 Found 279 hits with Last Name = 'freisheim' and Initial = 'jh'
Found 279 hits with Last Name = 'freisheim' and Initial = 'jh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50018235

(5-Amino-2-{4-[(2,4-diamino-5-chloro-quinazolin-6-y...)Show SMILES NCCCC(NC(=O)c1ccc(NCc2ccc3nc(N)nc(N)c3c2Cl)cc1)C(O)=O Show InChI InChI=1S/C21H24ClN7O3/c22-17-12(5-8-14-16(17)18(24)29-21(25)28-14)10-26-13-6-3-11(4-7-13)19(30)27-15(20(31)32)2-1-9-23/h3-8,15,26H,1-2,9-10,23H2,(H,27,30)(H,31,32)(H4,24,25,28,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase |
J Med Chem 32: 1559-65 (1989)
BindingDB Entry DOI: 10.7270/Q22806MJ |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50002472
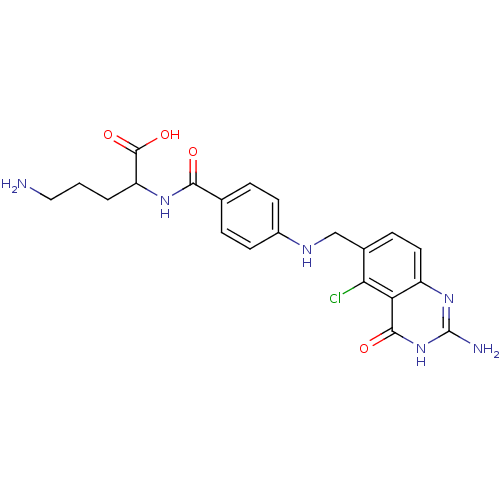
(5-Amino-2-{4-[(2-amino-5-chloro-4-oxo-3,4-dihydro-...)Show SMILES NCCCC(NC(=O)c1ccc(NCc2ccc3nc(N)[nH]c(=O)c3c2Cl)cc1)C(O)=O Show InChI InChI=1S/C21H23ClN6O4/c22-17-12(5-8-14-16(17)19(30)28-21(24)27-14)10-25-13-6-3-11(4-7-13)18(29)26-15(20(31)32)2-1-9-23/h3-8,15,25H,1-2,9-10,23H2,(H,26,29)(H,31,32)(H3,24,27,28,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase |
J Med Chem 32: 1559-65 (1989)
BindingDB Entry DOI: 10.7270/Q22806MJ |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Mus musculus) | BDBM50002471
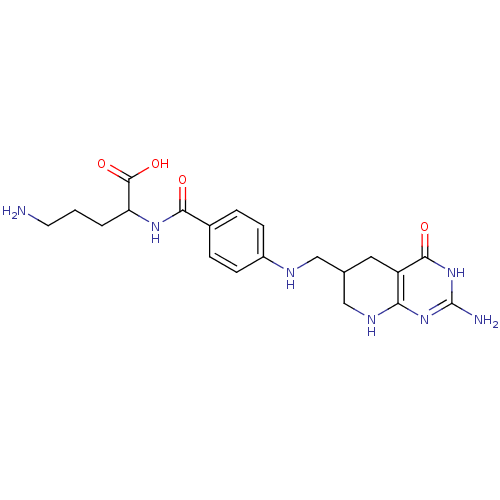
(5-Amino-2-{4-[(2-amino-4-oxo-2,3,4,4a,5,6,7,8-octa...)Show SMILES NCCCC(NC(=O)c1ccc(NCC2CNc3nc(N)[nH]c(=O)c3C2)cc1)C(O)=O Show InChI InChI=1S/C20H27N7O4/c21-7-1-2-15(19(30)31)25-17(28)12-3-5-13(6-4-12)23-9-11-8-14-16(24-10-11)26-20(22)27-18(14)29/h3-6,11,15,23H,1-2,7-10,21H2,(H,25,28)(H,30,31)(H4,22,24,26,27,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
In vitro inhibition of mouse liver Folyl-polyglutamate synthase |
J Med Chem 35: 1578-88 (1992)
BindingDB Entry DOI: 10.7270/Q2028S6J |
More data for this
Ligand-Target Pair | |
Trifunctional purine biosynthetic protein adenosine-3
(Mus musculus) | BDBM50005868

(2-{4-[(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[...)Show SMILES Nc1nc2NCC(CNc3ccc(cc3)C(=O)NC(CCP(O)(O)=O)C(O)=O)Cc2c(=O)[nH]1 Show InChI InChI=1S/C19H25N6O7P/c20-19-24-15-13(17(27)25-19)7-10(9-22-15)8-21-12-3-1-11(2-4-12)16(26)23-14(18(28)29)5-6-33(30,31)32/h1-4,10,14,21H,5-9H2,(H,23,26)(H,28,29)(H2,30,31,32)(H4,20,22,24,25,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
In vitro inhibition of Glycinamide ribonucleotide formyltransferase from L1210 murine leukemia cells |
J Med Chem 35: 1578-88 (1992)
BindingDB Entry DOI: 10.7270/Q2028S6J |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405088
(CHEMBL31004)Show SMILES CC1(C)N=C(N)N=C(N)N1c1ccc(CSc2ccccc2)cc1 |t:3,6| Show InChI InChI=1S/C18H21N5S/c1-18(2)22-16(19)21-17(20)23(18)14-10-8-13(9-11-14)12-24-15-6-4-3-5-7-15/h3-11H,12H2,1-2H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405074
