

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM50080514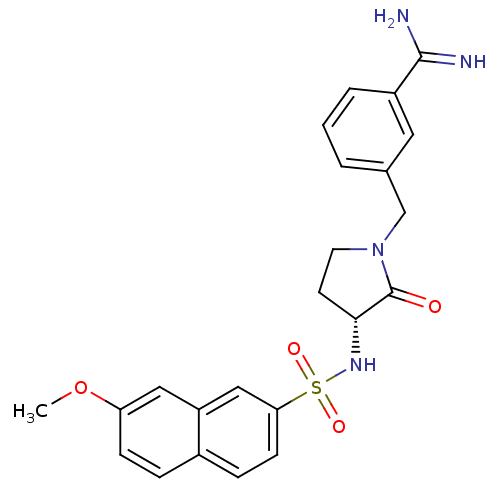 (3-[(R)-3-(7-Methoxy-naphthalene-2-sulfonylamino)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of human Coagulation factor Xa | J Med Chem 42: 3557-71 (1999) Article DOI: 10.1021/jm990040h BindingDB Entry DOI: 10.7270/Q2M04640 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50588316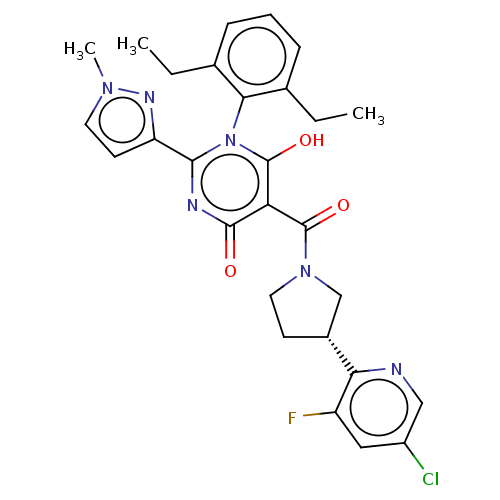 (CHEMBL5170657) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01504 BindingDB Entry DOI: 10.7270/Q21V5JX5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50567169 (CHEMBL4873876) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]Apelin-13 from human APJ receptor stably expressed in human HEK293 cell membrane incubated for 120 mins by TopCount scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01878 BindingDB Entry DOI: 10.7270/Q20G3PXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50014619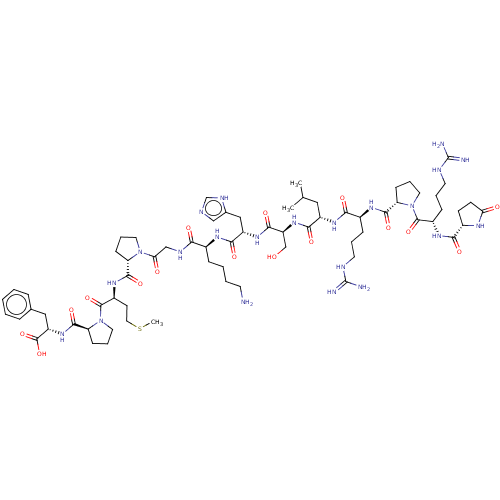 (CHEMBL3184840) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]Apelin-13 from human APJ receptor stably expressed in human HEK293 cell membrane incubated for 120 mins by TopCount scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01878 BindingDB Entry DOI: 10.7270/Q20G3PXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057761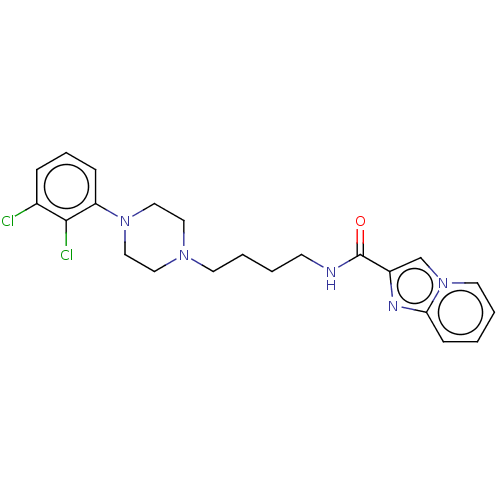 (CHEMBL3322994) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IABN from human D3R expressed in HEK293 cell membranes by gamma counting method | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14059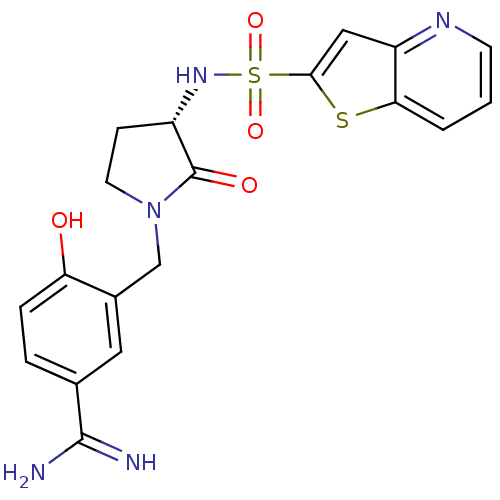 (4-hydroxy-3-[[(3S)-2-oxo-3-(9-thia-5-azabicyclo[4....