 Found 8064 hits with Last Name = 'kan' and Initial = 'j'
Found 8064 hits with Last Name = 'kan' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Coagulation factor Xa (serine protease) was determined |
Bioorg Med Chem Lett 12: 1511-5 (2002)
BindingDB Entry DOI: 10.7270/Q2P84B6X |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50183266
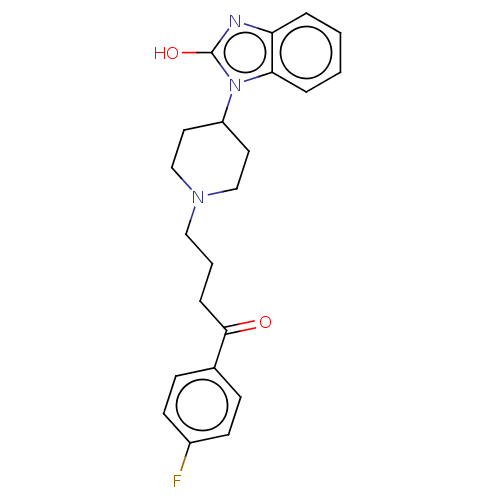
(Anquil | Benperidol | Benquil | MCN-JR-4584 | R-45...)Show SMILES Oc1nc2ccccc2n1C1CCN(CCCC(=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C22H24FN3O2/c23-17-9-7-16(8-10-17)21(27)6-3-13-25-14-11-18(12-15-25)26-20-5-2-1-4-19(20)24-22(26)28/h1-2,4-5,7-10,18H,3,6,11-15H2,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human dopamine D2 receptor by radioligand displacement assay |
Bioorg Med Chem 24: 3671-9 (2016)
Article DOI: 10.1016/j.bmc.2016.06.011
BindingDB Entry DOI: 10.7270/Q2W66NPV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50193861
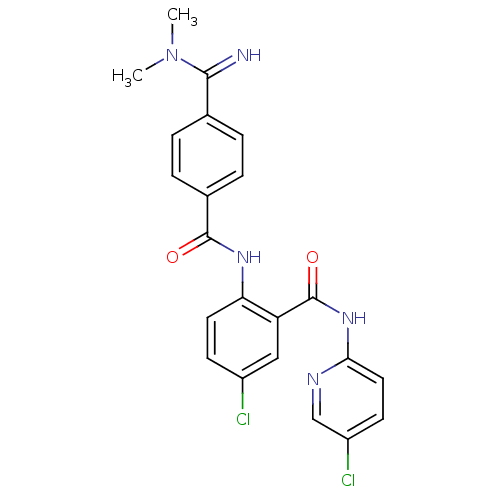
(5-chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N-dimet...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H19Cl2N5O2/c1-29(2)20(25)13-3-5-14(6-4-13)21(30)27-18-9-7-15(23)11-17(18)22(31)28-19-10-8-16(24)12-26-19/h3-12,25H,1-2H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249120
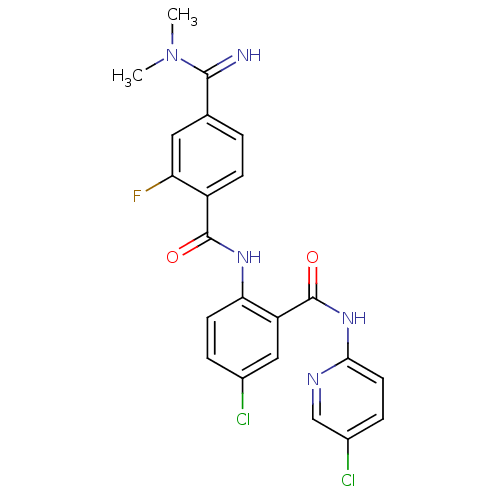
(CHEMBL472967 | N-(4-chloro-2-(5-chloropyridin-2-yl...)Show SMILES CN(C)C(=N)c1ccc(C(=O)Nc2ccc(Cl)cc2C(=O)Nc2ccc(Cl)cn2)c(F)c1 Show InChI InChI=1S/C22H18Cl2FN5O2/c1-30(2)20(26)12-3-6-15(17(25)9-12)21(31)28-18-7-4-13(23)10-16(18)22(32)29-19-8-5-14(24)11-27-19/h3-11,26H,1-2H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50183266

(Anquil | Benperidol | Benquil | MCN-JR-4584 | R-45...)Show SMILES Oc1nc2ccccc2n1C1CCN(CCCC(=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C22H24FN3O2/c23-17-9-7-16(8-10-17)21(27)6-3-13-25-14-11-18(12-15-25)26-20-5-2-1-4-19(20)24-22(26)28/h1-2,4-5,7-10,18H,3,6,11-15H2,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human dopamine D4 receptor by radioligand displacement assay |
Bioorg Med Chem 24: 3671-9 (2016)
Article DOI: 10.1016/j.bmc.2016.06.011
BindingDB Entry DOI: 10.7270/Q2W66NPV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM19023

(1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...)Show SMILES COc1ccc(cc1)-n1nc(C(N)=O)c2CCN(C(=O)c12)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249423
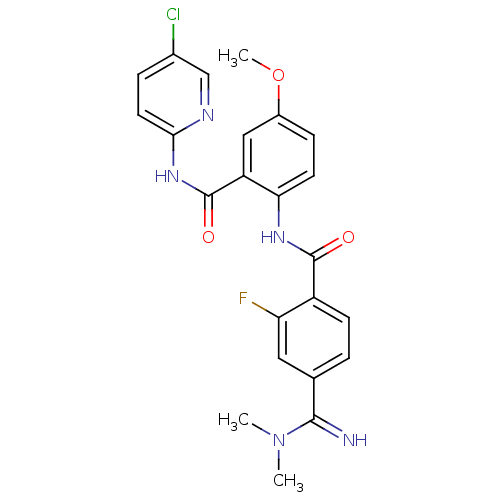
(CHEMBL515919 | N-(2-(5-chloropyridin-2-ylcarbamoyl...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2F)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H21ClFN5O3/c1-30(2)21(26)13-4-7-16(18(25)10-13)22(31)28-19-8-6-15(33-3)11-17(19)23(32)29-20-9-5-14(24)12-27-20/h4-12,26H,1-3H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249298
