 Found 5608 hits with Last Name = 'xia' and Initial = 'j'
Found 5608 hits with Last Name = 'xia' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50096906
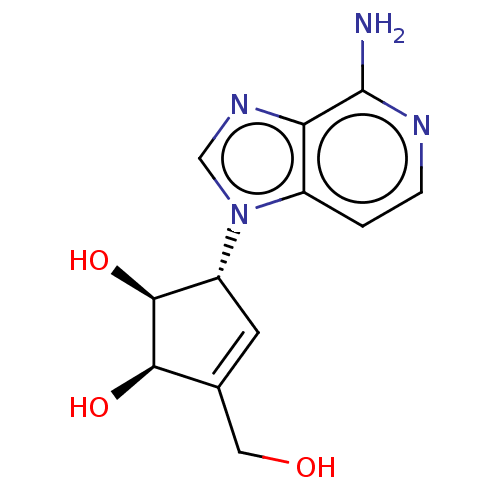
(CHEMBL154745 | US10227373, Compound D-3-Deazaisone...)Show SMILES Nc1nccc2n(cnc12)[C@@H]1C=C(CO)[C@@H](O)[C@H]1O |t:13| Show InChI InChI=1S/C12H14N4O3/c13-12-9-7(1-2-14-12)16(5-15-9)8-3-6(4-17)10(18)11(8)19/h1-3,5,8,10-11,17-19H,4H2,(H2,13,14)/t8-,10-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM85335
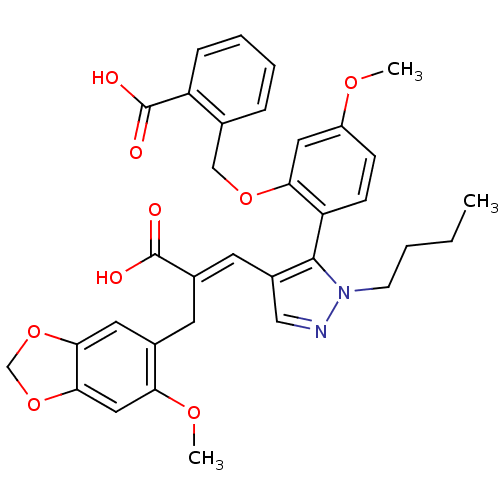
(SB 234551 | SB-234551)Show SMILES CCCCn1ncc(\C=C(/Cc2cc3OCOc3cc2OC)C(O)=O)c1-c1ccc(OC)cc1OCc1ccccc1C(O)=O |(5.96,3.45,;6.87,2.2,;8.4,2.36,;9.31,1.12,;10.84,1.28,;11.61,2.61,;13.11,2.29,;13.27,.76,;14.61,-.01,;15.94,.76,;15.94,2.3,;17.28,3.07,;18.61,2.3,;19.94,3.07,;21.41,2.6,;22.31,3.84,;21.41,5.09,;19.94,4.61,;18.61,5.38,;17.28,4.61,;15.94,5.38,;15.94,6.92,;17.28,-.01,;17.28,-1.55,;18.61,.76,;11.87,.13,;11.55,-1.37,;12.69,-2.4,;12.37,-3.91,;10.91,-4.38,;10.59,-5.89,;11.73,-6.92,;9.76,-3.35,;10.08,-1.85,;8.94,-.82,;7.47,-1.29,;6.33,-.26,;6.65,1.24,;5.51,2.27,;4.04,1.8,;3.72,.29,;4.86,-.74,;4.54,-2.24,;3.08,-2.72,;5.69,-3.28,)| Show InChI InChI=1S/C34H34N2O9/c1-4-5-12-36-32(27-11-10-25(41-2)16-29(27)43-19-21-8-6-7-9-26(21)34(39)40)24(18-35-36)14-23(33(37)38)13-22-15-30-31(45-20-44-30)17-28(22)42-3/h6-11,14-18H,4-5,12-13,19-20H2,1-3H3,(H,37,38)(H,39,40)/b23-14+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 286: 650-6 (1998)
BindingDB Entry DOI: 10.7270/Q2V40SSK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336407
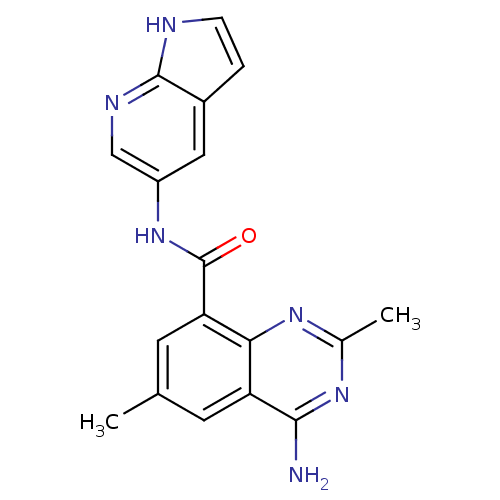
(4-amino-2,6-dimethyl-N-(1H-pyrrolo[2,3-b]pyridin-5...)Show SMILES Cc1cc(C(=O)Nc2cnc3[nH]ccc3c2)c2nc(C)nc(N)c2c1 Show InChI InChI=1S/C18H16N6O/c1-9-5-13-15(22-10(2)23-16(13)19)14(6-9)18(25)24-12-7-11-3-4-20-17(11)21-8-12/h3-8H,1-2H3,(H,20,21)(H,24,25)(H2,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha by fluorescene polarization assay |
Bioorg Med Chem Lett 21: 1270-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.026
BindingDB Entry DOI: 10.7270/Q2H1329M |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50041617
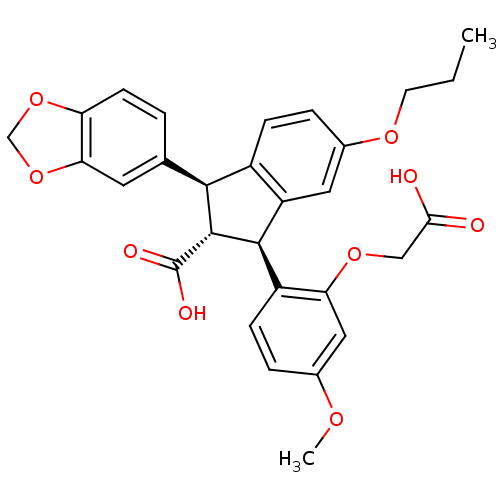
((1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-(2-carboxymet...)Show SMILES CCCOc1ccc2[C@@H]([C@H]([C@@H](c2c1)c1ccc(OC)cc1OCC(O)=O)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H28O9/c1-3-10-35-18-6-7-19-21(12-18)27(20-8-5-17(34-2)13-23(20)36-14-25(30)31)28(29(32)33)26(19)16-4-9-22-24(11-16)38-15-37-22/h4-9,11-13,26-28H,3,10,14-15H2,1-2H3,(H,30,31)(H,32,33)/t26-,27+,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 286: 650-6 (1998)