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity against serine protease factor Xa (fXa) | Bioorg Med Chem Lett 9: 2753-8 (1999) BindingDB Entry DOI: 10.7270/Q2W66K02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50081505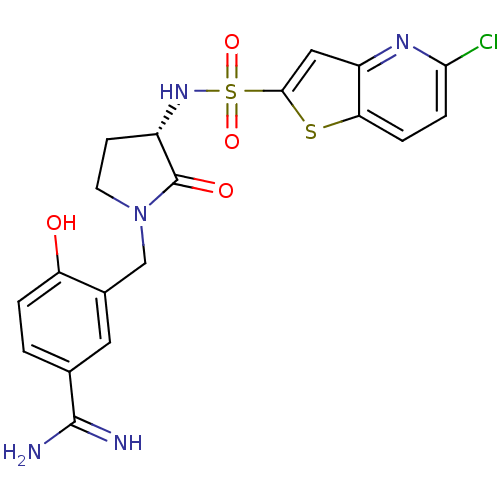 (3-[(S)-3-(5-Chloro-thieno[3,2-b]pyridine-2-sulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity against serine protease factor Xa (fXa) | Bioorg Med Chem Lett 9: 2753-8 (1999) BindingDB Entry DOI: 10.7270/Q2W66K02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50081499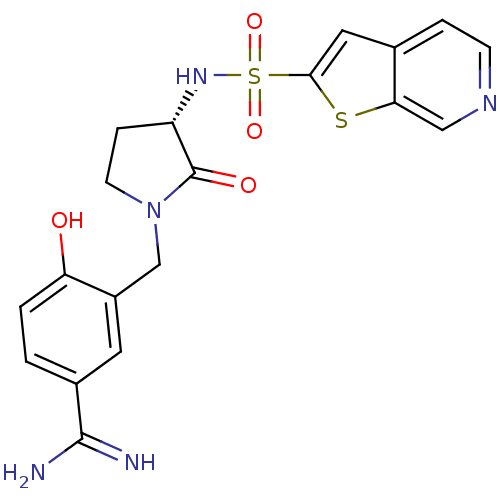 (4-Hydroxy-3-[(S)-2-oxo-3-(thieno[2,3-c]pyridine-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity against serine protease Coagulation factor X | Bioorg Med Chem Lett 9: 2753-8 (1999) BindingDB Entry DOI: 10.7270/Q2W66K02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50081512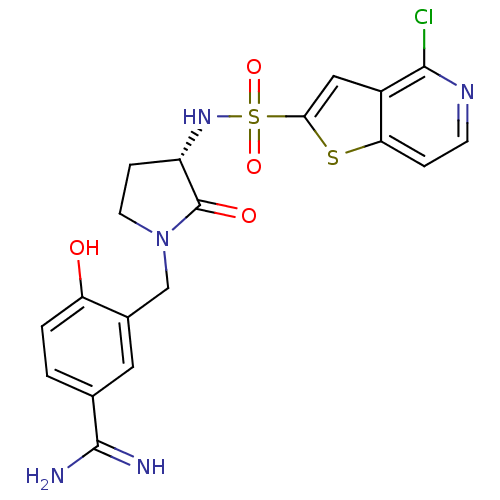 (3-[(S)-3-(4-Chloro-thieno[3,2-c]pyridine-2-sulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity against serine protease factor Xa (fXa) | Bioorg Med Chem Lett 9: 2753-8 (1999) BindingDB Entry DOI: 10.7270/Q2W66K02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057773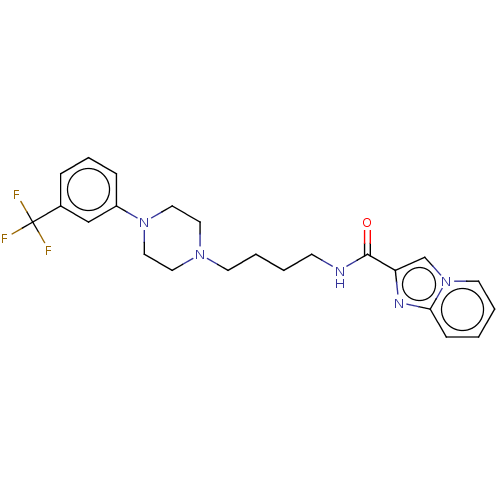 (CHEMBL3322995) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IABN from human D3R expressed in HEK293 cell membranes by gamma counting method | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13286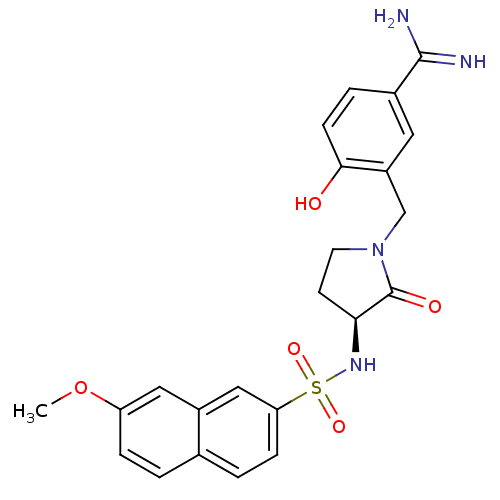 (4-Hydroxy-3-[3-(S)-(7-methoxynaphthalen-2-ylsulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity against serine protease Coagulation factor X | Bioorg Med Chem Lett 9: 2753-8 (1999) BindingDB Entry DOI: 10.7270/Q2W66K02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50081517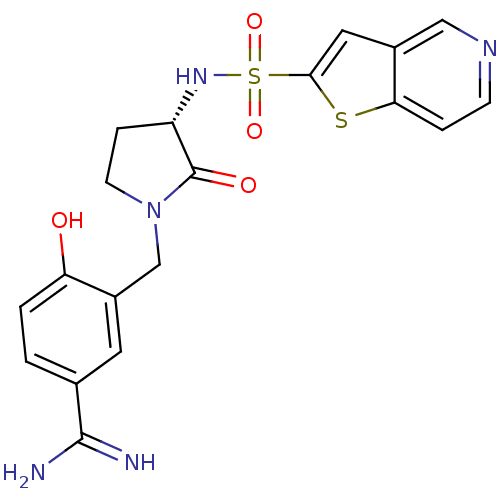 (4-Hydroxy-3-[(S)-2-oxo-3-(thieno[3,2-c]pyridine-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity against serine protease factor Xa (fXa) | Bioorg Med Chem Lett 9: 2753-8 (1999) BindingDB Entry DOI: 10.7270/Q2W66K02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50081510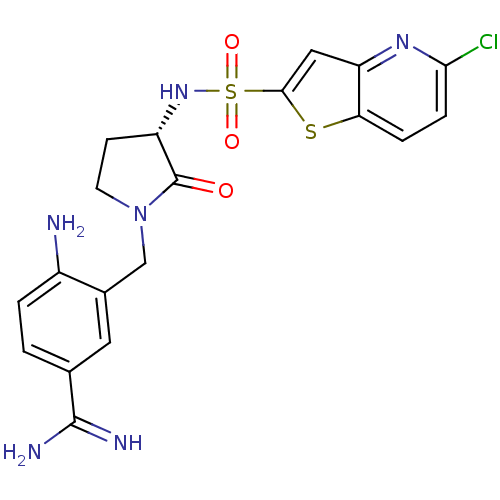 (4-Amino-3-[(S)-3-(5-chloro-thieno[3,2-b]pyridine-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity against serine protease factor Xa (fXa) | Bioorg Med Chem Lett 9: 2753-8 (1999) BindingDB Entry DOI: 10.7270/Q2W66K02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057772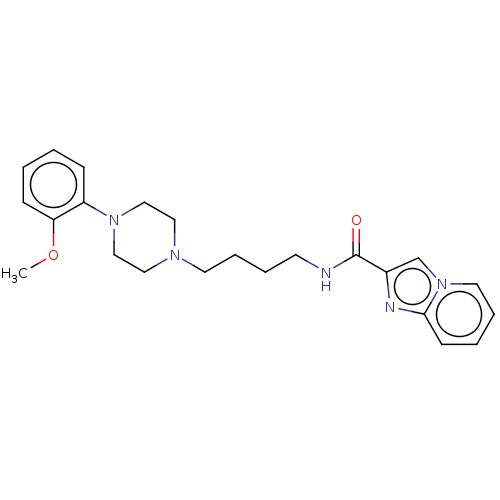 (CHEMBL3322993) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IABN from human D3R expressed in HEK293 cell membranes by gamma counting method | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50017021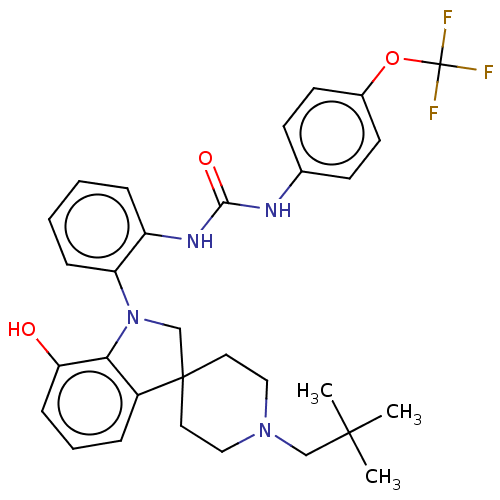 (CHEMBL3287047 | US9428504, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to P2Y1 receptor in human platelets | Bioorg Med Chem Lett 24: 1294-8 (2014) Article DOI: 10.1016/j.bmcl.2014.01.066 BindingDB Entry DOI: 10.7270/Q20G3MQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057762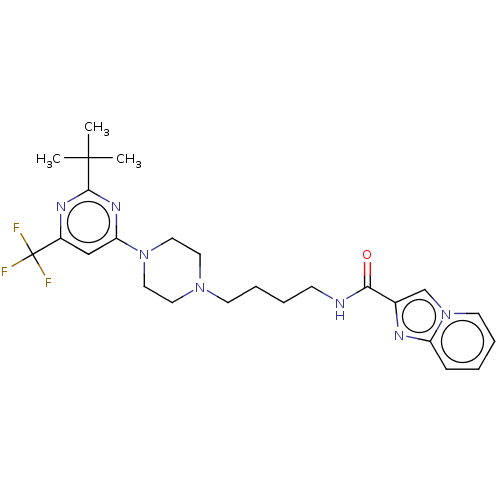 (CHEMBL3323011 | US9598387, Compound 113 | US996974...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IABN from human D3R expressed in HEK293 cell membranes by gamma counting method | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50445214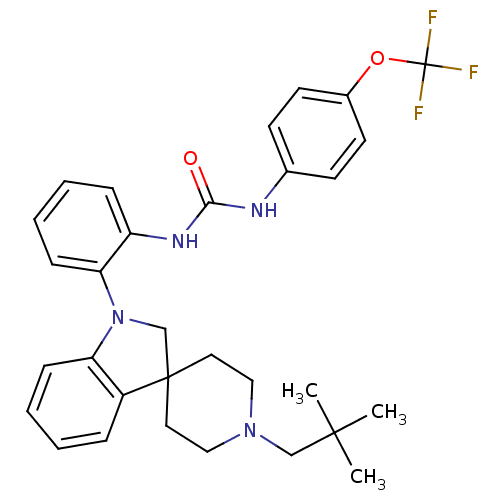 (CHEMBL3104636) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | J Med Chem 56: 9275-95 (2013) Article DOI: 10.1021/jm4013906 BindingDB Entry DOI: 10.7270/Q2KW5HHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057770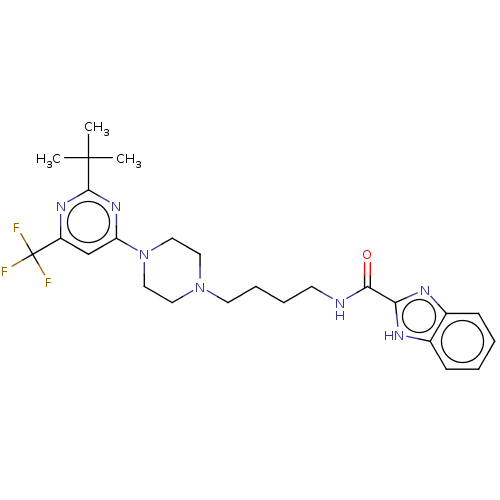 (CHEMBL3323013) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IABN from human D3R expressed in HEK293 cell membranes by gamma counting method | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50445208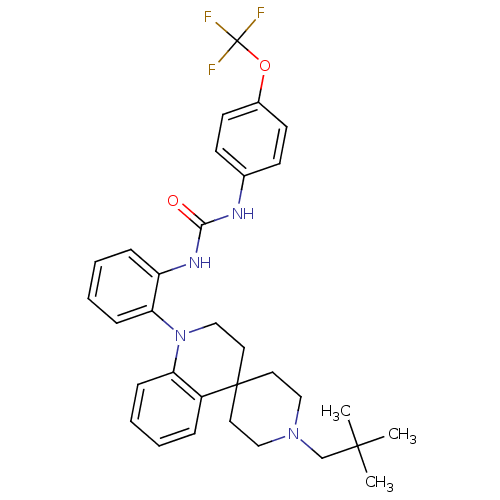 (CHEMBL3105199) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | J Med Chem 56: 9275-95 (2013) Article DOI: 10.1021/jm4013906 BindingDB Entry DOI: 10.7270/Q2KW5HHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50081506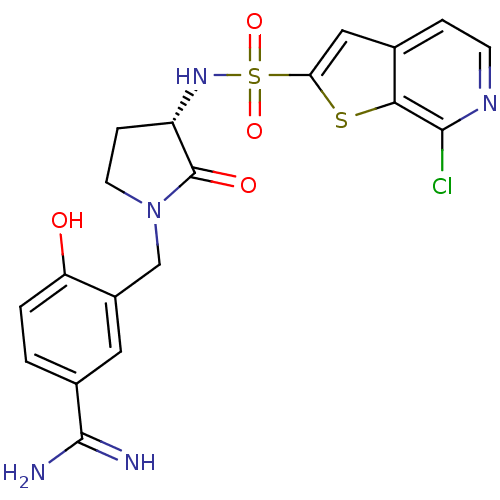 (3-[(S)-3-(7-Chloro-thieno[2,3-c]pyridine-2-sulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity against serine protease factor Xa (fXa) | Bioorg Med Chem Lett 9: 2753-8 (1999) BindingDB Entry DOI: 10.7270/Q2W66K02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50081515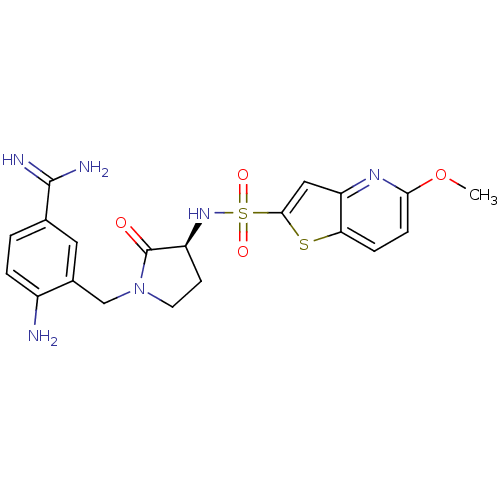 (4-Amino-3-[(S)-3-(5-methoxy-thieno[3,2-b]pyridine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity against serine protease factor Xa (fXa) | Bioorg Med Chem Lett 9: 2753-8 (1999) BindingDB Entry DOI: 10.7270/Q2W66K02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50445201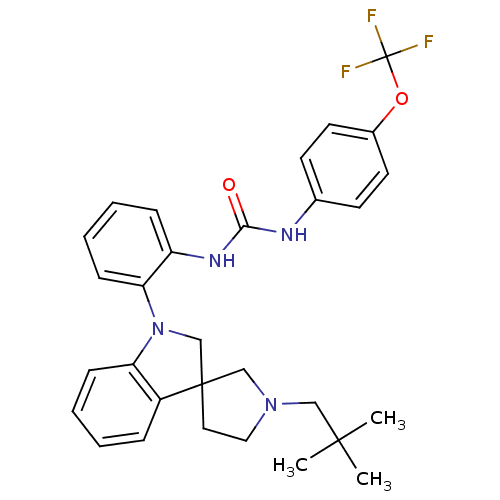 (CHEMBL3105201) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | J Med Chem 56: 9275-95 (2013) Article DOI: 10.1021/jm4013906 BindingDB Entry DOI: 10.7270/Q2KW5HHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50445213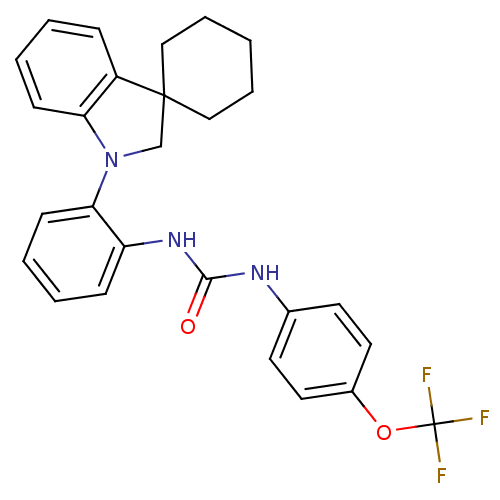 (CHEMBL3104624) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | J Med Chem 56: 9275-95 (2013) Article DOI: 10.1021/jm4013906 BindingDB Entry DOI: 10.7270/Q2KW5HHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50429537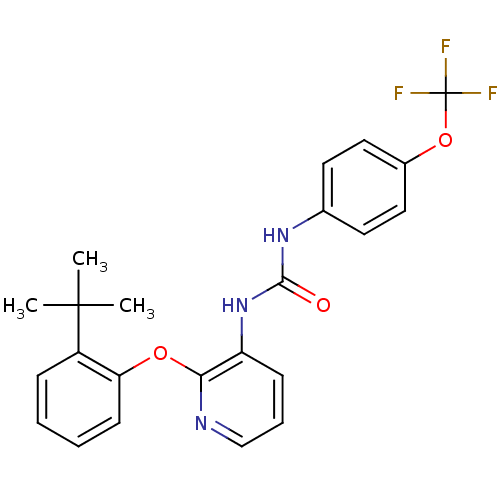 (CHEMBL2333770) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells assessed as residual [beta-33P] bound to plate after 1 hr by... | J Med Chem 56: 1704-14 (2013) Article DOI: 10.1021/jm301708u BindingDB Entry DOI: 10.7270/Q2ST7R6G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50429537 (CHEMBL2333770) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | J Med Chem 56: 9275-95 (2013) Article DOI: 10.1021/jm4013906 BindingDB Entry DOI: 10.7270/Q2KW5HHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50429537 (CHEMBL2333770) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human P2Y1 receptor | Bioorg Med Chem Lett 23: 3239-43 (2013) Article DOI: 10.1016/j.bmcl.2013.03.125 BindingDB Entry DOI: 10.7270/Q2PN9714 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057789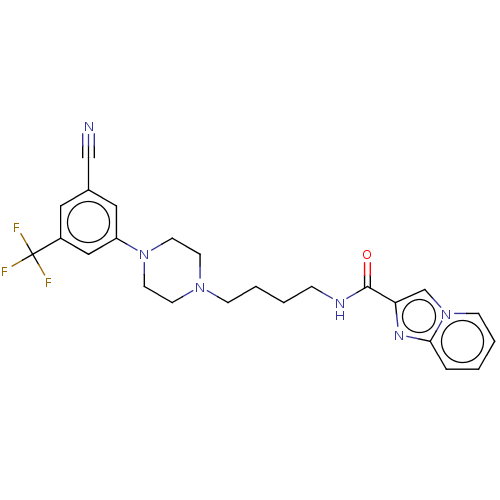 (CHEMBL3322996) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IABN from human D3R expressed in HEK293 cell membranes by gamma counting method | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50445211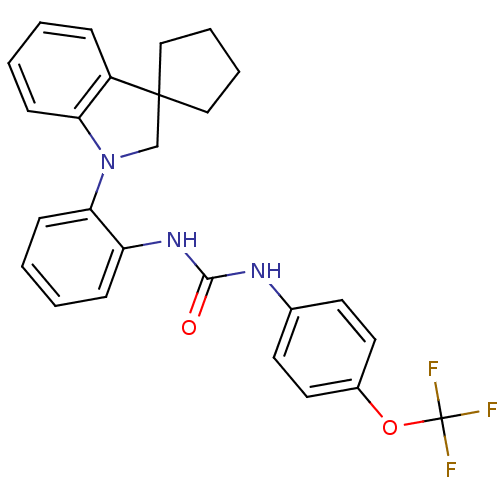 (CHEMBL3103636) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | J Med Chem 56: 9275-95 (2013) Article DOI: 10.1021/jm4013906 BindingDB Entry DOI: 10.7270/Q2KW5HHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50433798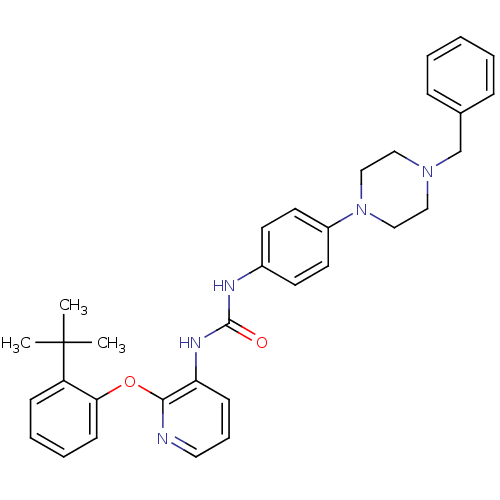 (CHEMBL2381894) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human P2Y1 receptor | Bioorg Med Chem Lett 23: 3239-43 (2013) Article DOI: 10.1016/j.bmcl.2013.03.125 BindingDB Entry DOI: 10.7270/Q2PN9714 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50429535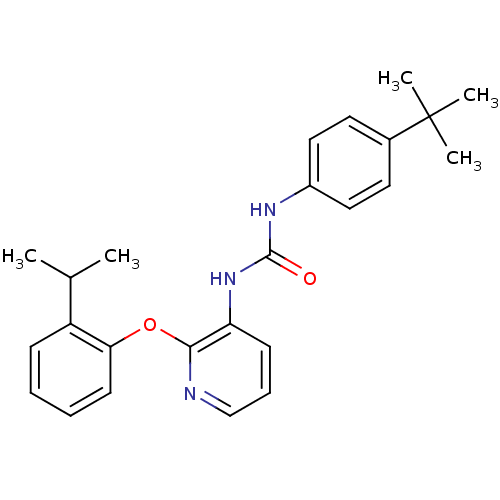 (CHEMBL2333772) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells assessed as residual [beta-33P] bound to plate after 1 hr by... | J Med Chem 56: 1704-14 (2013) Article DOI: 10.1021/jm301708u BindingDB Entry DOI: 10.7270/Q2ST7R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50429541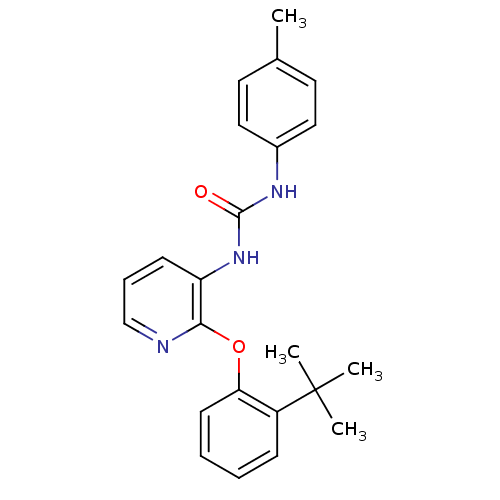 (CHEMBL2333766) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells assessed as residual [beta-33P] bound to plate after 1 hr by... | J Med Chem 56: 1704-14 (2013) Article DOI: 10.1021/jm301708u BindingDB