

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50009228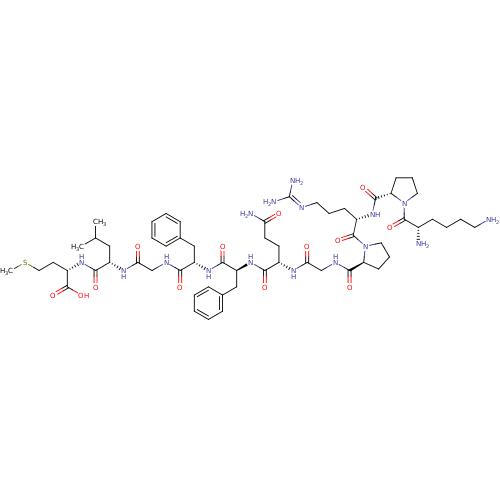 (CHEMBL413846 | SP (substance P) | Spantide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for tachykinin receptor 1 from rat forebrain tissue, [125I]-BH-SP as radioligand | J Med Chem 34: 1751-3 (1991) BindingDB Entry DOI: 10.7270/Q2JQ11MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50001450 ((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Inhibition of Tachykinin receptor 1 of rat forebrain tissue | J Med Chem 35: 1273-9 (1992) BindingDB Entry DOI: 10.7270/Q2222SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50317611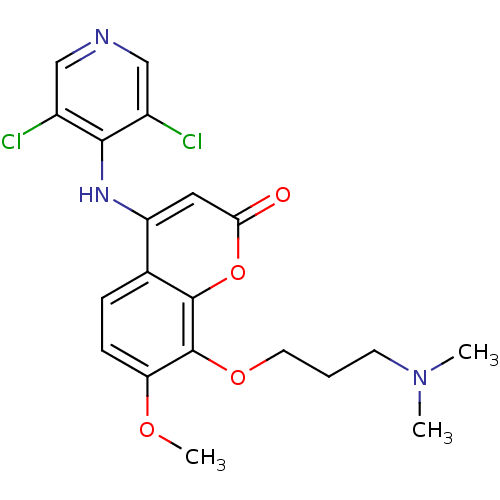 (4-(3,5-dichloropyridin-4-ylamino)-8-(3-(dimethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. Curated by ChEMBL | Assay Description Inhibition of human His6-tagged PDE4B (152-487) expressed in Escherichia coli BL21 (DE3) after 1 hr | Bioorg Med Chem Lett 20: 2928-32 (2010) Article DOI: 10.1016/j.bmcl.2010.03.023 BindingDB Entry DOI: 10.7270/Q2VD6ZMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50317609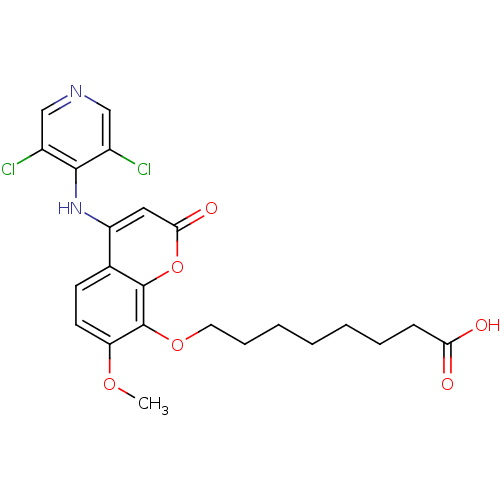 (8-(4-(3,5-dichloropyridin-4-ylamino)-7-methoxy-2-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. Curated by ChEMBL | Assay Description Inhibition of human His6-tagged PDE4B (152-487) expressed in Escherichia coli BL21 (DE3) after 1 hr | Bioorg Med Chem Lett 20: 2928-32 (2010) Article DOI: 10.1016/j.bmcl.2010.03.023 BindingDB Entry DOI: 10.7270/Q2VD6ZMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM14775 (3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. Curated by ChEMBL | Assay Description Inhibition of human His6-tagged PDE4B (152-487) expressed in Escherichia coli BL21 (DE3) after 1 hr | Bioorg Med Chem Lett 20: 2928-32 (2010) Article DOI: 10.1016/j.bmcl.2010.03.023 BindingDB Entry DOI: 10.7270/Q2VD6ZMF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50317610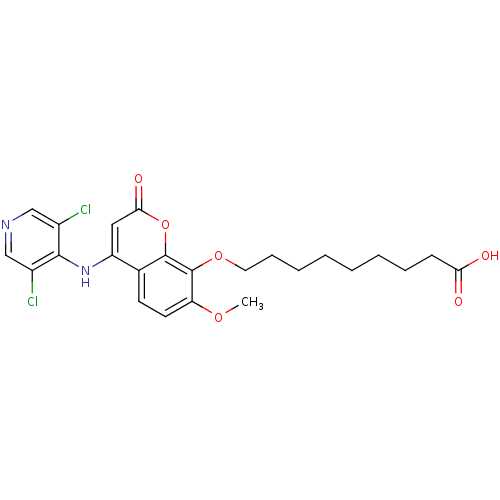 (9-(4-(3,5-dichloropyridin-4-ylamino)-7-methoxy-2-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. Curated