BindingDB Entry DOI: 10.7270/Q2V40SSK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336405
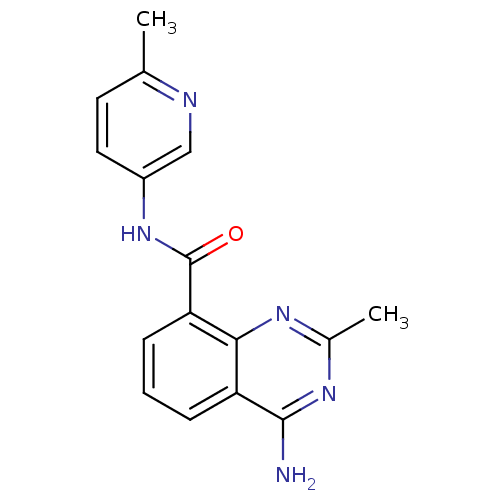
(4-amino-2-methyl-N-(6-methylpyridin-3-yl)quinazoli...)Show InChI InChI=1S/C16H15N5O/c1-9-6-7-11(8-18-9)21-16(22)13-5-3-4-12-14(13)19-10(2)20-15(12)17/h3-8H,1-2H3,(H,21,22)(H2,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha by fluorescene polarization assay |
Bioorg Med Chem Lett 21: 1270-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.026
BindingDB Entry DOI: 10.7270/Q2H1329M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336410
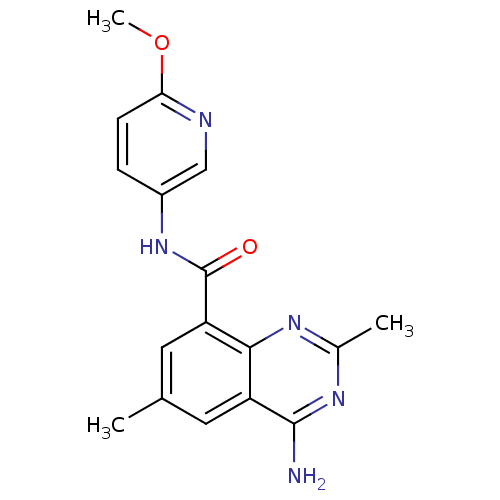
(4-amino-N-(6-methoxypyridin-3-yl)-2,6-dimethylquin...)Show InChI InChI=1S/C17H17N5O2/c1-9-6-12-15(20-10(2)21-16(12)18)13(7-9)17(23)22-11-4-5-14(24-3)19-8-11/h4-8H,1-3H3,(H,22,23)(H2,18,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha by fluorescene polarization assay |
Bioorg Med Chem Lett 21: 1270-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.026
BindingDB Entry DOI: 10.7270/Q2H1329M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336406
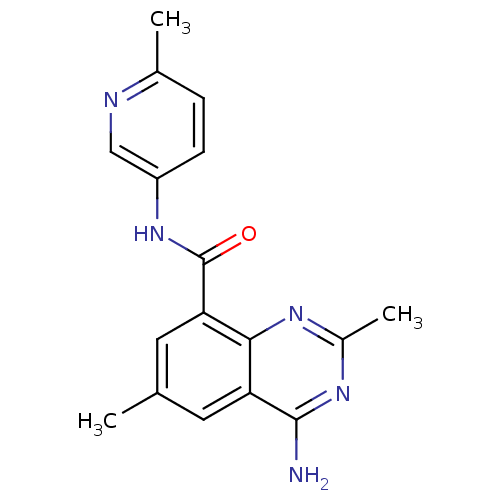
(4-amino-2,6-dimethyl-N-(6-methylpyridin-3-yl)quina...)Show InChI InChI=1S/C17H17N5O/c1-9-6-13-15(20-11(3)21-16(13)18)14(7-9)17(23)22-12-5-4-10(2)19-8-12/h4-8H,1-3H3,(H,22,23)(H2,18,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha by fluorescene polarization assay |
Bioorg Med Chem Lett 21: 1270-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.026
BindingDB Entry DOI: 10.7270/Q2H1329M |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50322229
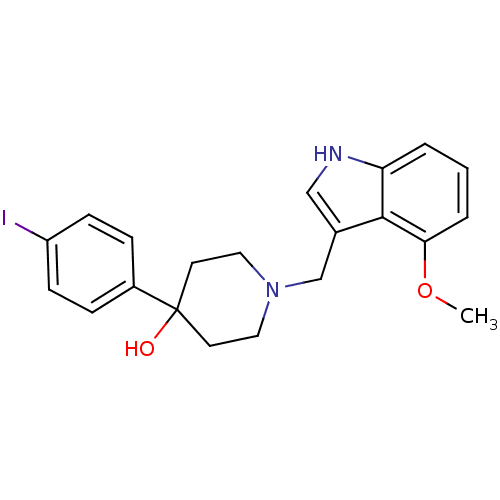
(4-(4-Iodophenyl)-1-((4-methoxy-1H-indol-3-yl)methy...)Show SMILES COc1cccc2[nH]cc(CN3CCC(O)(CC3)c3ccc(I)cc3)c12 Show InChI InChI=1S/C21H23IN2O2/c1-26-19-4-2-3-18-20(19)15(13-23-18)14-24-11-9-21(25,10-12-24)16-5-7-17(22)8-6-16/h2-8,13,23,25H,9-12,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]methylspiperone from human D2L receptor expressed in HEK cell membrane after 90 mins by scintillation counting analysis |
J Med Chem 57: 3450-63 (2014)
Article DOI: 10.1021/jm500126s
BindingDB Entry DOI: 10.7270/Q2MW2JN2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336418
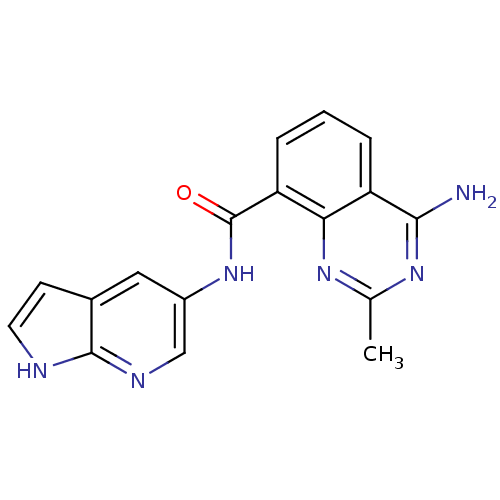
(4-amino-2-methyl-N-(1H-pyrrolo[2,3-b]pyridin-5-yl)...)Show InChI InChI=1S/C17H14N6O/c1-9-21-14-12(15(18)22-9)3-2-4-13(14)17(24)23-11-7-10-5-6-19-16(10)20-8-11/h2-8H,1H3,(H,19,20)(H,23,24)(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha by fluorescene polarization assay |