Entry DOI: 10.7270/Q2ST7R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13279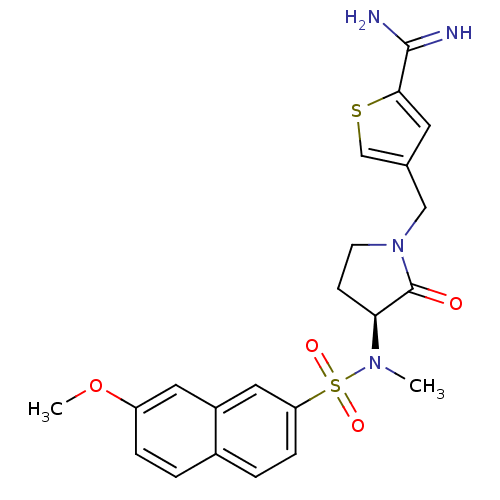 (4-{[(3S)-3-[(7-methoxynaphthalene-2-)(methyl)sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of human Coagulation factor Xa | J Med Chem 42: 3557-71 (1999) Article DOI: 10.1021/jm990040h BindingDB Entry DOI: 10.7270/Q2M04640 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50433804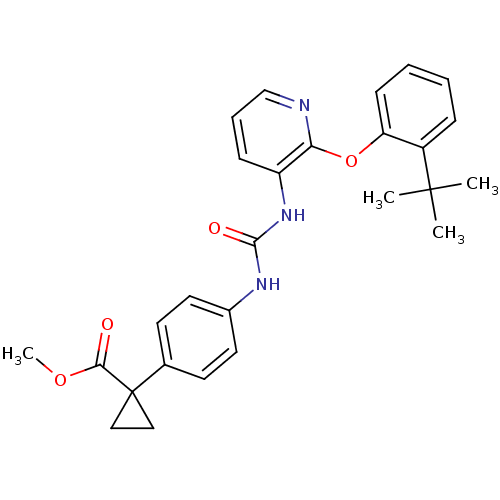 (CHEMBL2381888) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human P2Y1 receptor | Bioorg Med Chem Lett 23: 3239-43 (2013) Article DOI: 10.1016/j.bmcl.2013.03.125 BindingDB Entry DOI: 10.7270/Q2PN9714 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50445210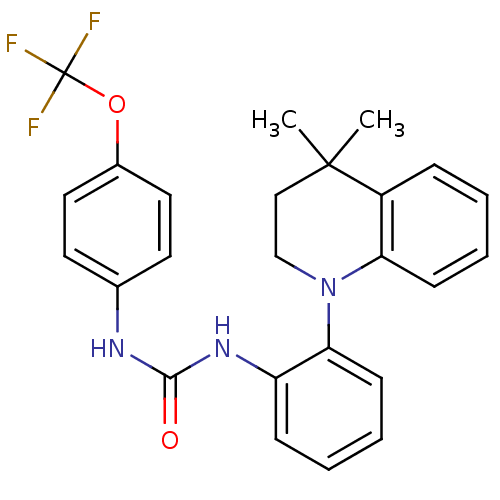 (CHEMBL3102866) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | J Med Chem 56: 9275-95 (2013) Article DOI: 10.1021/jm4013906 BindingDB Entry DOI: 10.7270/Q2KW5HHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50445207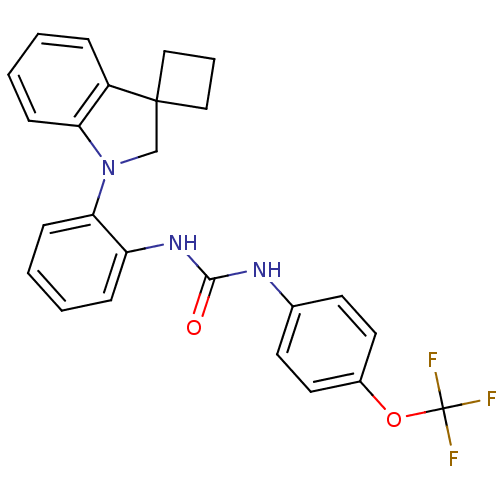 (CHEMBL3103635) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | J Med Chem 56: 9275-95 (2013) Article DOI: 10.1021/jm4013906 BindingDB Entry DOI: 10.7270/Q2KW5HHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50429536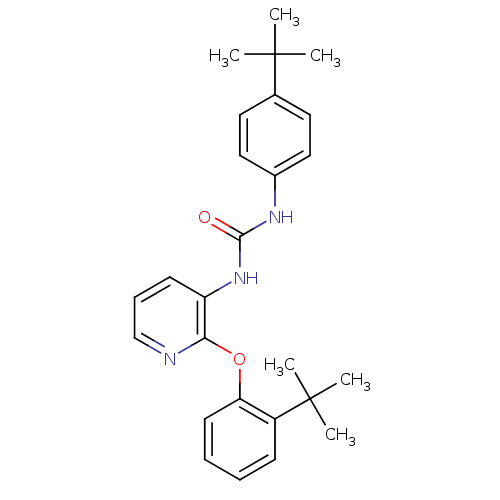 (CHEMBL2333771) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells assessed as residual [beta-33P] bound to plate after 1 hr by... | J Med Chem 56: 1704-14 (2013) Article DOI: 10.1021/jm301708u BindingDB Entry DOI: 10.7270/Q2ST7R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057763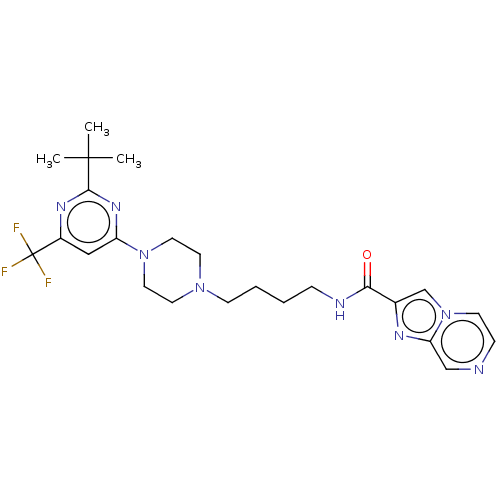 (CHEMBL3323016 | US9598387, Compound 115 | US996974...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IABN from human D3R expressed in HEK293 cell membranes by gamma counting method | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057777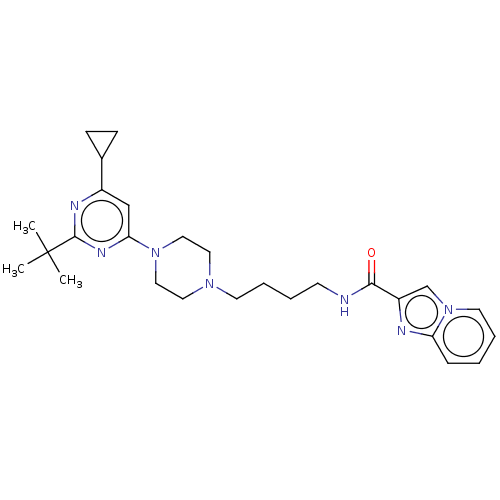 (CHEMBL3323010 | US9598387, Compound 112 | US996974...