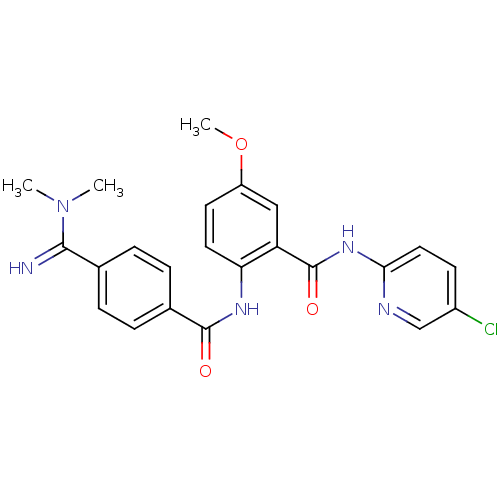
(BEVYXXA | CHEMBL512351 | N-(5-chloropyridin-2-yl)-...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H22ClN5O3/c1-29(2)21(25)14-4-6-15(7-5-14)22(30)27-19-10-9-17(32-3)12-18(19)23(31)28-20-11-8-16(24)13-26-20/h4-13,25H,1-3H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50113598
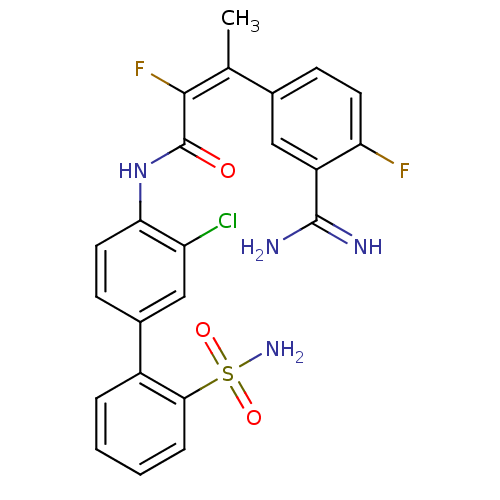
((Z)-3-(3-Carbamimidoyl-4-fluoro-phenyl)-2-fluoro-b...)Show SMILES C\C(=C(/F)C(=O)Nc1ccc(cc1Cl)-c1ccccc1S(N)(=O)=O)c1ccc(F)c(c1)C(N)=N Show InChI InChI=1S/C23H19ClF2N4O3S/c1-12(13-6-8-18(25)16(10-13)22(27)28)21(26)23(31)30-19-9-7-14(11-17(19)24)15-4-2-3-5-20(15)34(29,32)33/h2-11H,1H3,(H3,27,28)(H,30,31)(H2,29,32,33)/b21-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against factor Xa,activity expressed as Ki nM |
Bioorg Med Chem Lett 12: 1511-5 (2002)
BindingDB Entry DOI: 10.7270/Q2P84B6X |
More data for this
Ligand-Target Pair | |
Muscarinic receptor M1
(Bos taurus) | BDBM50010096
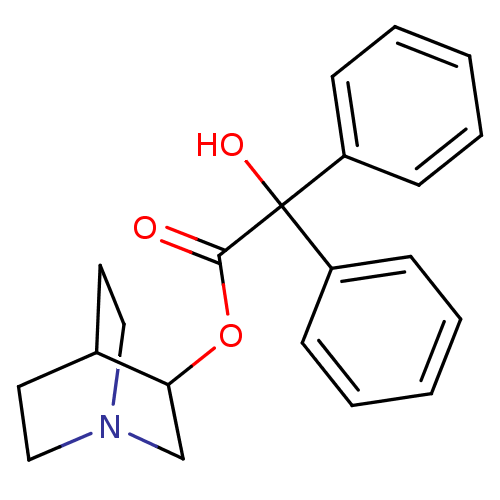
(CHEMBL12980 | Hydroxy-diphenyl-acetic acid 1-aza-b...)Show SMILES OC(C(=O)OC1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |(5.41,-4.43,;4.65,-5.78,;6.2,-5.78,;6.97,-7.12,;6.97,-4.45,;8.52,-4.1,;8.52,-2.56,;9.86,-1.78,;11.19,-2.56,;11.19,-4.1,;9.86,-4.88,;9.09,-3.78,;9.09,-2.9,;3.1,-5.81,;2.36,-7.19,;.82,-7.2,;.01,-5.88,;.78,-4.52,;2.32,-4.49,;4.65,-7.23,;3.39,-7.96,;3.38,-9.41,;4.64,-10.15,;5.91,-9.42,;5.91,-7.97,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor M1 by displacement of [3H]pirenzepine in bovine striatum |
J Med Chem 31: 1463-6 (1988)
BindingDB Entry DOI: 10.7270/Q2DV1KGN |
More data for this
Ligand-Target Pair | |
Muscarinic receptor M1
(Bos taurus) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor M1 by displacement of [3H]pirenzepine in bovine striatum |
J Med Chem 31: 1463-6 (1988)
BindingDB Entry DOI: 10.7270/Q2DV1KGN |
More data for this
Ligand-Target Pair | |
Muscarinic receptor M1
(Bos taurus) | BDBM50296314
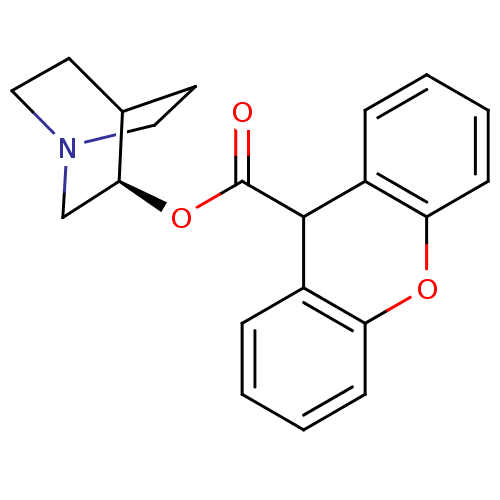
((3R)-1-Azabicyclo[2.2.2]oct-3-yl9H-xanthene-9-carb...)Show SMILES O=C(O[C@H]1CN2CCC1CC2)C1c2ccccc2Oc2ccccc12 |r,wD:3.2,(-1.78,.66,;-3.13,-.09,;-3.15,-1.63,;-1.81,-2.4,;-1.81,-3.94,;-.48,-4.7,;.85,-3.94,;.85,-2.4,;-.48,-1.62,;-.06,-2.86,;-1.11,-3.21,;-4.45,.7,;-5.79,-.04,;-5.82,-1.58,;-7.17,-2.33,;-8.5,-1.53,;-8.47,.02,;-7.11,.76,;-7.09,2.3,;-5.74,3.04,;-5.72,4.57,;-4.38,5.32,;-3.05,4.53,;-3.07,2.99,;-4.42,2.25,)| Show InChI InChI=1S/C21H21NO3/c23-21(25-19-13-22-11-9-14(19)10-12-22)20-15-5-1-3-7-17(15)24-18-8-4-2-6-16(18)20/h1-8,14,19-20H,9-13H2/t19-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor M1 by displacement of [3H]pirenzepine in bovine striatum |