Bioorg Med Chem Lett 21: 1270-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.026
BindingDB Entry DOI: 10.7270/Q2H1329M |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]methylspiperone from human D2L receptor expressed in HEK cell membrane after 90 mins by scintillation counting analysis |
J Med Chem 57: 3450-63 (2014)
Article DOI: 10.1021/jm500126s
BindingDB Entry DOI: 10.7270/Q2MW2JN2 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50061077
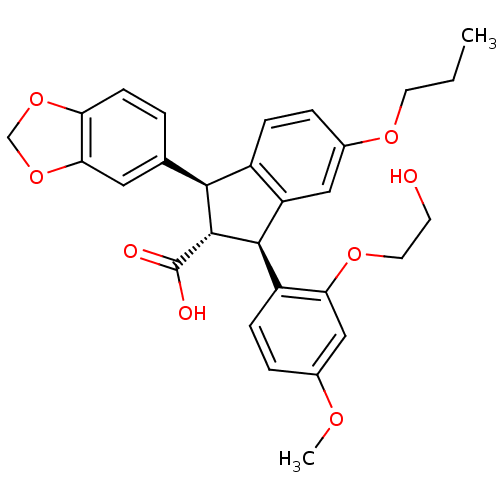
((1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-[2-(2-hydroxy...)Show SMILES CCCOc1ccc2[C@@H]([C@H]([C@@H](c2c1)c1ccc(OC)cc1OCCO)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H30O8/c1-3-11-34-19-6-7-20-22(14-19)27(21-8-5-18(33-2)15-24(21)35-12-10-30)28(29(31)32)26(20)17-4-9-23-25(13-17)37-16-36-23/h4-9,13-15,26-28,30H,3,10-12,16H2,1-2H3,(H,31,32)/t26-,27+,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 286: 650-6 (1998)
BindingDB Entry DOI: 10.7270/Q2V40SSK |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]methylspiperone from human D3 receptor expressed in HEK293 cell membrane after 90 mins by scintillation counting analysis |
J Med Chem 57: 3450-63 (2014)
Article DOI: 10.1021/jm500126s
BindingDB Entry DOI: 10.7270/Q2MW2JN2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50336404
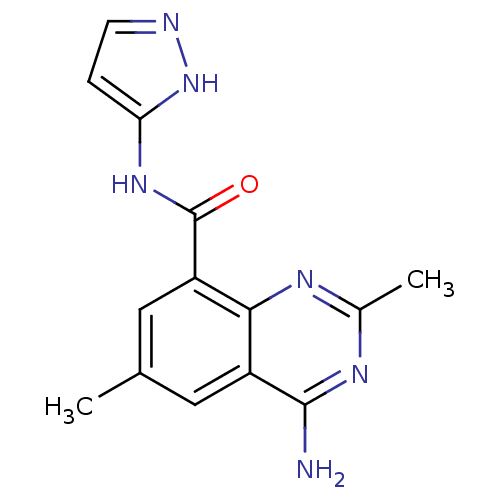
(4-amino-2,6-dimethyl-N-(1H-pyrazol-5-yl)quinazolin...)Show InChI InChI=1S/C14H14N6O/c1-7-5-9-12(17-8(2)18-13(9)15)10(6-7)14(21)19-11-3-4-16-20-11/h3-6H,1-2H3,(H2,15,17,18)(H2,16,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 21: 1270-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.026
BindingDB Entry DOI: 10.7270/Q2H1329M |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM155254
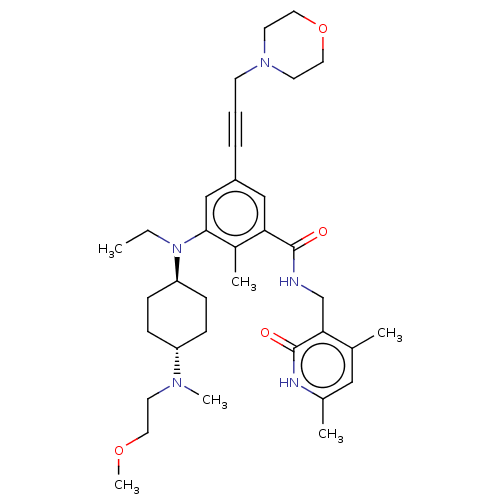
(US10098888, Compound 2 | US9006242, 2)Show SMILES CCN([C@H]1CC[C@@H](CC1)N(C)CCOC)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)C#CCN1CCOCC1 |r,wU:3.2,wD:6.9,(-6,1.54,;-4.67,.77,;-3.33,1.54,;-3.33,3.08,;-4.67,3.85,;-4.67,5.39,;-3.33,6.16,;-2,5.39,;-2,3.85,;-3.33,7.7,;-2,8.47,;-4.67,8.47,;-6,7.7,;-7.34,8.47,;-8.67,7.7,;-2,.77,;-.67,1.54,;.67,.77,;.67,-.77,;-.67,-1.54,;-.67,-3.08,;.67,-3.85,;-2,-3.85,;-2,-5.39,;-3.33,-6.16,;-3.33,-7.7,;-2,-8.47,;-4.67,-8.47,;-6,-7.7,;-7.34,-8.47,;-6,-6.16,;-4.67,-5.39,;-4.67,-3.85,;-2,-.77,;-3.33,-1.54,;2,1.54,;3.33,2.31,;4.67,3.08,;6,2.31,;6,.77,;7.34,,;8.67,.77,;8.67,2.31,;7.34,3.08,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50600091

