8 | USRE48059, Compound of Example 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description 5-HT2A: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-Ketanserin (final concentration 1 to 3 nM), 20 ... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM194787 (US9206167, 8 | USRE48059, Compound of Example 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Leysen J E et al. (Leysen J E, Niemegeers C J E, Van Nueten J M and Laduron P M. [3H] Ketanserin (... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194784 (US9206167, 5 | USRE48059, Compound of Example 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194808 (US10501452, Compound B | US9206167, 30 | USRE48059...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194795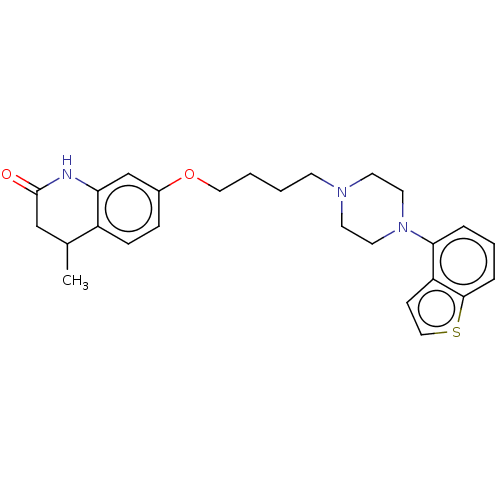 (US9206167, 16 | USRE48059, Compound of Example 16) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194795 (US9206167, 16 | USRE48059, Compound of Example 16) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194802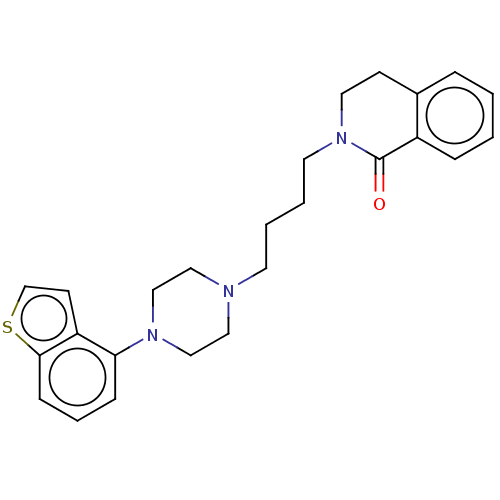 (US9206167, 23 | USRE48059, Compound of Example 23) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194808 (US10501452, Compound B | US9206167, 30 | USRE48059...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194802 (US9206167, 23 | USRE48059, Compound of Example 23) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50065563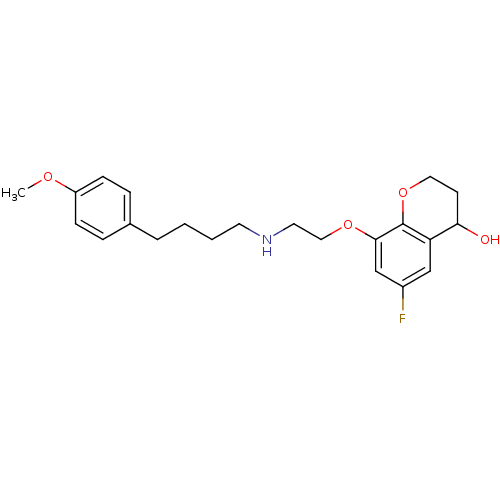 (6-Fluoro-8-{2-[4-(4-methoxy-phenyl)-butylamino]-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50065563 (6-Fluoro-8-{2-[4-(4-methoxy-phenyl)-butylamino]-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35667 (AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194756 (US9206167, 69) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194785 (US9206167, 6 | USRE48059, Compound of Example 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194785 (US9206167, 6 | USRE48059, Compound of Example 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM448323 (USRE48059, Compound of Example 69) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 648 total ) | Next | Last >> |