J Med Chem 31: 1463-6 (1988)
BindingDB Entry DOI: 10.7270/Q2DV1KGN |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50057512
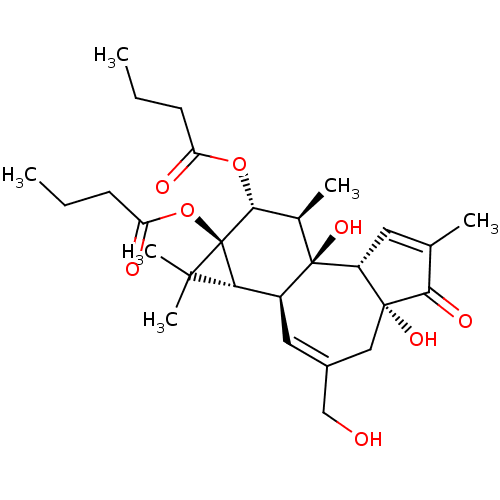
((1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-4a,7b-dihydroxy-3-...)Show SMILES CCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(=O)CCC |c:23,t:12| Show InChI InChI=1S/C28H40O8/c1-7-9-20(30)35-24-16(4)27(34)18(22-25(5,6)28(22,24)36-21(31)10-8-2)12-17(14-29)13-26(33)19(27)11-15(3)23(26)32/h11-12,16,18-19,22,24,29,33-34H,7-10,13-14H2,1-6H3/t16-,18+,19-,22?,24-,26-,27-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Binding Affinity against protein kinase C alpha |
J Med Chem 43: 921-44 (2000)
BindingDB Entry DOI: 10.7270/Q2RN372X |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50393252
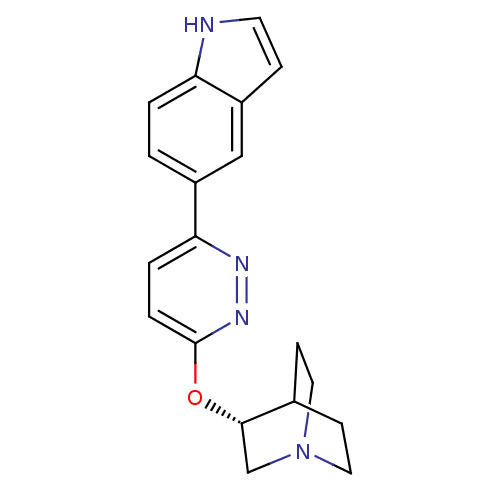
(CHEMBL2151570)Show SMILES C1CN2CCC1[C@H](C2)Oc1ccc(nn1)-c1ccc2[nH]ccc2c1 |r,wU:6.9,(10.31,-30.96,;10.31,-32.5,;11.64,-33.26,;10.9,-31.9,;12.4,-31.51,;11.64,-30.18,;12.97,-30.96,;12.97,-32.5,;14.31,-30.2,;15.64,-30.97,;16.97,-30.21,;18.3,-30.98,;18.3,-32.52,;16.96,-33.29,;15.63,-32.51,;19.62,-33.3,;19.61,-34.84,;20.94,-35.61,;22.28,-34.84,;23.75,-35.31,;24.65,-34.07,;23.75,-32.82,;22.28,-33.3,;20.95,-32.53,)| Show InChI InChI=1S/C19H20N4O/c1-2-16-15(5-8-20-16)11-14(1)17-3-4-19(22-21-17)24-18-12-23-9-6-13(18)7-10-23/h1-5,8,11,13,18,20H,6-7,9-10,12H2/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mahidol University
Curated by ChEMBL
| Assay Description
Displacement of [3H]A-585539 from alpha7 nAChR in human cerebral cortex membranes after 75 mins by scintillation counting |
ACS Med Chem Lett 7: 890-895 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00146
BindingDB Entry DOI: 10.7270/Q2ZW1QDQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50010096

(CHEMBL12980 | Hydroxy-diphenyl-acetic acid 1-aza-b...)Show SMILES OC(C(=O)OC1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |(5.41,-4.43,;4.65,-5.78,;6.2,-5.78,;6.97,-7.12,;6.97,-4.45,;8.52,-4.1,;8.52,-2.56,;9.86,-1.78,;11.19,-2.56,;11.19,-4.1,;9.86,-4.88,;9.09,-3.78,;9.09,-2.9,;3.1,-5.81,;2.36,-7.19,;.82,-7.2,;.01,-5.88,;.78,-4.52,;2.32,-4.49,;4.65,-7.23,;3.39,-7.96,;3.38,-9.41,;4.64,-10.15,;5.91,-9.42,;5.91,-7.97,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Evaluated for the inhibition of adenylate cyclase at M2 receptor in rat heart |
J Med Chem 31: 1463-6 (1988)
BindingDB Entry DOI: 10.7270/Q2DV1KGN |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50183266