(CHEMBL5191406)Show SMILES COCCNc1nc2ccc(cc2n1-c1ncnc(N)n1)C#CC1(O)CCCCC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116700
BindingDB Entry DOI: 10.7270/Q2FN1B89 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50061718
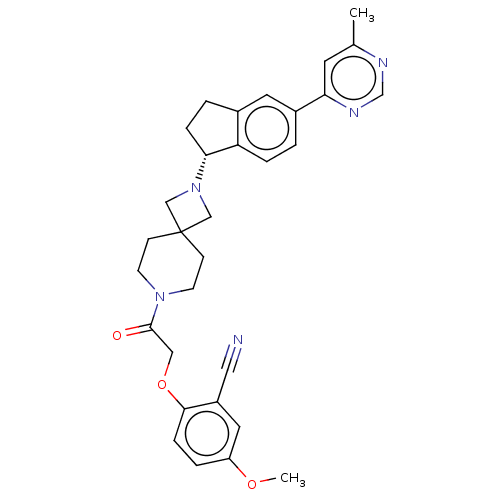
(CHEMBL3394200)Show SMILES COc1ccc(OCC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3cc(C)ncn3)CC2)c(c1)C#N |r| Show InChI InChI=1S/C31H33N5O3/c1-21-13-27(34-20-33-21)23-3-6-26-22(14-23)4-7-28(26)36-18-31(19-36)9-11-35(12-10-31)30(37)17-39-29-8-5-25(38-2)15-24(29)16-32/h3,5-6,8,13-15,20,28H,4,7,9-12,17-19H2,1-2H3/t28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHS-R1a expressed in HEK293 cells after 8 hrs by SPA method |
ACS Med Chem Lett 6: 156-61 (2015)
Article DOI: 10.1021/ml500414n
BindingDB Entry DOI: 10.7270/Q2N58P10 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50019921
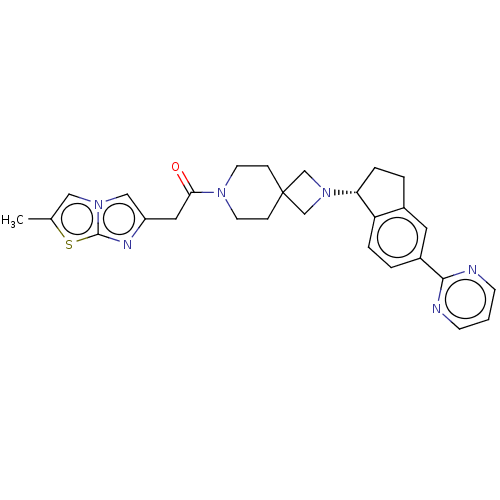
(CHEMBL3287213)Show SMILES Cc1cn2cc(CC(=O)N3CCC4(CN(C4)[C@@H]4CCc5cc(ccc45)-c4ncccn4)CC3)nc2s1 |r| Show InChI InChI=1S/C28H30N6OS/c1-19-15-33-16-22(31-27(33)36-19)14-25(35)32-11-7-28(8-12-32)17-34(18-28)24-6-4-20-13-21(3-5-23(20)24)26-29-9-2-10-30-26/h2-3,5,9-10,13,15-16,24H,4,6-8,11-12,14,17-18H2,1H3/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]ghrelin from human ghrelin receptor expressed in HEK293 cells after 8 hrs by scintillation proximity assay |
ACS Med Chem Lett 5: 474-9 (2014)
Article DOI: 10.1021/ml400473x
BindingDB Entry DOI: 10.7270/Q2MS3VB3 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50032651
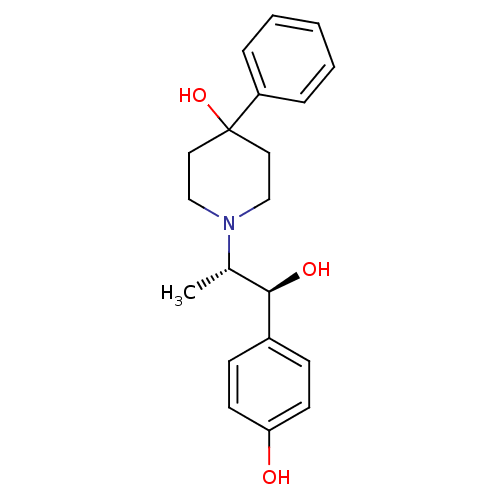
(1-((1S,2S)-1-hydroxy-1-(4-hydroxyphenyl)propan-2-y...)Show SMILES C[C@@H]([C@@H](O)c1ccc(O)cc1)N1CCC(O)(CC1)c1ccccc1 Show InChI InChI=1S/C20H25NO3/c1-15(19(23)16-7-9-18(22)10-8-16)21-13-11-20(24,12-14-21)17-5-3-2-4-6-17/h2-10,15,19,22-24H,11-14H2,1H3/t15-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50336418