(CHEMBL34627)Show SMILES CC1(C)N=C(N)N=C(N)N1c1ccc(OCc2ccc(cc2)C(N)=O)cc1 |t:3,6| Show InChI InChI=1S/C19H22N6O2/c1-19(2)24-17(21)23-18(22)25(19)14-7-9-15(10-8-14)27-11-12-3-5-13(6-4-12)16(20)26/h3-10H,11H2,1-2H3,(H2,20,26)(H4,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405052
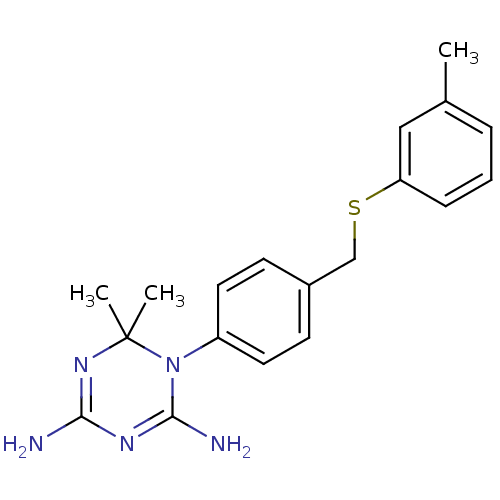
(CHEMBL283916)Show SMILES Cc1cccc(SCc2ccc(cc2)N2C(N)=NC(N)=NC2(C)C)c1 |c:17,20| Show InChI InChI=1S/C19H23N5S/c1-13-5-4-6-16(11-13)25-12-14-7-9-15(10-8-14)24-18(21)22-17(20)23-19(24,2)3/h4-11H,12H2,1-3H3,(H4,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405070

(CHEMBL30681)Show SMILES CC1(C)N=C(N)N=C(N)N1c1ccc(OCc2ccc(cc2)S(N)(=O)=O)cc1 |t:3,6| Show InChI InChI=1S/C18H22N6O3S/c1-18(2)23-16(19)22-17(20)24(18)13-5-7-14(8-6-13)27-11-12-3-9-15(10-4-12)28(21,25)26/h3-10H,11H2,1-2H3,(H2,21,25,26)(H4,19,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Trifunctional purine biosynthetic protein adenosine-3
(Mus musculus) | BDBM50024475
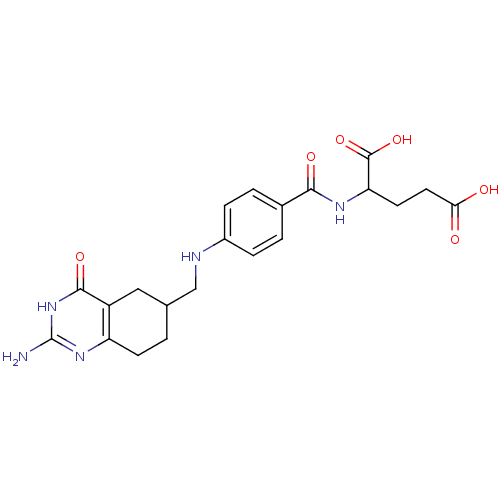
(2-{4-[(2-Amino-4-oxo-1,4,5,6,7,8-hexahydro-quinazo...)Show SMILES Nc1nc2CCC(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)Cc2c(=O)[nH]1 Show InChI InChI=1S/C21H25N5O6/c22-21-25-15-6-1-11(9-14(15)19(30)26-21)10-23-13-4-2-12(3-5-13)18(29)24-16(20(31)32)7-8-17(27)28/h2-5,11,16,23H,1,6-10H2,(H,24,29)(H,27,28)(H,31,32)(H3,22,25,26,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
In vitro inhibition of mouse GAR transformylase |
J Med Chem 35: 1578-88 (1992)
BindingDB Entry DOI: 10.7270/Q2028S6J |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405102
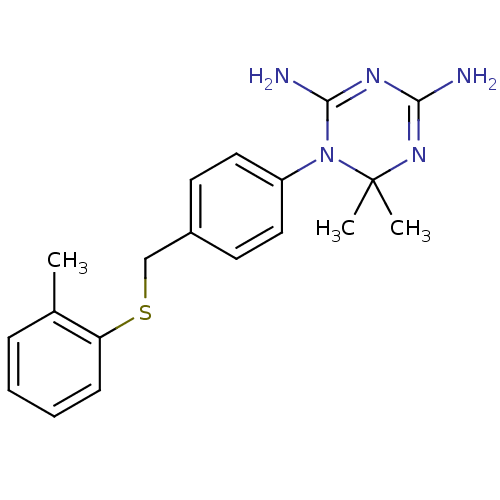
(CHEMBL30475)Show SMILES Cc1ccccc1SCc1ccc(cc1)N1C(N)=NC(N)=NC1(C)C |c:19,22| Show InChI InChI=1S/C19H23N5S/c1-13-6-4-5-7-16(13)25-12-14-8-10-15(11-9-14)24-18(21)22-17(20)23-19(24,2)3/h4-11H,12H2,1-3H3,(H4,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405072
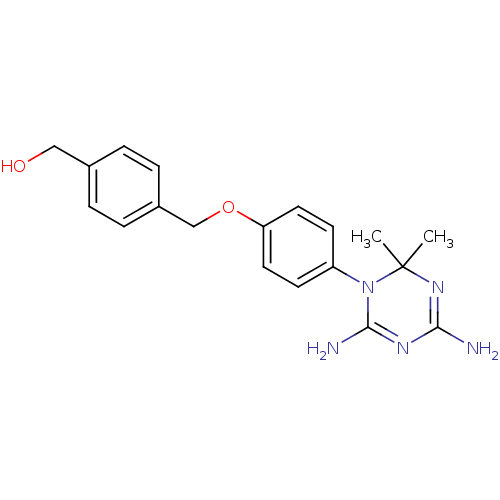
(CHEMBL33010)Show SMILES CC1(C)N=C(N)N=C(N)N1c1ccc(OCc2ccc(CO)cc2)cc1 |t:3,6| Show InChI InChI=1S/C19H23N5O2/c1-19(2)23-17(20)22-18(21)24(19)15-7-9-16(10-8-15)26-12-14-5-3-13(11-25)4-6-14/h3-10,25H,11-12H2,1-2H3,(H4,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405055
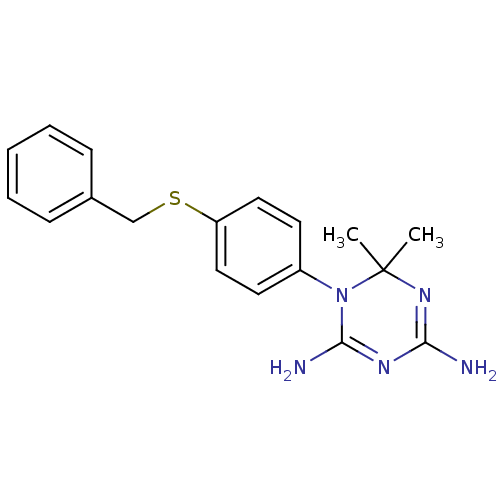
(CHEMBL283173)Show SMILES CC1(C)N=C(N)N=C(N)N1c1ccc(SCc2ccccc2)cc1 |t:3,6| Show InChI InChI=1S/C18H21N5S/c1-18(2)22-16(19)21-17(20)23(18)14-8-10-15(11-9-14)24-12-13-6-4-3-5-7-13/h3-11H,12H2,1-2H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405049
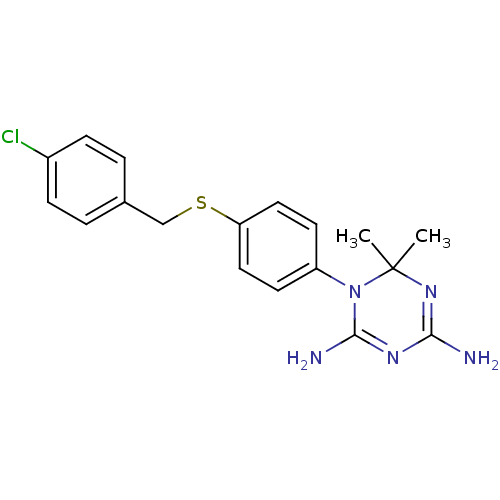
(CHEMBL285019)Show SMILES CC1(C)N=C(N)N=C(N)N1c1ccc(SCc2ccc(Cl)cc2)cc1 |t:3,6| Show InChI InChI=1S/C18H20ClN5S/c1-18(2)23-16(20)22-17(21)24(18)14-7-9-15(10-8-14)25-11-12-3-5-13(19)6-4-12/h3-10H,11H2,1-2H3,(H4,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405043