(Anquil | Benperidol | Benquil | MCN-JR-4584 | R-45...)Show SMILES Oc1nc2ccccc2n1C1CCN(CCCC(=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C22H24FN3O2/c23-17-9-7-16(8-10-17)21(27)6-3-13-25-14-11-18(12-15-25)26-20-5-2-1-4-19(20)24-22(26)28/h1-2,4-5,7-10,18H,3,6,11-15H2,(H,24,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IABN from human recombinant dopamine D3 receptor expressed in HEK293 cell membrane incubated for 60 mins filtration binding as... |
Bioorg Med Chem 24: 3671-9 (2016)
Article DOI: 10.1016/j.bmc.2016.06.011
BindingDB Entry DOI: 10.7270/Q2W66NPV |
More data for this
Ligand-Target Pair | |
Muscarinic receptor M1
(Bos taurus) | BDBM50405720
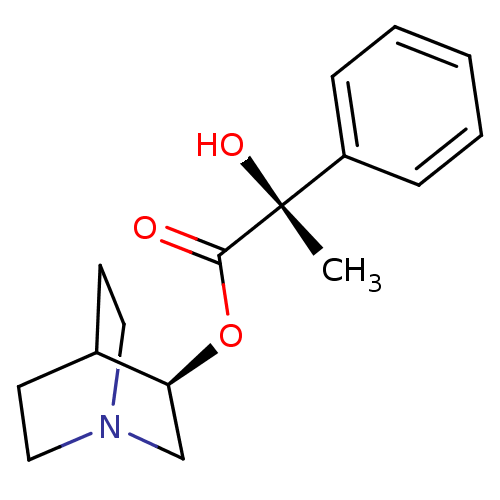
(CHEMBL2115342)Show SMILES C[C@](O)(C(=O)O[C@H]1CN2CCC1CC2)c1ccccc1 |wU:1.2,wD:6.5,1.1,THB:5:6:10.9:12.13,(-.9,-11.74,;-.13,-10.4,;.64,-9.06,;1.2,-11.18,;1.2,-12.72,;2.53,-10.4,;4.08,-10.4,;4.03,-11.69,;5.38,-10.64,;6.78,-11.57,;6.74,-10.39,;5.41,-9.62,;5.49,-8.49,;4.44,-9.01,;-1.47,-9.63,;-2.81,-10.4,;-4.14,-9.63,;-4.14,-8.07,;-2.81,-7.3,;-1.47,-8.07,)| Show InChI InChI=1S/C16H21NO3/c1-16(19,13-5-3-2-4-6-13)15(18)20-14-11-17-9-7-12(14)8-10-17/h2-6,12,14,19H,7-11H2,1H3/t14-,16+/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor M1 by displacement of [3H]pirenzepine in bovine striatum |
J Med Chem 31: 1463-6 (1988)
BindingDB Entry DOI: 10.7270/Q2DV1KGN |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553855
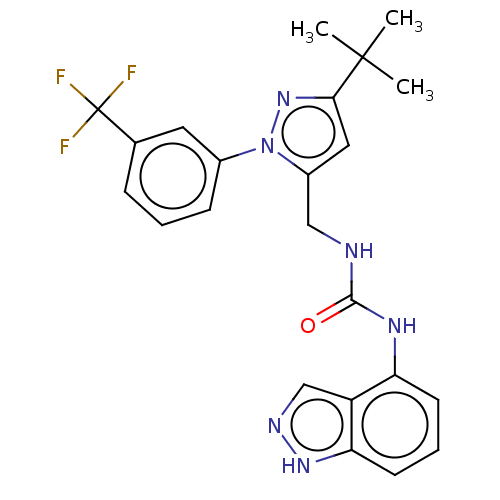
(CHEMBL4782906)Show SMILES CC(C)(C)c1cc(CNC(=O)Nc2cccc3[nH]ncc23)n(n1)-c1cccc(c1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM176564
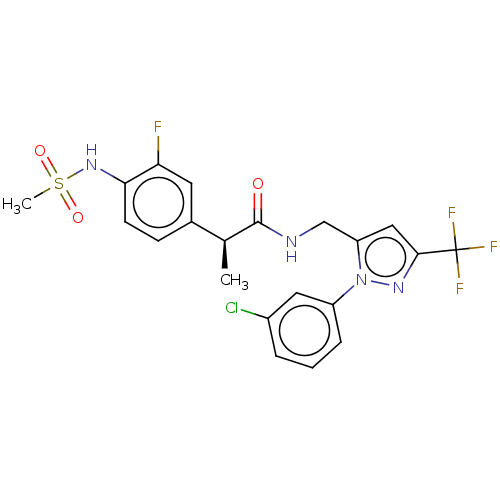
(US9120756, 26)Show SMILES C[C@H](C(=O)NCc1cc(nn1-c1cccc(Cl)c1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C21H19ClF4N4O3S/c1-12(13-6-7-18(17(23)8-13)29-34(2,32)33)20(31)27-11-16-10-19(21(24,25)26)28-30(16)15-5-3-4-14(22)9-15/h3-10,12,29H,11H2,1-2H3,(H,27,31)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128266
BindingDB Entry DOI: 10.7270/Q2CV4NG9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398494

(CHEMBL2177429)Show SMILES C[C@H](C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128266
BindingDB Entry DOI: 10.7270/Q2CV4NG9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50232114
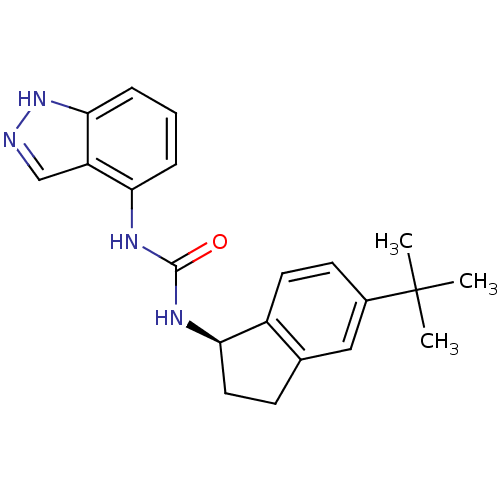
((R)-1-(5-tert-butyl-2,3-dihydro-1H-inden-1-yl)-3-(...)Show SMILES CC(C)(C)c1ccc2[C@@H](CCc2c1)NC(=O)Nc1cccc2[nH]ncc12 Show InChI InChI=1S/C21H24N4O/c1-21(2,3)14-8-9-15-13(11-14)7-10-18(15)24-20(26)23-17-5-4-6-19-16(17)12-22-25-19/h4-6,8-9,11-12,18H,7,10H2,1-3H3,(H,22,25)(H2,23,24,26)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM176555
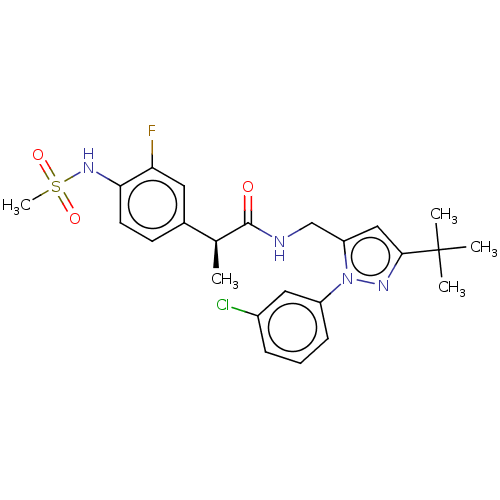