(4-amino-2-methyl-N-(1H-pyrrolo[2,3-b]pyridin-5-yl)...)Show InChI InChI=1S/C17H14N6O/c1-9-21-14-12(15(18)22-9)3-2-4-13(14)17(24)23-11-7-10-5-6-19-16(10)20-8-11/h2-8H,1H3,(H,19,20)(H,23,24)(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 21: 1270-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.026
BindingDB Entry DOI: 10.7270/Q2H1329M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336408

(4-amino-2,6-dimethyl-N-(1H-pyrazol-4-yl)quinazolin...)Show InChI InChI=1S/C14H14N6O/c1-7-3-10-12(18-8(2)19-13(10)15)11(4-7)14(21)20-9-5-16-17-6-9/h3-6H,1-2H3,(H,16,17)(H,20,21)(H2,15,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha by fluorescene polarization assay |
Bioorg Med Chem Lett 21: 1270-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.026
BindingDB Entry DOI: 10.7270/Q2H1329M |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50474703
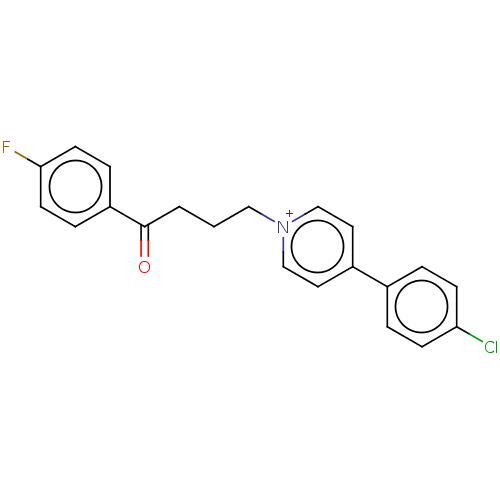
(CHEMBL1269)Show SMILES Fc1ccc(cc1)C(=O)CCC[n+]1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H18ClFNO/c22-19-7-3-16(4-8-19)17-11-14-24(15-12-17)13-1-2-21(25)18-5-9-20(23)10-6-18/h3-12,14-15H,1-2,13H2/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human Dopamine receptor D4 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336420
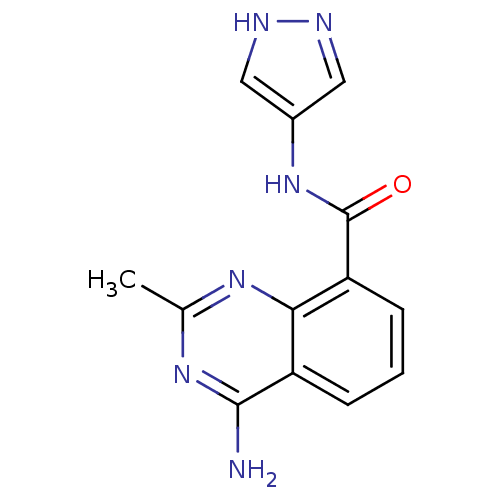
(4-amino-2-methyl-N-(1H-pyrazol-4-yl)quinazoline-8-...)Show InChI InChI=1S/C13H12N6O/c1-7-17-11-9(12(14)18-7)3-2-4-10(11)13(20)19-8-5-15-16-6-8/h2-6H,1H3,(H,15,16)(H,19,20)(H2,14,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha by fluorescene polarization assay |
Bioorg Med Chem Lett 21: 1270-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.026
BindingDB Entry DOI: 10.7270/Q2H1329M |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50474703