by ChEMBL | Assay Description Inhibition of human His6-tagged PDE4B (152-487) expressed in Escherichia coli BL21 (DE3) after 1 hr | Bioorg Med Chem Lett 20: 2928-32 (2010) Article DOI: 10.1016/j.bmcl.2010.03.023 BindingDB Entry DOI: 10.7270/Q2VD6ZMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50317616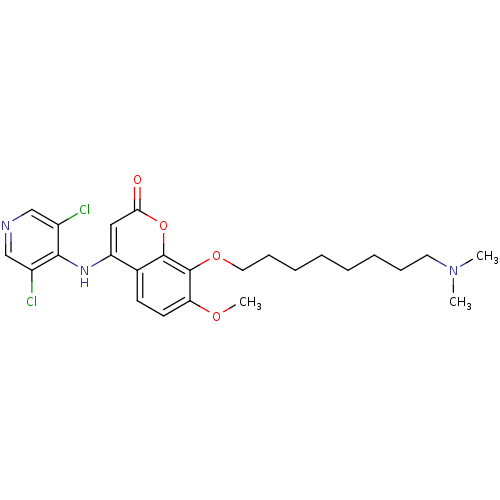 (4-(3,5-dichloropyridin-4-ylamino)-8-(8-(dimethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. Curated by ChEMBL | Assay Description Inhibition of human His6-tagged PDE4B (152-487) expressed in Escherichia coli BL21 (DE3) after 1 hr | Bioorg Med Chem Lett 20: 2928-32 (2010) Article DOI: 10.1016/j.bmcl.2010.03.023 BindingDB Entry DOI: 10.7270/Q2VD6ZMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50317615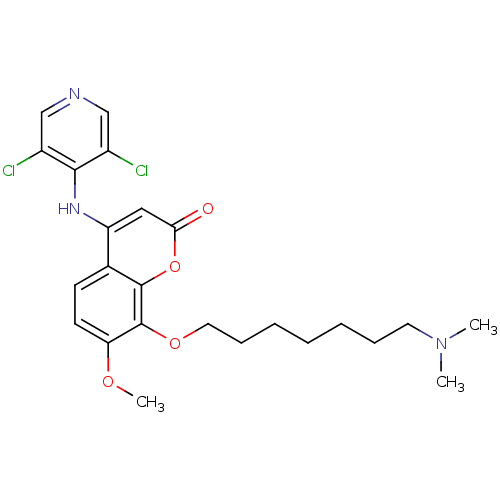 (4-(3,5-dichloropyridin-4-ylamino)-8-(7-(dimethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. Curated by ChEMBL | Assay Description Inhibition of human His6-tagged PDE4B (152-487) expressed in Escherichia coli BL21 (DE3) after 1 hr | Bioorg Med Chem Lett 20: 2928-32 (2010) Article DOI: 10.1016/j.bmcl.2010.03.023 BindingDB Entry DOI: 10.7270/Q2VD6ZMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50317604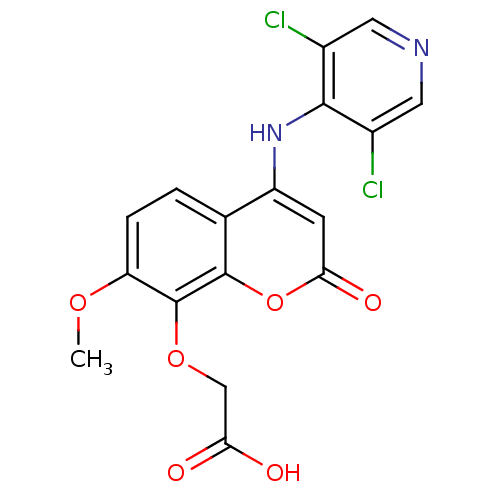 (2-(4-(3,5-dichloropyridin-4-ylamino)-7-methoxy-2-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. Curated by ChEMBL | Assay Description Inhibition of human His6-tagged PDE4B (152-487) expressed in Escherichia coli BL21 (DE3) after 1 hr | Bioorg Med Chem Lett 20: 2928-32 (2010) Article DOI: 10.1016/j.bmcl.2010.03.023 BindingDB Entry DOI: 10.7270/Q2VD6ZMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50317603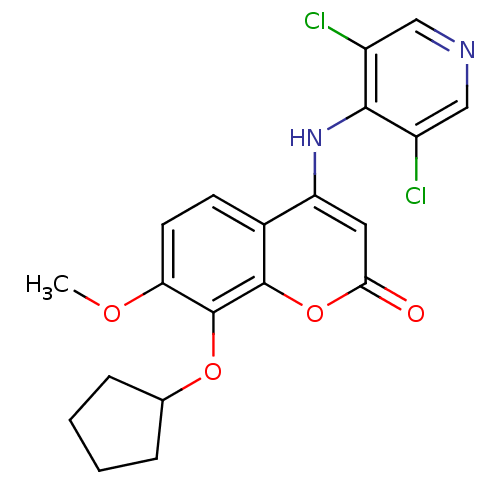 (8-(cyclopentyloxy)-4-(3,5-dichloropyridin-4-ylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. Curated by ChEMBL | Assay Description Inhibition of human His6-tagged PDE4B (152-487) expressed in Escherichia coli BL21 (DE3) after 1 hr | Bioorg Med Chem Lett 20: 2928-32 (2010) Article DOI: 10.1016/j.bmcl.2010.03.023 BindingDB Entry DOI: 