(US9120756, 17)Show SMILES C[C@H](C(=O)NCc1cc(nn1-c1cccc(Cl)c1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C24H28ClFN4O3S/c1-15(16-9-10-21(20(26)11-16)29-34(5,32)33)23(31)27-14-19-13-22(24(2,3)4)28-30(19)18-8-6-7-17(25)12-18/h6-13,15,29H,14H2,1-5H3,(H,27,31)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128266
BindingDB Entry DOI: 10.7270/Q2CV4NG9 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D3 receptor (unknown origin) |
Bioorg Med Chem 24: 3671-9 (2016)
Article DOI: 10.1016/j.bmc.2016.06.011
BindingDB Entry DOI: 10.7270/Q2W66NPV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Mus musculus) | BDBM50057512

((1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-4a,7b-dihydroxy-3-...)Show SMILES CCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(=O)CCC |c:23,t:12| Show InChI InChI=1S/C28H40O8/c1-7-9-20(30)35-24-16(4)27(34)18(22-25(5,6)28(22,24)36-21(31)10-8-2)12-17(14-29)13-26(33)19(27)11-15(3)23(26)32/h11-12,16,18-19,22,24,29,33-34H,7-10,13-14H2,1-6H3/t16-,18+,19-,22?,24-,26-,27-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from mouse wild type C1b domain of PKCdelta expressed in Escherichia coli |
J Med Chem 50: 3465-81 (2007)
Article DOI: 10.1021/jm0702579
BindingDB Entry DOI: 10.7270/Q2RF5TQ5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-HT2B receptor expressed in cell membranes after 1 hr by liquid scintillation counting |
Bioorg Med Chem 24: 3464-71 (2016)
Article DOI: 10.1016/j.bmc.2016.05.053
BindingDB Entry DOI: 10.7270/Q2X63PVP |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50296314

((3R)-1-Azabicyclo[2.2.2]oct-3-yl9H-xanthene-9-carb...)Show SMILES O=C(O[C@H]1CN2CCC1CC2)C1c2ccccc2Oc2ccccc12 |r,wD:3.2,(-1.78,.66,;-3.13,-.09,;-3.15,-1.63,;-1.81,-2.4,;-1.81,-3.94,;-.48,-4.7,;.85,-3.94,;.85,-2.4,;-.48,-1.62,;-.06,-2.86,;-1.11,-3.21,;-4.45,.7,;-5.79,-.04,;-5.82,-1.58,;-7.17,-2.33,;-8.5,-1.53,;-8.47,.02,;-7.11,.76,;-7.09,2.3,;-5.74,3.04,;-5.72,4.57,;-4.38,5.32,;-3.05,4.53,;-3.07,2.99,;-4.42,2.25,)| Show InChI InChI=1S/C21H21NO3/c23-21(25-19-13-22-11-9-14(19)10-12-22)20-15-5-1-3-7-17(15)24-18-8-4-2-6-16(18)20/h1-8,14,19-20H,9-13H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor M2 by displacement of [3H]QNB in rat myocardium |
J Med Chem 31: 1463-6 (1988)
BindingDB Entry DOI: 10.7270/Q2DV1KGN |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM7840
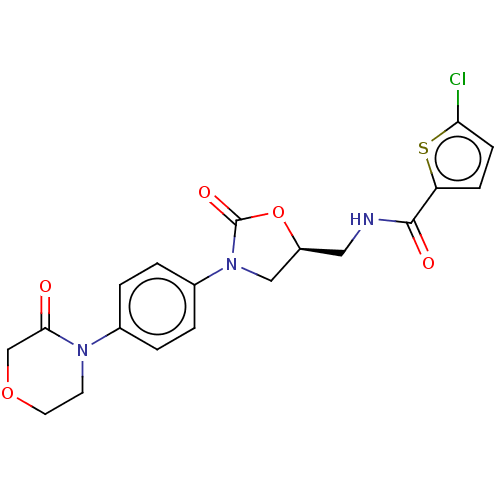
(RIVAROXABAN | US8822458, 44 | US8822458, 97)Show SMILES Clc1ccc(s1)C(=O)NC[C@H]1CN(C(=O)O1)c1ccc(cc1)N1CCOCC1=O |r| Show InChI InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553837

(CHEMBL4752231)Show SMILES Cn1ncc2c(NC(=O)NCc3cc(nn3-c3cccc(Cl)c3)C(C)(C)C)cccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards dopamine receptor D2 in rat striatal membranes by [3H]-sulpiride displacement. |