(CHEMBL1269)Show SMILES Fc1ccc(cc1)C(=O)CCC[n+]1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H18ClFNO/c22-19-7-3-16(4-8-19)17-11-14-24(15-12-17)13-1-2-21(25)18-5-9-20(23)10-6-18/h3-12,14-15H,1-2,13H2/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human Dopamine receptor D2 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50474703

(CHEMBL1269)Show SMILES Fc1ccc(cc1)C(=O)CCC[n+]1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H18ClFNO/c22-19-7-3-16(4-8-19)17-11-14-24(15-12-17)13-1-2-21(25)18-5-9-20(23)10-6-18/h3-12,14-15H,1-2,13H2/q+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]7-OH-DPAT from human Dopamine receptor D3 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50440261
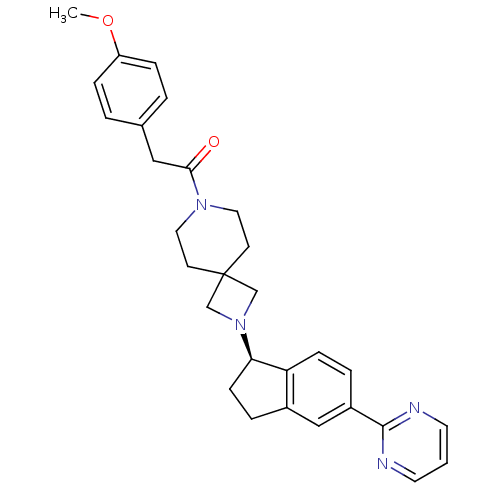
(CHEMBL2426677)Show SMILES COc1ccc(CC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3ncccn3)CC2)cc1 |r| Show InChI InChI=1S/C29H32N4O2/c1-35-24-7-3-21(4-8-24)17-27(34)32-15-11-29(12-16-32)19-33(20-29)26-10-6-22-18-23(5-9-25(22)26)28-30-13-2-14-31-28/h2-5,7-9,13-14,18,26H,6,10-12,15-17,19-20H2,1H3/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]ghrelin from human ghrelin receptor expressed in HEK293 cells after 8 hrs by scintillation proximity assay |
ACS Med Chem Lett 5: 474-9 (2014)
Article DOI: 10.1021/ml400473x
BindingDB Entry DOI: 10.7270/Q2MS3VB3 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50061725
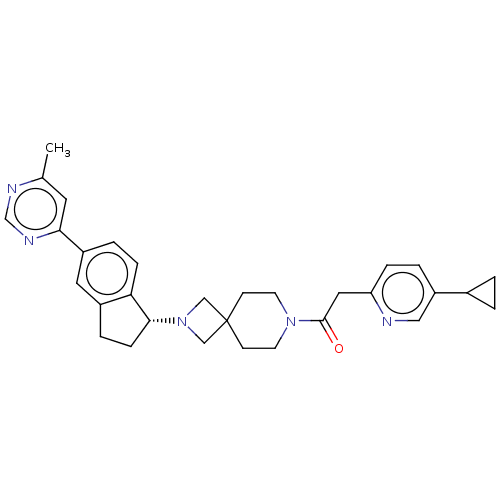
(CHEMBL3394195)Show SMILES Cc1cc(ncn1)-c1ccc2[C@@H](CCc2c1)N1CC2(C1)CCN(CC2)C(=O)Cc1ccc(cn1)C1CC1 |r| Show InChI InChI=1S/C31H35N5O/c1-21-14-28(34-20-33-21)24-5-8-27-23(15-24)6-9-29(27)36-18-31(19-36)10-12-35(13-11-31)30(37)16-26-7-4-25(17-32-26)22-2-3-22/h4-5,7-8,14-15,17,20,22,29H,2-3,6,9-13,16,18-19H2,1H3/t29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHS-R1a expressed in HEK293 cells after 8 hrs by SPA method |
ACS Med Chem Lett 6: 156-61 (2015)
Article DOI: 10.1021/ml500414n
BindingDB Entry DOI: 10.7270/Q2N58P10 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50019921

(CHEMBL3287213)Show SMILES Cc1cn2cc(CC(=O)N3CCC4(CN(C4)[C@@H]4CCc5cc(ccc45)-c4ncccn4)CC3)nc2s1 |r| Show InChI InChI=1S/C28H30N6OS/c1-19-15-33-16-22(31-27(33)36-19)14-25(35)32-11-7-28(8-12-32)17-34(18-28)24-6-4-20-13-21(3-5-23(20)24)26-29-9-2-10-30-26/h2-3,5,9-10,13,15-16,24H,4,6-8,11-12,14,17-18H2,1H3/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human ghrelin receptor expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP-gamma-S b... |
ACS Med Chem Lett 5: 474-9 (2014)
Article DOI: 10.1021/ml400473x
BindingDB Entry DOI: 10.7270/Q2MS3VB3 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50019926
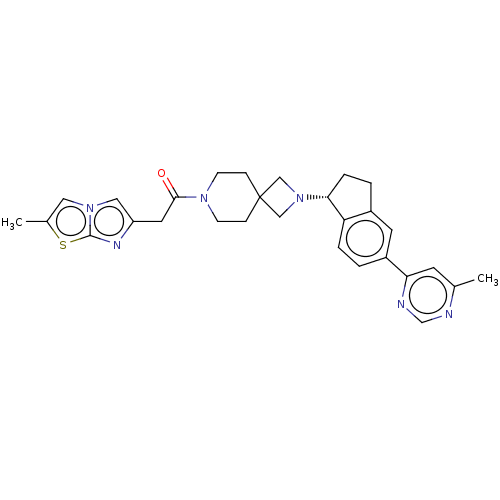
(CHEMBL3287218)Show SMILES Cc1cn2cc(CC(=O)N3CCC4(CN(C4)[C@@H]4CCc5cc(ccc45)-c4cc(C)ncn4)CC3)nc2s1 |r| Show InChI InChI=1S/C29H32N6OS/c1-19-11-25(31-18-30-19)22-3-5-24-21(12-22)4-6-26(24)35-16-29(17-35)7-9-33(10-8-29)27(36)13-23-15-34-14-20(2)37-28(34)32-23/h3,5,11-12,14-15,18,26H,4,6-10,13,16-17H2,1-2H3/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]ghrelin from human ghrelin receptor expressed in HEK293 cells after 8 hrs by scintillation proximity assay |
ACS Med Chem Lett 5: 474-9 (2014)
Article DOI: 10.1021/ml400473x
BindingDB Entry DOI: 10.7270/Q2MS3VB3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50019926

(CHEMBL3287218)Show SMILES Cc1cn2cc(CC(=O)N3CCC4(CN(C4)[C@@H]4CCc5cc(ccc45)-c4cc(C)ncn4)CC3)nc2s1 |r| Show InChI InChI=1S/C29H32N6OS/c1-19-11-25(31-18-30-19)22-3-5-24-21(12-22)4-6-26(24)35-16-29(17-35)7-9-33(10-8-29)27(36)13-23-15-34-14-20(2)37-28(34)32-23/h3,5,11-12,14-15,18,26H,4,6-10,13,16-17H2,1-2H3/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human ghrelin receptor expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP-gamma-S b... |
ACS Med Chem Lett 5: 474-9 (2014)
Article DOI: 10.1021/ml400473x
BindingDB Entry DOI: 10.7270/Q2MS3VB3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]7-OH-DPAT from human Dopamine receptor D3 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50185474