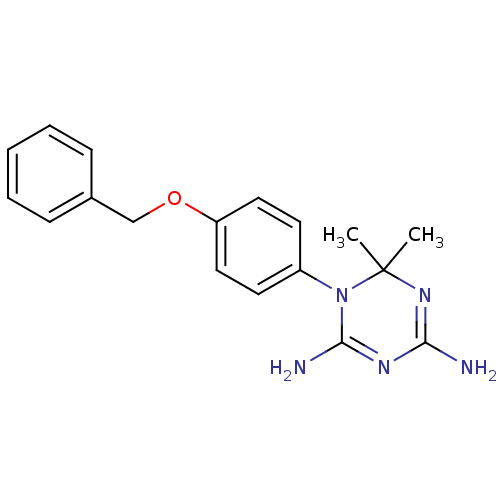
(1-(4-(Benzyloxy)phenyl)-6,6-dimethyl-1,6-dihydro-1...)Show SMILES CC1(C)N=C(N)N=C(N)N1c1ccc(OCc2ccccc2)cc1 |t:3,6| Show InChI InChI=1S/C18H21N5O/c1-18(2)22-16(19)21-17(20)23(18)14-8-10-15(11-9-14)24-12-13-6-4-3-5-7-13/h3-11H,12H2,1-2H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50016343
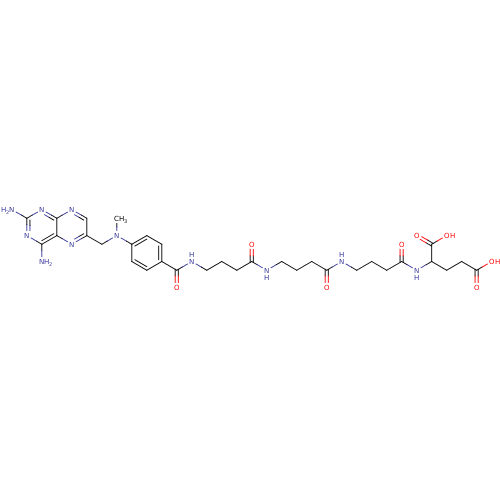
(2-{4-[4-(4-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-m...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NCCCC(=O)NCCCC(=O)NCCCC(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C32H43N11O8/c1-43(18-20-17-38-29-27(39-20)28(33)41-32(34)42-29)21-10-8-19(9-11-21)30(49)37-16-3-6-24(45)35-14-2-5-23(44)36-15-4-7-25(46)40-22(31(50)51)12-13-26(47)48/h8-11,17,22H,2-7,12-16,18H2,1H3,(H,35,45)(H,36,44)(H,37,49)(H,40,46)(H,47,48)(H,50,51)(H4,33,34,38,41,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit thymidylate synthetase isolated from MTX-resistant Lactobacillus casei |
J Med Chem 29: 1872-6 (1986)
BindingDB Entry DOI: 10.7270/Q2JH3K4Q |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Mus musculus) | BDBM50011885
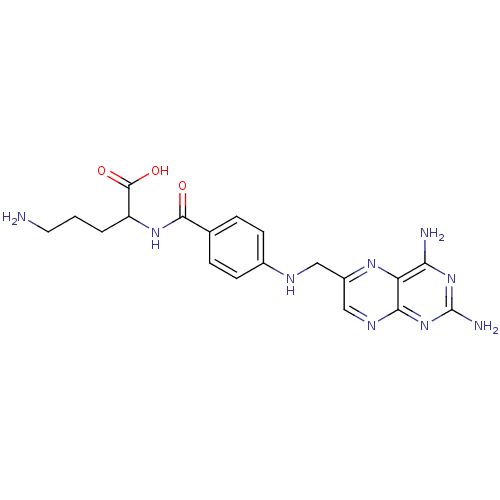
(5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-am...)Show SMILES NCCCC(NC(=O)c1ccc(NCc2cnc3nc(N)nc(N)c3n2)cc1)C(O)=O Show InChI InChI=1S/C19H23N9O3/c20-7-1-2-13(18(30)31)26-17(29)10-3-5-11(6-4-10)23-8-12-9-24-16-14(25-12)15(21)27-19(22)28-16/h3-6,9,13,23H,1-2,7-8,20H2,(H,26,29)(H,30,31)(H4,21,22,24,27,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit Folyl-polyglutamate synthase from mouse liver |
J Med Chem 29: 655-60 (1986)
BindingDB Entry DOI: 10.7270/Q2RR1ZTR |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50016340
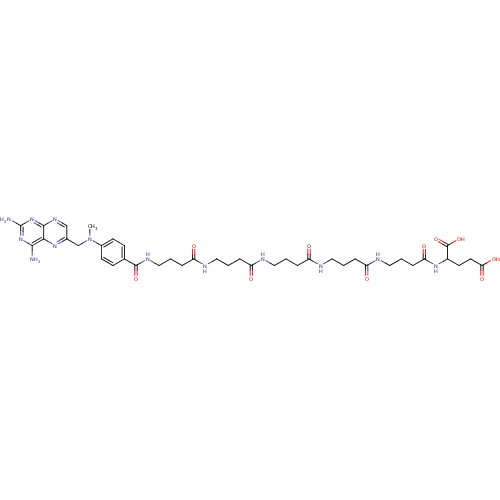
(2-[4-(4-{4-[4-(4-{4-[(2,4-Diamino-pteridin-6-ylmet...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NCCCC(=O)NCCCC(=O)NCCCC(=O)NCCCC(=O)NCCCC(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C40H57N13O10/c1-53(24-26-23-48-37-35(49-26)36(41)51-40(42)52-37)27-14-12-25(13-15-27)38(61)47-22-5-10-32(57)45-20-3-8-30(55)43-18-2-7-29(54)44-19-4-9-31(56)46-21-6-11-33(58)50-28(39(62)63)16-17-34(59)60/h12-15,23,28H,2-11,16-22,24H2,1H3,(H,43,55)(H,44,54)(H,45,57)(H,46,56)(H,47,61)(H,50,58)(H,59,60)(H,62,63)(H4,41,42,48,51,52) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit thymidylate synthetase isolated from MTX-resistant Lactobacillus casei |
J Med Chem 29: 1872-6 (1986)
BindingDB Entry DOI: 10.7270/Q2JH3K4Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50016339