J Med Chem 36: 3073-6 (1993)
BindingDB Entry DOI: 10.7270/Q2RF5T2N |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Evaluated for the phosphatidyl inositol turnover at Muscarinic acetylcholine receptor M1 in rat cortex |
J Med Chem 31: 1463-6 (1988)
BindingDB Entry DOI: 10.7270/Q2DV1KGN |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553861
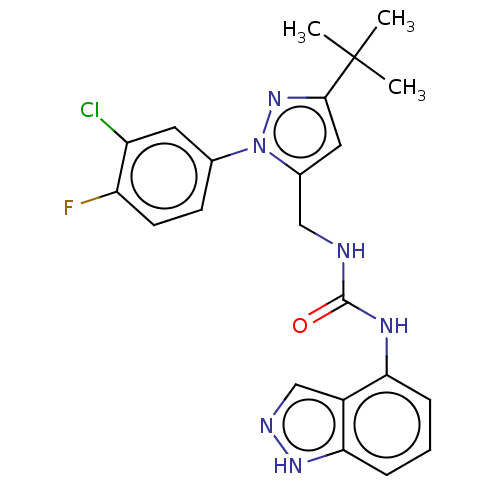
(CHEMBL4792344)Show SMILES CC(C)(C)c1cc(CNC(=O)Nc2cccc3[nH]ncc23)n(n1)-c1ccc(F)c(Cl)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50048803

(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553860

(CHEMBL4745407)Show SMILES CC(C)(C)c1cc(CNC(=O)Nc2cccc3[nH]ncc23)n(n1)-c1ccc(F)c(F)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
Muscarinic receptor M1
(Bos taurus) | BDBM50367742
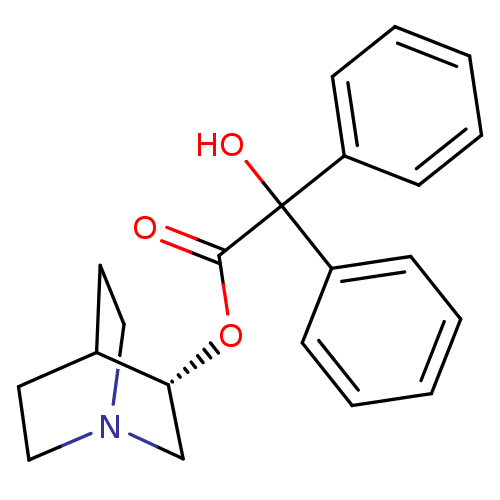
(CHEMBL1788199)Show SMILES OC(C(=O)O[C@@H]1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |r,wD:5.4,(5.41,-4.43,;4.65,-5.78,;6.2,-5.78,;6.97,-7.12,;6.97,-4.45,;8.52,-4.1,;8.52,-2.56,;9.86,-1.78,;11.19,-2.56,;11.19,-4.1,;9.86,-4.88,;9.09,-3.78,;9.09,-2.9,;3.1,-5.81,;2.36,-7.19,;.82,-7.2,;.01,-5.88,;.78,-4.52,;2.32,-4.49,;4.65,-7.23,;3.39,-7.96,;3.38,-9.41,;4.64,-10.15,;5.91,-9.42,;5.91,-7.97,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2/t19-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor M1 by displacement of [3H]pirenzepine in bovine striatum |
J Med Chem 31: 1463-6 (1988)
BindingDB Entry DOI: 10.7270/Q2DV1KGN |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553836
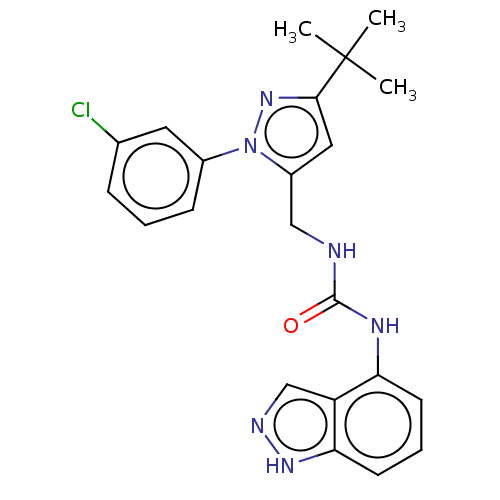
(CHEMBL4753881)Show SMILES CC(C)(C)c1cc(CNC(=O)Nc2cccc3[nH]ncc23)n(n1)-c1cccc(Cl)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553868

(CHEMBL4763969)Show SMILES Cn1ncc2c(NC(=O)NCc3cc(nn3-c3ccc(F)c(Cl)c3)C(C)(C)C)cccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001786
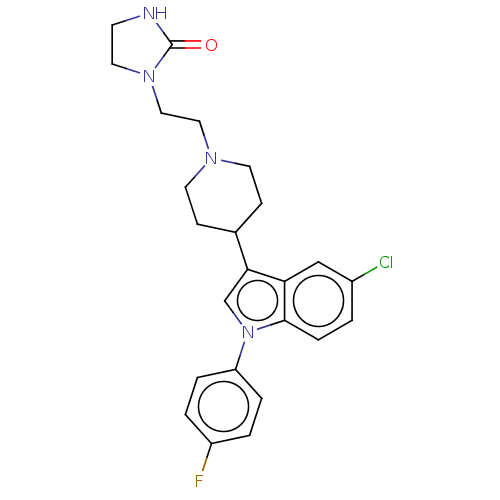
(1-(2-{4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl...)Show SMILES Fc1ccc(cc1)-n1cc(C2CCN(CCN3CCNC3=O)CC2)c2cc(Cl)ccc12 Show InChI InChI=1S/C24H26ClFN4O/c25-18-1-6-23-21(15-18)22(16-30(23)20-4-2-19(26)3-5-20)17-7-10-28(11-8-17)13-14-29-12-9-27-24(29)31/h1-6,15-17H,7-14H2,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553862
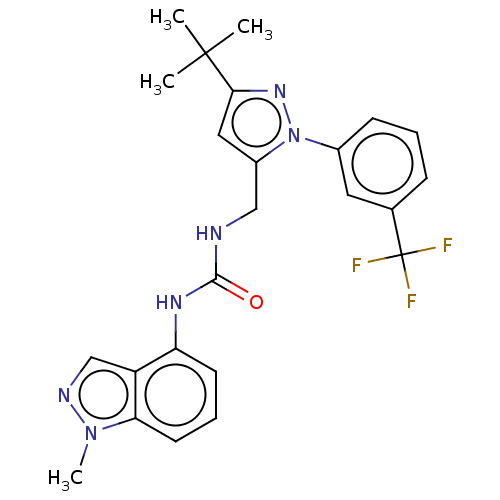
(CHEMBL4756342)Show SMILES Cn1ncc2c(NC(=O)NCc3cc(nn3-c3cccc(c3)C(F)(F)F)C(C)(C)C)cccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553843
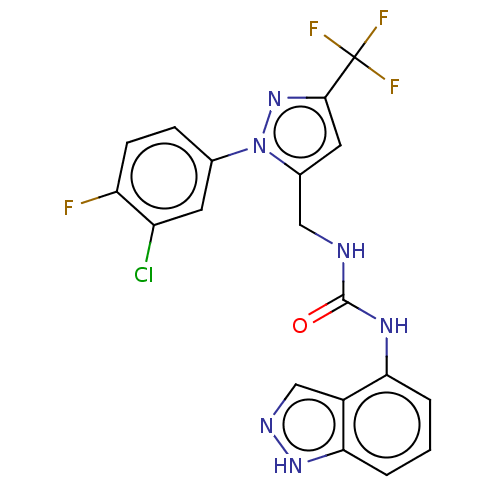
(CHEMBL4763915)Show SMILES Fc1ccc(cc1Cl)-n1nc(cc1CNC(=O)Nc1cccc2[nH]ncc12)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50001775