((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)C1)c1ccc(Cl)cc1 Show InChI InChI=1S/C20H21ClFNO2/c21-17-7-5-16(6-8-17)20(25)11-13-23(14-20)12-1-2-19(24)15-3-9-18(22)10-4-15/h3-10,25H,1-2,11-14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]7-OH-DPAT from human Dopamine receptor D3 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50474702

(CHEMBL358190)Show SMILES OCCC1(CCN(CCCC(=O)c2ccc(F)cc2)C1)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H25ClFNO2/c23-19-7-5-18(6-8-19)22(12-15-26)11-14-25(16-22)13-1-2-21(27)17-3-9-20(24)10-4-17/h3-10,26H,1-2,11-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human Dopamine receptor D4 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50474706
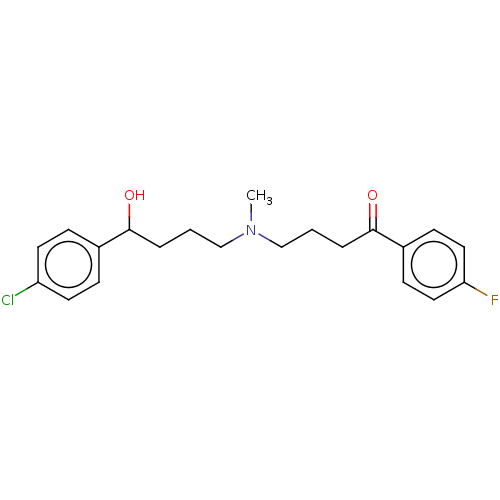
(CHEMBL148626)Show InChI InChI=1S/C21H25ClFNO2/c1-24(14-2-4-20(25)16-6-10-18(22)11-7-16)15-3-5-21(26)17-8-12-19(23)13-9-17/h6-13,20,25H,2-5,14-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human Dopamine receptor D4 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50474708
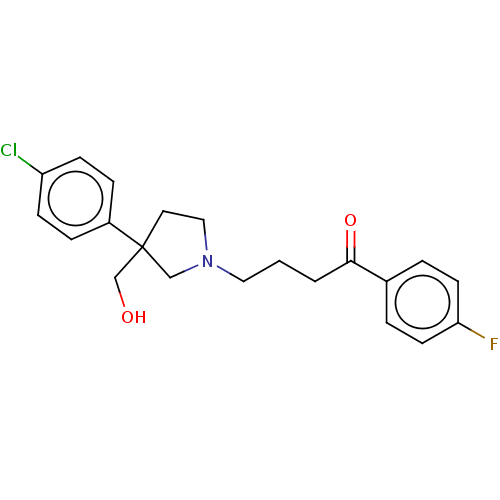
(CHEMBL149877)Show SMILES OCC1(CCN(CCCC(=O)c2ccc(F)cc2)C1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(15-25)11-13-24(14-21)12-1-2-20(26)16-3-9-19(23)10-4-16/h3-10,25H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]7-OH-DPAT from human Dopamine receptor D3 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50474706

(CHEMBL148626)Show InChI InChI=1S/C21H25ClFNO2/c1-24(14-2-4-20(25)16-6-10-18(22)11-7-16)15-3-5-21(26)17-8-12-19(23)13-9-17/h6-13,20,25H,2-5,14-15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]7-OH-DPAT from human Dopamine receptor D3 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 6.87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human Dopamine receptor D2 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50440261

(CHEMBL2426677)Show SMILES COc1ccc(CC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3ncccn3)CC2)cc1 |r| Show InChI InChI=1S/C29H32N4O2/c1-35-24-7-3-21(4-8-24)17-27(34)32-15-11-29(12-16-32)19-33(20-29)26-10-6-22-18-23(5-9-25(22)26)28-30-13-2-14-31-28/h2-5,7-9,13-14,18,26H,6,10-12,15-17,19-20H2,1H3/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHS-R1a expressed in HEK293 cells after 8 hrs by SPA method |
ACS Med Chem Lett 6: 156-61 (2015)
Article DOI: 10.1021/ml500414n
BindingDB Entry DOI: 10.7270/Q2N58P10 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50474705
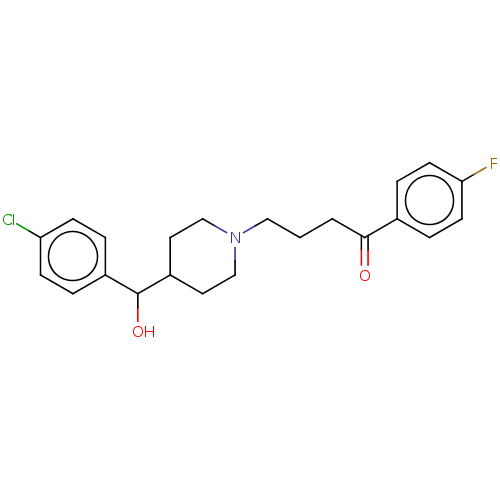
(CHEMBL357956)Show SMILES OC(C1CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H25ClFNO2/c23-19-7-3-17(4-8-19)22(27)18-11-14-25(15-12-18)13-1-2-21(26)16-5-9-20(24)10-6-16/h3-10,18,22,27H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human Dopamine receptor D4 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50474700
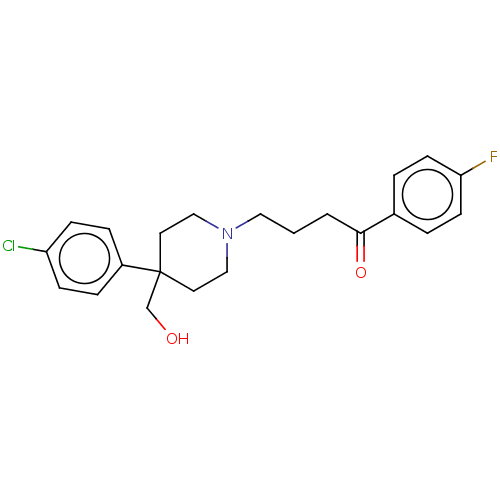
(CHEMBL149721)Show SMILES OCC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H25ClFNO2/c23-19-7-5-18(6-8-19)22(16-26)11-14-25(15-12-22)13-1-2-21(27)17-3-9-20(24)10-4-17/h3-10,26H,1-2,11-16H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]7-OH-DPAT from human Dopamine receptor D3 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336404