(2-(4-{4-[4-(4-{4-[(2,4-Diamino-pteridin-6-ylmethyl...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NCCCC(=O)NCCCC(=O)NCCCC(=O)NCCCC(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C36H50N12O9/c1-48(21-23-20-43-33-31(44-23)32(37)46-36(38)47-33)24-12-10-22(11-13-24)34(55)42-19-4-8-28(51)40-17-2-6-26(49)39-16-3-7-27(50)41-18-5-9-29(52)45-25(35(56)57)14-15-30(53)54/h10-13,20,25H,2-9,14-19,21H2,1H3,(H,39,49)(H,40,51)(H,41,50)(H,42,55)(H,45,52)(H,53,54)(H,56,57)(H4,37,38,43,46,47) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit thymidylate synthetase isolated from MTX-resistant Lactobacillus casei |
J Med Chem 29: 1872-6 (1986)
BindingDB Entry DOI: 10.7270/Q2JH3K4Q |
More data for this
Ligand-Target Pair | |
Trifunctional purine biosynthetic protein adenosine-3
(Mus musculus) | BDBM50005865
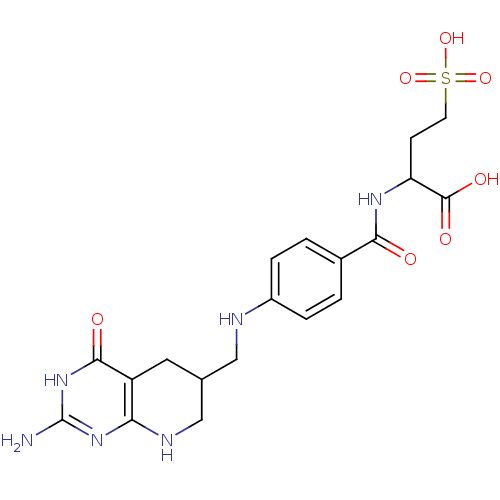
(2-{4-[(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[...)Show SMILES Nc1nc2NCC(CNc3ccc(cc3)C(=O)NC(CCS(O)(=O)=O)C(O)=O)Cc2c(=O)[nH]1 Show InChI InChI=1S/C19H24N6O7S/c20-19-24-15-13(17(27)25-19)7-10(9-22-15)8-21-12-3-1-11(2-4-12)16(26)23-14(18(28)29)5-6-33(30,31)32/h1-4,10,14,21H,5-9H2,(H,23,26)(H,28,29)(H,30,31,32)(H4,20,22,24,25,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
In vitro inhibition of Glycinamide ribonucleotide formyltransferase from L1210 murine leukemia cells |
J Med Chem 35: 1578-88 (1992)
BindingDB Entry DOI: 10.7270/Q2028S6J |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50016342

(2-[4-(4-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-meth...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NCCCC(=O)NCCCC(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C28H36N10O7/c1-38(15-17-14-33-25-23(34-17)24(29)36-28(30)37-25)18-8-6-16(7-9-18)26(43)32-13-2-4-20(39)31-12-3-5-21(40)35-19(27(44)45)10-11-22(41)42/h6-9,14,19H,2-5,10-13,15H2,1H3,(H,31,39)(H,32,43)(H,35,40)(H,41,42)(H,44,45)(H4,29,30,33,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit thymidylate synthetase isolated from MTX-resistant Lactobacillus casei |
J Med Chem 29: 1872-6 (1986)
BindingDB Entry DOI: 10.7270/Q2JH3K4Q |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Mus musculus) | BDBM50020107
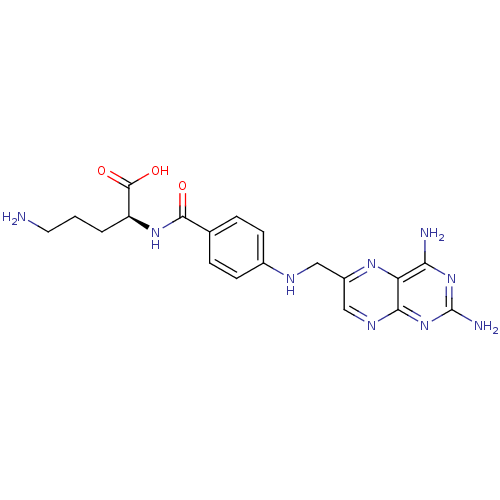
(5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-am...)Show SMILES NCCC[C@H](NC(=O)c1ccc(NCc2cnc3nc(N)nc(N)c3n2)cc1)C(O)=O Show InChI InChI=1S/C19H23N9O3/c20-7-1-2-13(18(30)31)26-17(29)10-3-5-11(6-4-10)23-8-12-9-24-16-14(25-12)15(21)27-19(22)28-16/h3-6,9,13,23H,1-2,7-8,20H2,(H,26,29)(H,30,31)(H4,21,22,24,27,28)/t13-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory constant against purified Folyl-polyglutamate synthase obtained from mouse |
J Med Chem 31: 1338-44 (1988)
BindingDB Entry DOI: 10.7270/Q22J6CFD |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50020107