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IABN from human D3R expressed in HEK293 cell membranes by gamma counting method | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057762 (CHEMBL3323011 | US9598387, Compound 113 | US996974...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IABN from wild type human D3R expressed in HEK293 cell membranes by gamma counting method | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50445209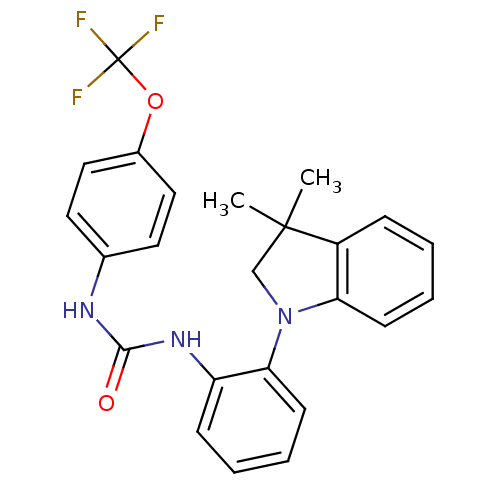 (CHEMBL3103625 | US9540323, Comparator 2 | US954032...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | J Med Chem 56: 9275-95 (2013) Article DOI: 10.1021/jm4013906 BindingDB Entry DOI: 10.7270/Q2KW5HHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057753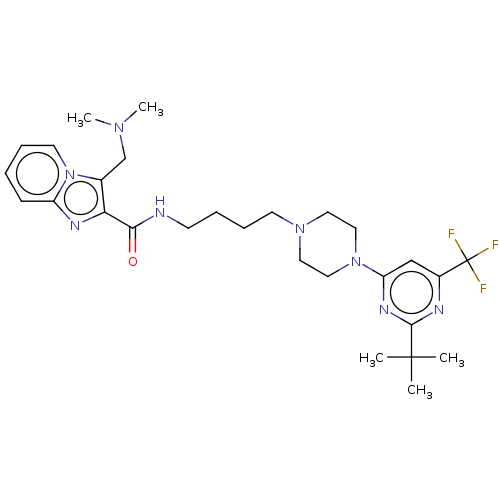 (CHEMBL3323112 | US9598387, Compound 120 | US996974...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IABN from wild type human D3R expressed in HEK293 cell membranes by gamma counting method | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057779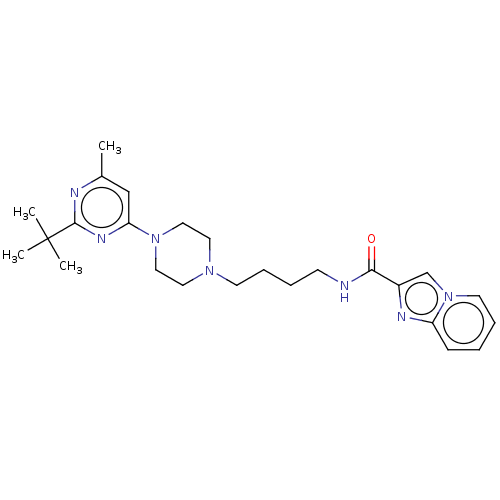 (CHEMBL3323008 | US9598387, Compound 111 | US996974...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IABN from human D3R expressed in HEK293 cell membranes by gamma counting method | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057759 (CHEMBL3323117) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IABN from human D3R expressed in HEK293 cell membranes by gamma counting method | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057790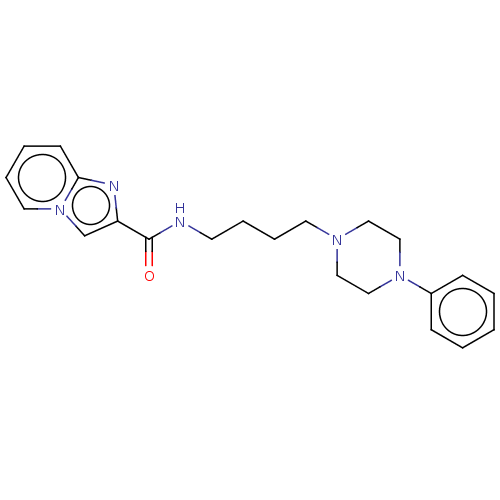 (CHEMBL3322992) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IABN from human D3R expressed in HEK293 cell membranes by gamma counting method | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057767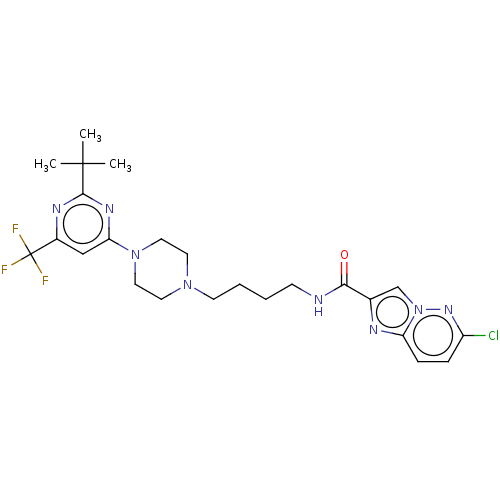 (CHEMBL3323017 | US9598387, Compound 116 | US996974...