((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards 5-hydroxytryptamine 2 receptor in rat striatal membranes by [3H]ketanserin displacement. |
J Med Chem 36: 3073-6 (1993)
BindingDB Entry DOI: 10.7270/Q2RF5T2N |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor M2 by displacement of [3H]QNB in rat myocardium |
J Med Chem 31: 1463-6 (1988)
BindingDB Entry DOI: 10.7270/Q2DV1KGN |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50036649
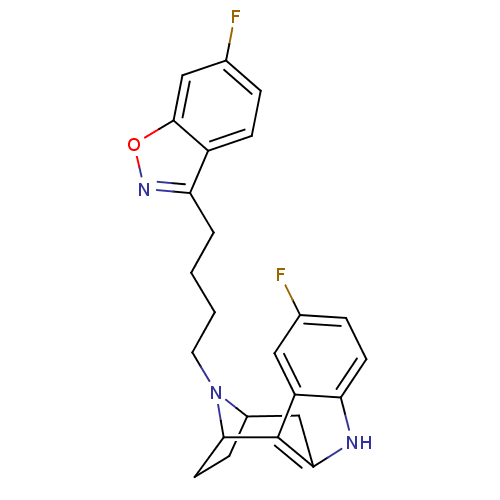
((7R,10S)-1-(6-fluorobenzo[d]isoxazol-3-yl)-4-[5-fl...)Show SMILES Fc1ccc2c(CCCCN3C4CCC3c3c(C4)[nH]c4ccc(F)cc34)noc2c1 |TLB:9:10:15.16.17:13.12,THB:25:15:10:13.12,18:16:10:13.12| Show InChI InChI=1S/C24H23F2N3O/c25-14-5-8-19-18(11-14)24-21(27-19)13-16-6-9-22(24)29(16)10-2-1-3-20-17-7-4-15(26)12-23(17)30-28-20/h4-5,7-8,11-12,16,22,27H,1-3,6,9-10,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards dopamine receptor D2 in rat striatal membranes by [3H]-sulpiride displacement. |
J Med Chem 36: 3073-6 (1993)
BindingDB Entry DOI: 10.7270/Q2RF5T2N |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50036649

((7R,10S)-1-(6-fluorobenzo[d]isoxazol-3-yl)-4-[5-fl...)Show SMILES Fc1ccc2c(CCCCN3C4CCC3c3c(C4)[nH]c4ccc(F)cc34)noc2c1 |TLB:9:10:15.16.17:13.12,THB:25:15:10:13.12,18:16:10:13.12| Show InChI InChI=1S/C24H23F2N3O/c25-14-5-8-19-18(11-14)24-21(27-19)13-16-6-9-22(24)29(16)10-2-1-3-20-17-7-4-15(26)12-23(17)30-28-20/h4-5,7-8,11-12,16,22,27H,1-3,6,9-10,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards dopamine receptor D2 in rat striatal membranes by [3H]-sulpiride displacement. |
J Med Chem 36: 3073-6 (1993)
BindingDB Entry DOI: 10.7270/Q2RF5T2N |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159507
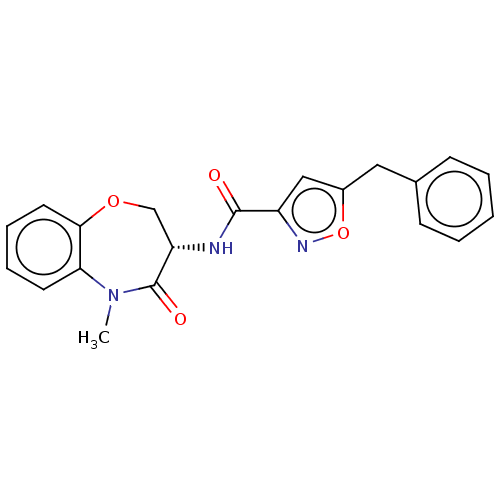
(CHEMBL3785703)Show SMILES CN1c2ccccc2OC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O |r| Show InChI InChI=1S/C21H19N3O4/c1-24-18-9-5-6-10-19(18)27-13-17(21(24)26)22-20(25)16-12-15(28-23-16)11-14-7-3-2-4-8-14/h2-10,12,17H,11,13H2,1H3,(H,22,25)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human RIP1 (1 to 375 residues) in presence of increasing ATP by ADP-Glo reagent based assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50036648
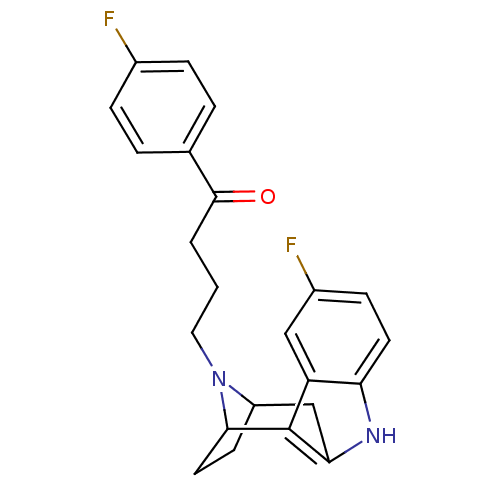
((7R,10S)-4-[5-fluoro-9,15-diazatetracyclo[10.2.1.0...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1C2CCC1c1c(C2)[nH]c2ccc(F)cc12 |TLB:11:12:17.18.19:15.14,THB:27:17:12:15.14,20:18:12:15.14| Show InChI InChI=1S/C23H22F2N2O/c24-15-5-3-14(4-6-15)22(28)2-1-11-27-17-8-10-21(27)23-18-12-16(25)7-9-19(18)26-20(23)13-17/h3-7,9,12,17,21,26H,1-2,8,10-11,13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards 5-hydroxytryptamine 2 receptor in rat striatal membranes by [3H]ketanserin displacement. |