(5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-am...)Show SMILES NCCC[C@H](NC(=O)c1ccc(NCc2cnc3nc(N)nc(N)c3n2)cc1)C(O)=O Show InChI InChI=1S/C19H23N9O3/c20-7-1-2-13(18(30)31)26-17(29)10-3-5-11(6-4-10)23-8-12-9-24-16-14(25-12)15(21)27-19(22)28-16/h3-6,9,13,23H,1-2,7-8,20H2,(H,26,29)(H,30,31)(H4,21,22,24,27,28)/t13-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory constant against purified Folyl-polyglutamate synthase obtained from human |
J Med Chem 31: 1338-44 (1988)
BindingDB Entry DOI: 10.7270/Q22J6CFD |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405073
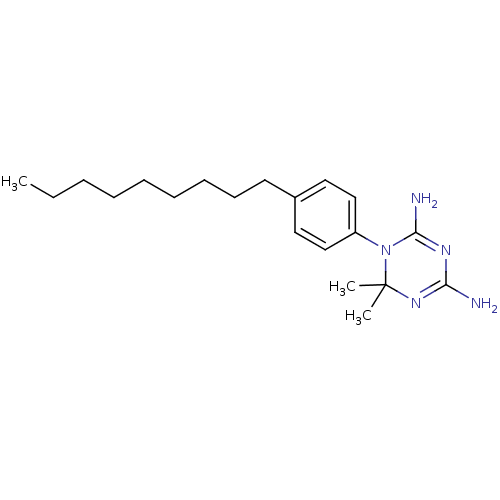
(CHEMBL33014)Show SMILES CCCCCCCCCc1ccc(cc1)N1C(N)=NC(N)=NC1(C)C |c:18,21| Show InChI InChI=1S/C20H33N5/c1-4-5-6-7-8-9-10-11-16-12-14-17(15-13-16)25-19(22)23-18(21)24-20(25,2)3/h12-15H,4-11H2,1-3H3,(H4,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50016341
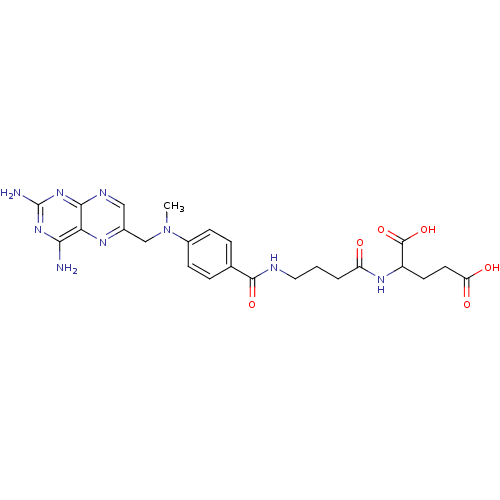
(2-(4-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NCCCC(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C24H29N9O6/c1-33(12-14-11-28-21-19(29-14)20(25)31-24(26)32-21)15-6-4-13(5-7-15)22(37)27-10-2-3-17(34)30-16(23(38)39)8-9-18(35)36/h4-7,11,16H,2-3,8-10,12H2,1H3,(H,27,37)(H,30,34)(H,35,36)(H,38,39)(H4,25,26,28,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 336 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit thymidylate synthetase isolated from MTX-resistant Lactobacillus casei |
J Med Chem 29: 1872-6 (1986)
BindingDB Entry DOI: 10.7270/Q2JH3K4Q |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405054
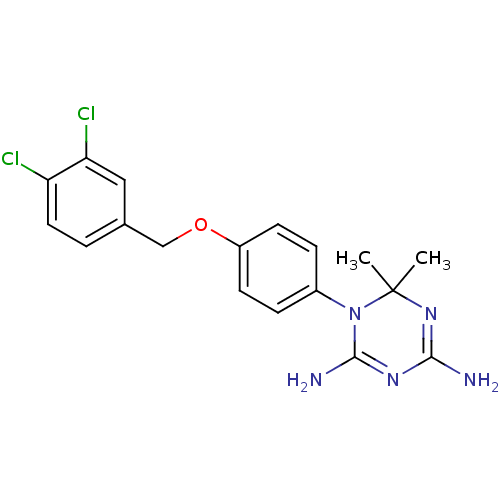
(CHEMBL7035)Show SMILES CC1(C)N=C(N)N=C(N)N1c1ccc(OCc2ccc(Cl)c(Cl)c2)cc1 |t:3,6| Show InChI InChI=1S/C18H19Cl2N5O/c1-18(2)24-16(21)23-17(22)25(18)12-4-6-13(7-5-12)26-10-11-3-8-14(19)15(20)9-11/h3-9H,10H2,1-2H3,(H4,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50018236
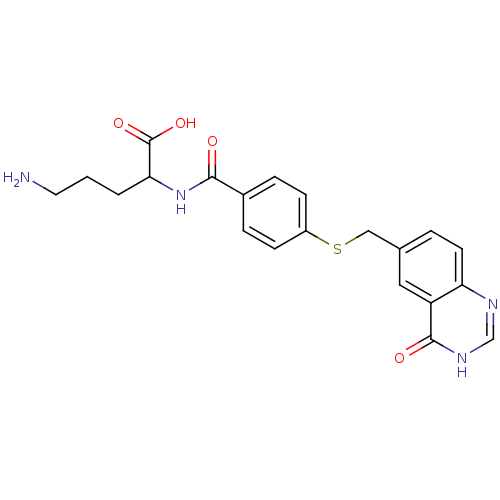
(5-Amino-2-[4-(4-oxo-3,4-dihydro-quinazolin-6-ylmet...)Show SMILES NCCCC(NC(=O)c1ccc(SCc2ccc3nc[nH]c(=O)c3c2)cc1)C(O)=O Show InChI InChI=1S/C21H22N4O4S/c22-9-1-2-18(21(28)29)25-19(26)14-4-6-15(7-5-14)30-11-13-3-8-17-16(10-13)20(27)24-12-23-17/h3-8,10,12,18H,1-2,9,11,22H2,(H,25,26)(H,28,29)(H,23,24,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase |
J Med Chem 32: 1559-65 (1989)
BindingDB Entry DOI: 10.7270/Q22806MJ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405046
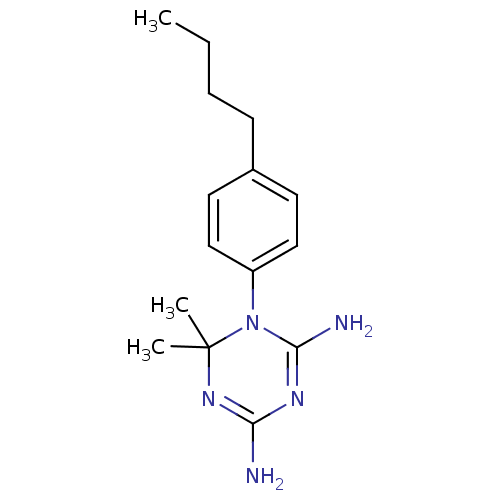
(CHEMBL6962)Show SMILES CCCCc1ccc(cc1)N1C(N)=NC(N)=NC1(C)C |c:13,16| Show InChI InChI=1S/C15H23N5/c1-4-5-6-11-7-9-12(10-8-11)20-14(17)18-13(16)19-15(20,2)3/h7-10H,4-6H2,1-3H3,(H4,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18792
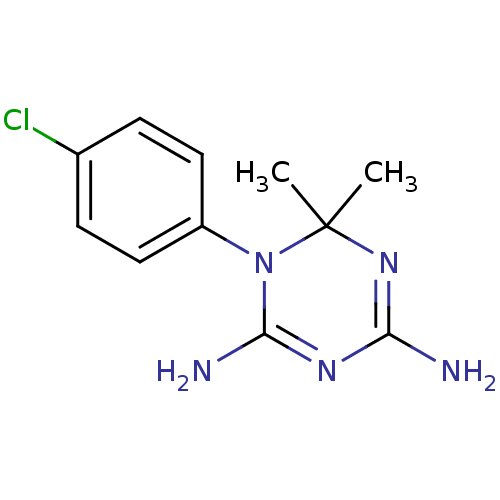
(1-(4-chlorophenyl)-6,6-dimethyl-1,6-dihydro-1,3,5-...)Show InChI InChI=1S/C11H14ClN5/c1-11(2)16-9(13)15-10(14)17(11)8-5-3-7(12)4-6-8/h3-6H,1-2H3,(H4,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50090050