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IABN from human D3R expressed in HEK293 cell membranes by gamma counting method | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50433789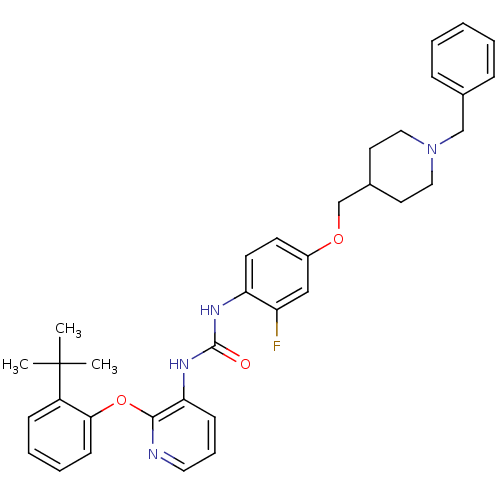 (CHEMBL2381904) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human P2Y1 receptor | Bioorg Med Chem Lett 23: 3239-43 (2013) Article DOI: 10.1016/j.bmcl.2013.03.125 BindingDB Entry DOI: 10.7270/Q2PN9714 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50080482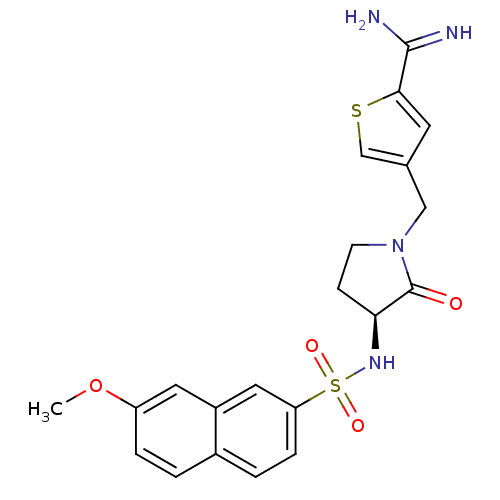 (4-[(S)-3-(7-Methoxy-naphthalene-2-sulfonylamino)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of human Coagulation factor Xa | J Med Chem 42: 3557-71 (1999) Article DOI: 10.1021/jm990040h BindingDB Entry DOI: 10.7270/Q2M04640 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057771 (CHEMBL3323012) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IABN from human D3R expressed in HEK293 cell membranes by gamma counting method | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50433797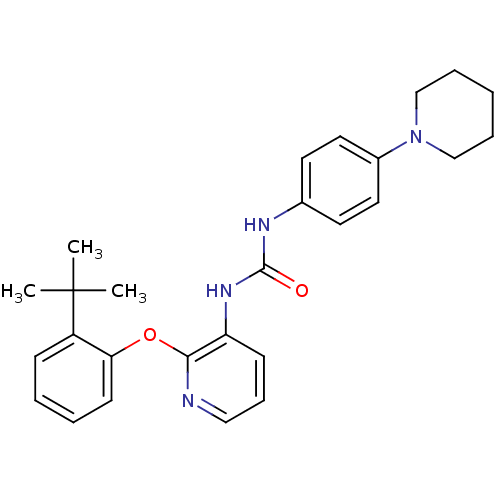 (CHEMBL2381895) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human P2Y1 receptor | Bioorg Med Chem Lett 23: 3239-43 (2013) Article DOI: 10.1016/j.bmcl.2013.03.125 BindingDB Entry DOI: 10.7270/Q2PN9714 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50445206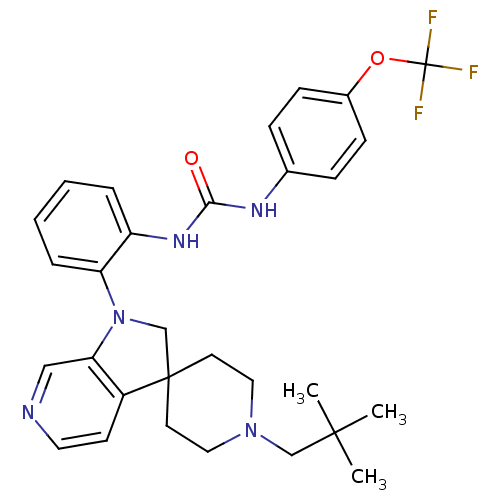 (CHEMBL3105195) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | J Med Chem 56: 9275-95 (2013) Article DOI: 10.1021/jm4013906 BindingDB Entry DOI: 10.7270/Q2KW5HHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 863 total ) | Next | Last >> |