(4-amino-2,6-dimethyl-N-(1H-pyrazol-5-yl)quinazolin...)Show InChI InChI=1S/C14H14N6O/c1-7-5-9-12(17-8(2)18-13(9)15)10(6-7)14(21)19-11-3-4-16-20-11/h3-6H,1-2H3,(H2,15,17,18)(H2,16,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha by fluorescene polarization assay |
Bioorg Med Chem Lett 21: 1270-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.026
BindingDB Entry DOI: 10.7270/Q2H1329M |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50474700

(CHEMBL149721)Show SMILES OCC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H25ClFNO2/c23-19-7-5-18(6-8-19)22(16-26)11-14-25(15-12-22)13-1-2-21(27)17-3-9-20(24)10-4-17/h3-10,26H,1-2,11-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human Dopamine receptor D4 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50474705

(CHEMBL357956)Show SMILES OC(C1CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H25ClFNO2/c23-19-7-3-17(4-8-19)22(27)18-11-14-25(15-12-18)13-1-2-21(26)16-5-9-20(24)10-6-16/h3-10,18,22,27H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]7-OH-DPAT from human Dopamine receptor D3 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50474709

(CHEMBL147829)Show SMILES OC1(Cc2ccc(Cl)cc2)CCN(CCCC(=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C22H25ClFNO2/c23-19-7-3-17(4-8-19)16-22(27)11-14-25(15-12-22)13-1-2-21(26)18-5-9-20(24)10-6-18/h3-10,27H,1-2,11-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human Dopamine receptor D4 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 7.27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human Dopamine receptor D4 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50474701

(CHEMBL147830)Show InChI InChI=1S/C21H23ClFNO/c22-19-7-3-16(4-8-19)17-11-14-24(15-12-17)13-1-2-21(25)18-5-9-20(23)10-6-18/h3-10,17H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]7-OH-DPAT from human Dopamine receptor D3 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50474708

(CHEMBL149877)Show SMILES OCC1(CCN(CCCC(=O)c2ccc(F)cc2)C1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(15-25)11-13-24(14-21)12-1-2-20(26)16-3-9-19(23)10-4-16/h3-10,25H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human Dopamine receptor D2 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50185474

((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)C1)c1ccc(Cl)cc1 Show InChI InChI=1S/C20H21ClFNO2/c21-17-7-5-16(6-8-17)20(25)11-13-23(14-20)12-1-2-19(24)15-3-9-18(22)10-4-15/h3-10,25H,1-2,11-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human Dopamine receptor D2 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50474702

(CHEMBL358190)Show SMILES OCCC1(CCN(CCCC(=O)c2ccc(F)cc2)C1)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H25ClFNO2/c23-19-7-5-18(6-8-19)22(12-15-26)11-14-25(16-22)13-1-2-21(27)17-3-9-20(24)10-4-17/h3-10,26H,1-2,11-16H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]7-OH-DPAT from human Dopamine receptor D3 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50050467
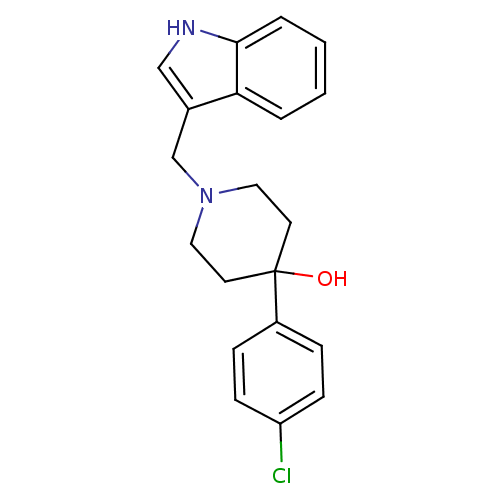
(1-((1H-indol-3-yl)methyl)-4-(4-chlorophenyl)piperi...)Show InChI InChI=1S/C20H21ClN2O/c21-17-7-5-16(6-8-17)20(24)9-11-23(12-10-20)14-15-13-22-19-4-2-1-3-18(15)19/h1-8,13,22,24H,9-12,14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]methylspiperone from human D2L receptor expressed in HEK cell membrane after 90 mins by scintillation counting analysis |
J Med Chem 57: 3450-63 (2014)
Article DOI: 10.1021/jm500126s
BindingDB Entry DOI: 10.7270/Q2MW2JN2 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50474706

(CHEMBL148626)Show InChI InChI=1S/C21H25ClFNO2/c1-24(14-2-4-20(25)16-6-10-18(22)11-7-16)15-3-5-21(26)17-8-12-19(23)13-9-17/h6-13,20,25H,2-5,14-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A and M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human Dopamine receptor D2 |
J Med Chem 47: 497-508 (2004)
Article DOI: 10.1021/jm0301033
BindingDB Entry DOI: 10.7270/Q2445Q6H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data