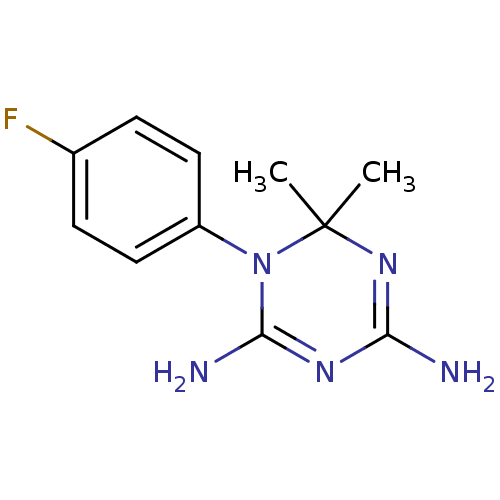
(1-(4-Fluoro-phenyl)-6,6-dimethyl-1,6-dihydro-[1,3,...)Show InChI InChI=1S/C11H14FN5/c1-11(2)16-9(13)15-10(14)17(11)8-5-3-7(12)4-6-8/h3-6H,1-2H3,(H4,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405051
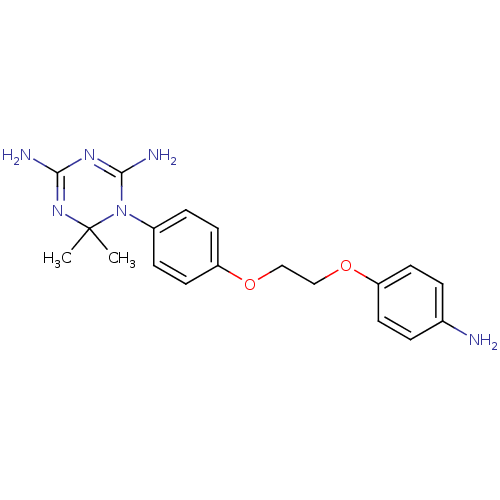
(CHEMBL33389)Show SMILES CC1(C)N=C(N)N=C(N)N1c1ccc(OCCOc2ccc(N)cc2)cc1 |t:3,6| Show InChI InChI=1S/C19H24N6O2/c1-19(2)24-17(21)23-18(22)25(19)14-5-9-16(10-6-14)27-12-11-26-15-7-3-13(20)4-8-15/h3-10H,11-12,20H2,1-2H3,(H4,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50090051
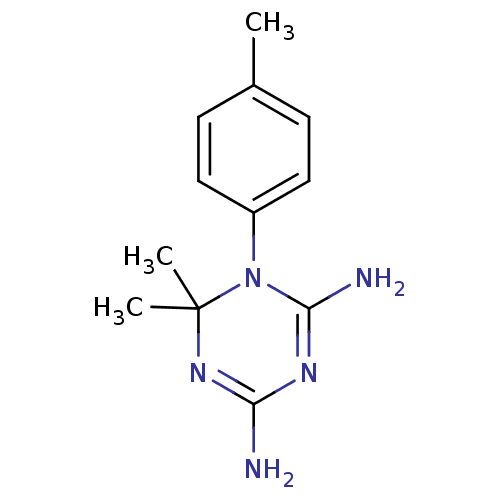
(6,6-Dimethyl-1-p-tolyl-1,6-dihydro-[1,3,5]triazine...)Show InChI InChI=1S/C12H17N5/c1-8-4-6-9(7-5-8)17-11(14)15-10(13)16-12(17,2)3/h4-7H,1-3H3,(H4,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50018232
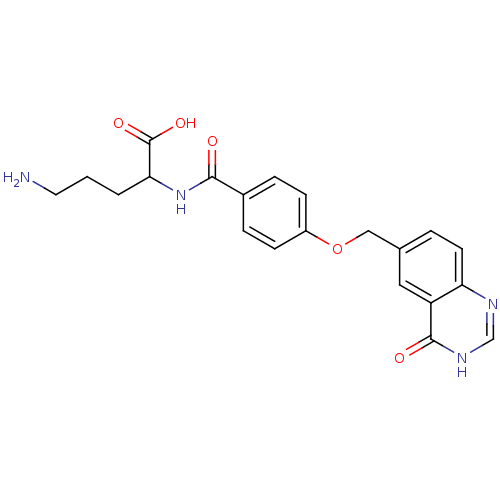
(5-Amino-2-[4-(4-oxo-3,4-dihydro-quinazolin-6-ylmet...)Show SMILES NCCCC(NC(=O)c1ccc(OCc2ccc3nc[nH]c(=O)c3c2)cc1)C(O)=O Show InChI InChI=1S/C21H22N4O5/c22-9-1-2-18(21(28)29)25-19(26)14-4-6-15(7-5-14)30-11-13-3-8-17-16(10-13)20(27)24-12-23-17/h3-8,10,12,18H,1-2,9,11,22H2,(H,25,26)(H,28,29)(H,23,24,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase |
J Med Chem 32: 1559-65 (1989)
BindingDB Entry DOI: 10.7270/Q22806MJ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405100
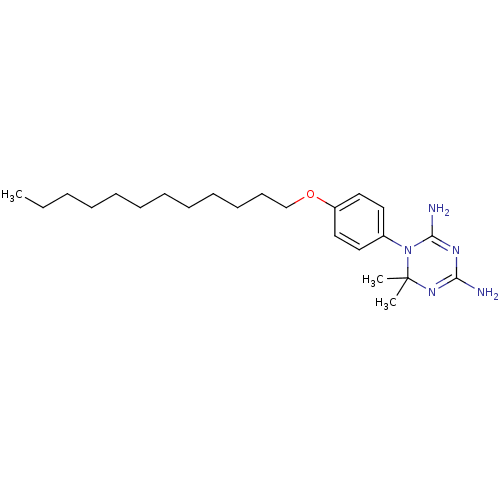
(CHEMBL32304)Show SMILES CCCCCCCCCCCCOc1ccc(cc1)N1C(N)=NC(N)=NC1(C)C |c:22,25| Show InChI InChI=1S/C23H39N5O/c1-4-5-6-7-8-9-10-11-12-13-18-29-20-16-14-19(15-17-20)28-22(25)26-21(24)27-23(28,2)3/h14-17H,4-13,18H2,1-3H3,(H4,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50090067
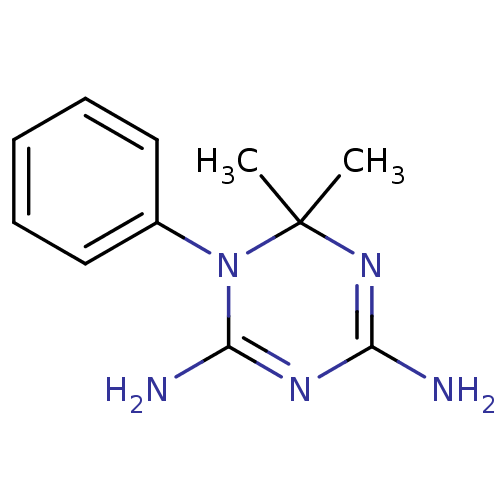
(6,6-Dimethyl-1-phenyl-1,6-dihydro-[1,3,5]triazine-...)Show InChI InChI=1S/C11H15N5/c1-11(2)15-9(12)14-10(13)16(11)8-6-4-3-5-7-8/h3-7H,1-2H3,(H4,12,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50090075
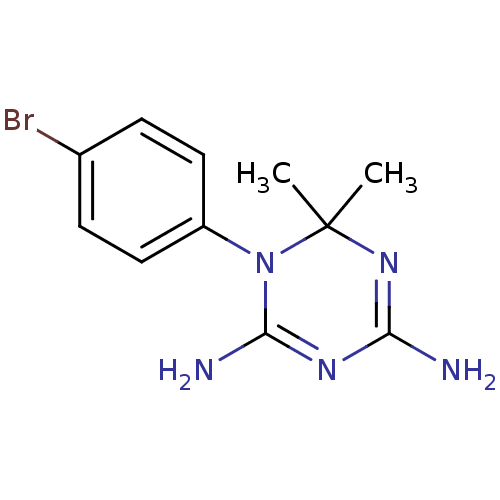
(1-(4-Bromo-phenyl)-6,6-dimethyl-1,6-dihydro-[1,3,5...)Show InChI InChI=1S/C11H14BrN5/c1-11(2)16-9(13)15-10(14)17(11)8-5-3-7(12)4-6-8/h3-6H,1-2H3,(H4,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50020101
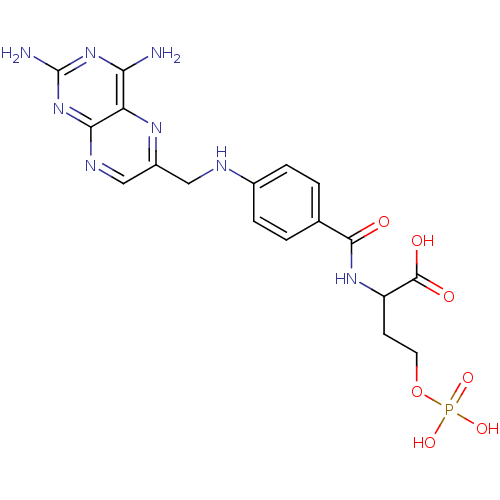
(2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-amino]-ben...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)NC(CCOP(O)(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C18H21N8O7P/c19-14-13-15(26-18(20)25-14)22-8-11(23-13)7-21-10-3-1-9(2-4-10)16(27)24-12(17(28)29)5-6-33-34(30,31)32/h1-4,8,12,21H,5-7H2,(H,24,27)(H,28,29)(H2,30,31,32)(H4,19,20,22,25,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory constant against purified Folyl-polyglutamate synthase obtained from human |
J Med Chem 31: 1338-44 (1988)