J Med Chem 36: 3073-6 (1993)
BindingDB Entry DOI: 10.7270/Q2RF5T2N |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Mus musculus) | BDBM50216859

(CHEMBL442232 | {2-(hydroxymethyl)-5-[5-methyl-3-(2...)Show SMILES CC(C)CC(C\C=C1\OC(CO)(COC(=O)C(C)(C)C)OC1=O)CC(C)C |w:9.11| Show InChI InChI=1S/C21H36O6/c1-14(2)10-16(11-15(3)4)8-9-17-18(23)27-21(12-22,26-17)13-25-19(24)20(5,6)7/h9,14-16,22H,8,10-13H2,1-7H3/b17-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from mouse wild type C1b domain of PKCdelta expressed in Escherichia coli |
J Med Chem 50: 3465-81 (2007)
Article DOI: 10.1021/jm0702579
BindingDB Entry DOI: 10.7270/Q2RF5TQ5 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Mus musculus) | BDBM50216857
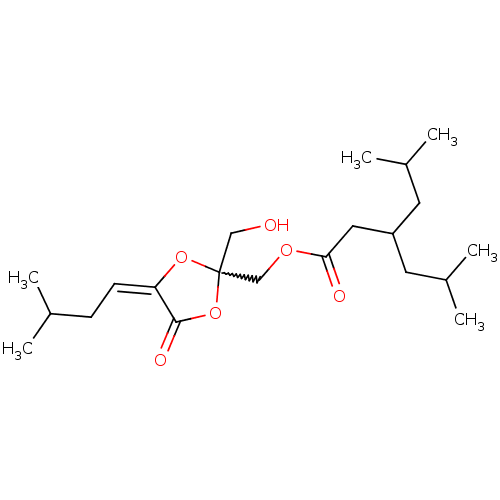
((2-(hydroxymethyl)-4-(3-methylbutylidene)-5-oxo-te...)Show SMILES CC(C)C\C=C1\OC(CO)(COC(=O)CC(CC(C)C)CC(C)C)OC1=O |w:7.9| Show InChI InChI=1S/C21H36O6/c1-14(2)7-8-18-20(24)27-21(12-22,26-18)13-25-19(23)11-17(9-15(3)4)10-16(5)6/h8,14-17,22H,7,9-13H2,1-6H3/b18-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from mouse wild type C1b domain of PKCdelta expressed in Escherichia coli |
J Med Chem 50: 3465-81 (2007)
Article DOI: 10.1021/jm0702579
BindingDB Entry DOI: 10.7270/Q2RF5TQ5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553833
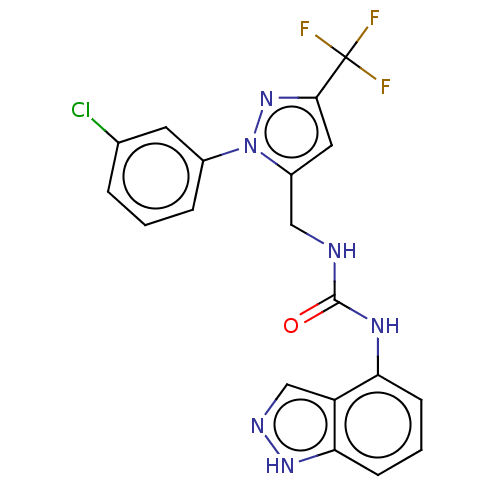
(CHEMBL4752959)Show SMILES FC(F)(F)c1cc(CNC(=O)Nc2cccc3[nH]ncc23)n(n1)-c1cccc(Cl)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50107120
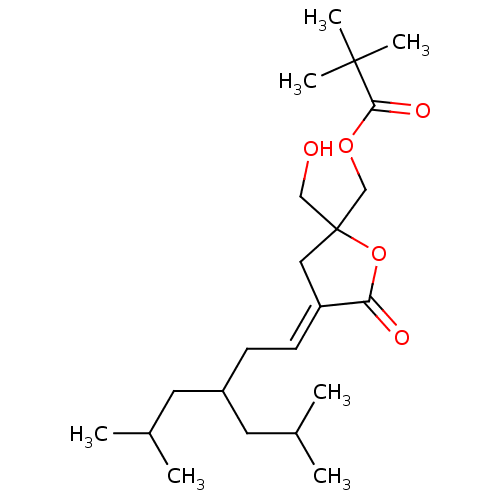
((E) 2,2-Dimethyl-propionic acid 2-hydroxymethyl-4-...)Show SMILES CC(C)CC(C\C=C1/CC(CO)(COC(=O)C(C)(C)C)OC1=O)CC(C)C Show InChI InChI=1S/C22H38O5/c1-15(2)10-17(11-16(3)4)8-9-18-12-22(13-23,27-19(18)24)14-26-20(25)21(5,6)7/h9,15-17,23H,8,10-14H2,1-7H3/b18-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to C1b domain of protein kinase C delta with phosphatidylserine |
J Med Chem 48: 5738-48 (2005)
Article DOI: 10.1021/jm050352m
BindingDB Entry DOI: 10.7270/Q27M07G5 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Mus musculus) | BDBM50216856
(CHEMBL231617 | Z-{2-(Hydroxymethyl)-4-[5-methyl-3-...)Show SMILES CC(C)CC(C\C=C1\CC(CO)(COC(=O)CC(C)C)OC1=O)CC(C)C |w:9.11| Show InChI InChI=1S/C22H38O5/c1-15(2)9-18(10-16(3)4)7-8-19-12-22(13-23,27-21(19)25)14-26-20(24)11-17(5)6/h8,15-18,23H,7,9-14H2,1-6H3/b19-8- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from mouse wild type C1b domain of PKCdelta expressed in Escherichia coli |
J Med Chem 50: 3465-81 (2007)
Article DOI: 10.1021/jm0702579
BindingDB Entry DOI: 10.7270/Q2RF5TQ5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data