BindingDB Entry DOI: 10.7270/Q22J6CFD |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405075
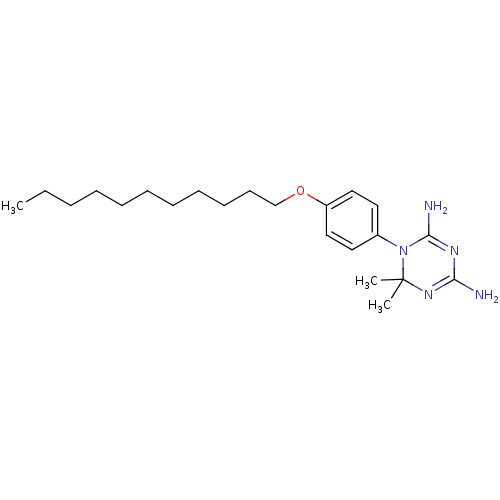
(CHEMBL34794)Show SMILES CCCCCCCCCCCOc1ccc(cc1)N1C(N)=NC(N)=NC1(C)C |c:21,24| Show InChI InChI=1S/C22H37N5O/c1-4-5-6-7-8-9-10-11-12-17-28-19-15-13-18(14-16-19)27-21(24)25-20(23)26-22(27,2)3/h13-16H,4-12,17H2,1-3H3,(H4,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405057

(CHEMBL6661)Show SMILES CC(C)(C)c1ccc(cc1)N1C(N)=NC(N)=NC1(C)C |c:13,16| Show InChI InChI=1S/C15H23N5/c1-14(2,3)10-6-8-11(9-7-10)20-13(17)18-12(16)19-15(20,4)5/h6-9H,1-5H3,(H4,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405098

(CHEMBL30370)Show SMILES CC1(C)N=C(N)N=C(N)N1c1ccc(OCC(=O)N2CCOCC2)cc1 |t:3,6| Show InChI InChI=1S/C17H24N6O3/c1-17(2)21-15(18)20-16(19)23(17)12-3-5-13(6-4-12)26-11-14(24)22-7-9-25-10-8-22/h3-6H,7-11H2,1-2H3,(H4,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405063
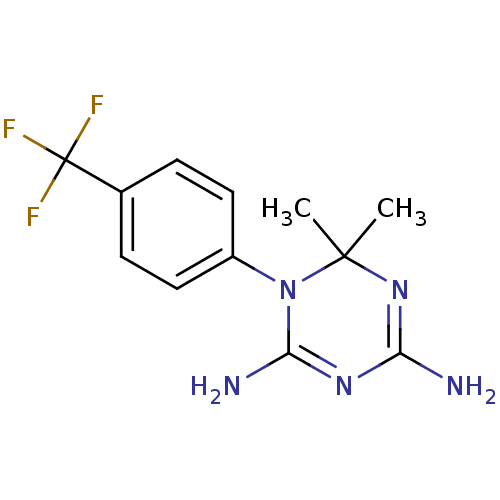
(CHEMBL427826)Show SMILES CC1(C)N=C(N)N=C(N)N1c1ccc(cc1)C(F)(F)F |t:3,6| Show InChI InChI=1S/C12H14F3N5/c1-11(2)19-9(16)18-10(17)20(11)8-5-3-7(4-6-8)12(13,14)15/h3-6H,1-2H3,(H4,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405045

(CHEMBL34680)Show SMILES CCCOc1ccc(cc1)N1C(N)=NC(N)=NC1(C)C |c:13,16| Show InChI InChI=1S/C14H21N5O/c1-4-9-20-11-7-5-10(6-8-11)19-13(16)17-12(15)18-14(19,2)3/h5-8H,4,9H2,1-3H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50018234
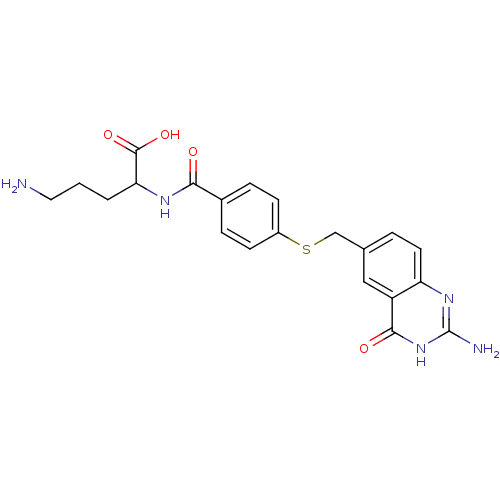
(5-Amino-2-[4-(2-amino-4-oxo-3,4-dihydro-quinazolin...)Show SMILES NCCCC(NC(=O)c1ccc(SCc2ccc3nc(N)[nH]c(=O)c3c2)cc1)C(O)=O Show InChI InChI=1S/C21H23N5O4S/c22-9-1-2-17(20(29)30)24-18(27)13-4-6-14(7-5-13)31-11-12-3-8-16-15(10-12)19(28)26-21(23)25-16/h3-8,10,17H,1-2,9,11,22H2,(H,24,27)(H,29,30)(H3,23,25,26,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase |
J Med Chem 32: 1559-65 (1989)
BindingDB Entry DOI: 10.7270/Q22806MJ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405062
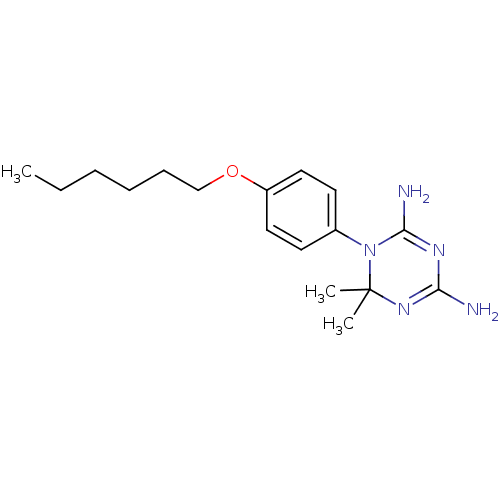
(CHEMBL33677)Show SMILES CCCCCCOc1ccc(cc1)N1C(N)=NC(N)=NC1(C)C |c:16,19| Show InChI InChI=1S/C17H27N5O/c1-4-5-6-7-12-23-14-10-8-13(9-11-14)22-16(19)20-15(18)21-17(22,2)3/h8-11H,4-7,12H2,1-3H3,(H4,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405048

(CHEMBL7341)Show InChI InChI=1S/C11H14IN5/c1-11(2)16-9(13)15-10(14)17(11)8-5-3-7(12)4-6-8/h3-6H,1-2H3,(H4,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Mus musculus) | BDBM50005868

(2-{4-[(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[...)Show SMILES Nc1nc2NCC(CNc3ccc(cc3)C(=O)NC(CCP(O)(O)=O)C(O)=O)Cc2c(=O)[nH]1 Show InChI InChI=1S/C19H25N6O7P/c20-19-24-15-13(17(27)25-19)7-10(9-22-15)8-21-12-3-1-11(2-4-12)16(26)23-14(18(28)29)5-6-33(30,31)32/h1-4,10,14,21H,5-9H2,(H,23,26)(H,28,29)(H2,30,31,32)(H4,20,22,24,25,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
In vitro inhibition of mouse liver Folyl-polyglutamate synthase |
J Med Chem 35: 1578-88 (1992)
BindingDB Entry DOI: 10.7270/Q2028S6J |
More data for this
Ligand-Target Pair | |
Trifunctional purine biosynthetic protein adenosine-3
(Mus musculus) | BDBM50002471

(5-Amino-2-{4-[(2-amino-4-oxo-2,3,4,4a,5,6,7,8-octa...)Show SMILES NCCCC(NC(=O)c1ccc(NCC2CNc3nc(N)[nH]c(=O)c3C2)cc1)C(O)=O Show InChI InChI=1S/C20H27N7O4/c21-7-1-2-15(19(30)31)25-17(28)12-3-5-13(6-4-12)23-9-11-8-14-16(24-10-11)26-20(22)27-18(14)29/h3-6,11,15,23H,1-2,7-10,21H2,(H,25,28)(H,30,31)(H4,22,24,26,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
In vitro inhibition of glycinamide ribonucleotide formyltransferase from L1210 murine leukemia cells |
J Med Chem 35: 1578-88 (1992)
BindingDB Entry DOI: 10.7270/Q2028S6J |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405047
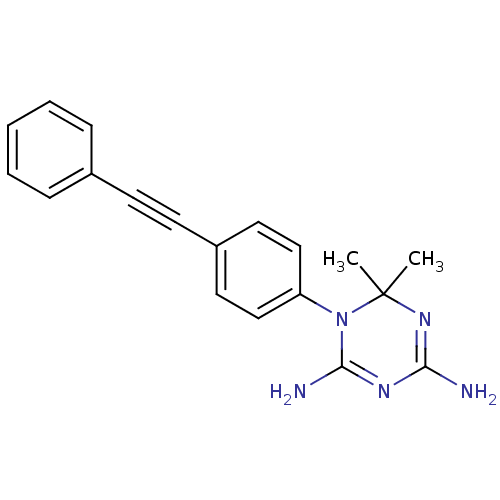
(CHEMBL284157)Show SMILES CC1(C)N=C(N)N=C(N)N1c1ccc(cc1)C#Cc1ccccc1 |t:3,6| Show InChI InChI=1S/C19H19N5/c1-19(2)23-17(20)22-18(21)24(19)16-12-10-15(11-13-16)9-8-14-6-4-3-5-7-14/h3-7,10-13H,1-2H3,(H4,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50020102
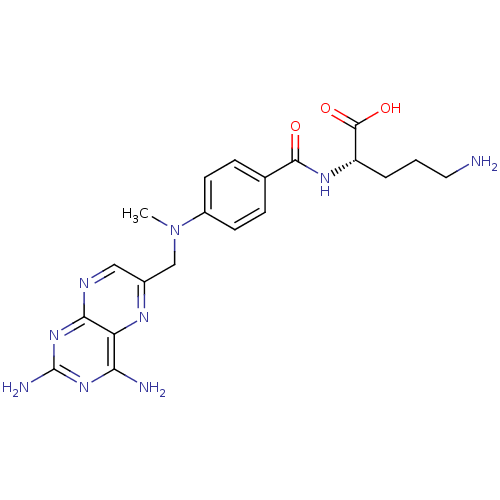
(5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-me...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCCN)C(O)=O Show InChI InChI=1S/C20H25N9O3/c1-29(10-12-9-24-17-15(25-12)16(22)27-20(23)28-17)13-6-4-11(5-7-13)18(30)26-14(19(31)32)3-2-8-21/h4-7,9,14H,2-3,8,10,21H2,1H3,(H,26,30)(H,31,32)(H4,22,23,24,27,28)/t14-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory constant against purified Folyl-polyglutamate synthase obtained from human |
J Med Chem 31: 1338-44 (1988)
BindingDB Entry DOI: 10.7270/Q22J6CFD |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Mus musculus) | BDBM50020102

(5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-me...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCCN)C(O)=O Show InChI InChI=1S/C20H25N9O3/c1-29(10-12-9-24-17-15(25-12)16(22)27-20(23)28-17)13-6-4-11(5-7-13)18(30)26-14(19(31)32)3-2-8-21/h4-7,9,14H,2-3,8,10,21H2,1H3,(H,26,30)(H,31,32)(H4,22,23,24,27,28)/t14-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory constant against purified Folyl-polyglutamate synthase obtained from rat |
J Med Chem 31: 1338-44 (1988)
BindingDB Entry DOI: 10.7270/Q22J6CFD |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405035

(CHEMBL266573)Show InChI InChI=1S/C12H17N5O/c1-12(2)16-10(13)15-11(14)17(12)8-4-6-9(18-3)7-5-8/h4-7H,1-3H3,(H4,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) |
J Med Chem 27: 144-9 (1984)
BindingDB Entry DOI: 10.7270/Q28K7C96 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data