10.7270/Q2VD6ZMF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50317614 (4-(3,5-dichloropyridin-4-ylamino)-8-(6-(dimethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. Curated by ChEMBL | Assay Description Inhibition of human His6-tagged PDE4B (152-487) expressed in Escherichia coli BL21 (DE3) after 1 hr | Bioorg Med Chem Lett 20: 2928-32 (2010) Article DOI: 10.1016/j.bmcl.2010.03.023 BindingDB Entry DOI: 10.7270/Q2VD6ZMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50017371 (1-[4-(3-{4-[Bis-(4-fluoro-phenyl)-hydroxy-methyl]-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Tritiated [3H]- mepyramine binding to histamine H1 receptor in guinea pig cerebral cortex | J Med Chem 32: 105-18 (1989) BindingDB Entry DOI: 10.7270/Q2WM1CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM76863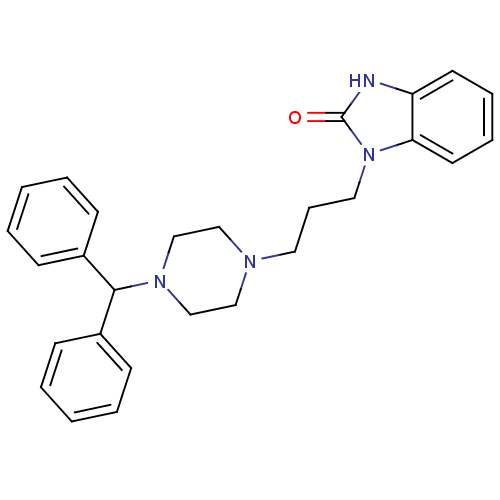 (3-[3-(4-benzhydrylpiperazin-1-yl)propyl]-1H-benzim...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Tritiated [3H]- mepyramine binding to Histamine H1 receptor in guinea pig cerebral cortex | J Med Chem 32: 105-18 (1989) BindingDB Entry DOI: 10.7270/Q2WM1CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50317608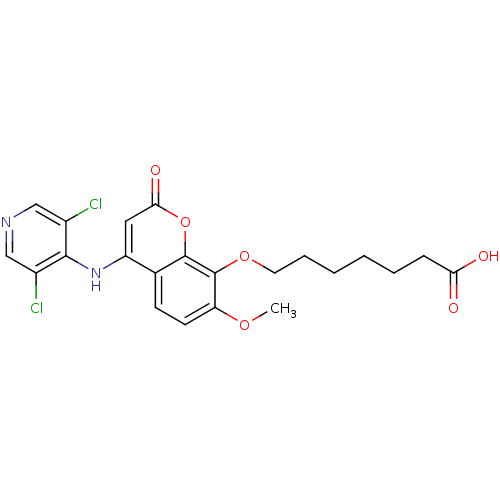 (7-(4-(3,5-dichloropyridin-4-ylamino)-7-methoxy-2-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. Curated by ChEMBL | Assay Description Inhibition of human His6-tagged PDE4B (152-487) expressed in Escherichia coli BL21 (DE3) after 1 hr | Bioorg Med Chem Lett 20: 2928-32 (2010) Article DOI: 10.1016/j.bmcl.2010.03.023 BindingDB Entry DOI: 10.7270/Q2VD6ZMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50317613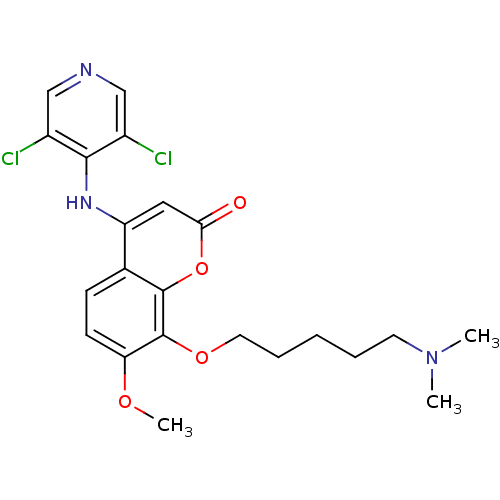 (4-(3,5-dichloropyridin-4-ylamino)-8-(5-(dimethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. Curated by ChEMBL | Assay Description Inhibition of human His6-tagged PDE4B (152-487) expressed in Escherichia coli BL21 (DE3) after 1 hr | Bioorg Med Chem Lett 20: 2928-32 (2010) Article DOI: 10.1016/j.bmcl.2010.03.023 BindingDB Entry DOI: 10.7270/Q2VD6ZMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50017374 (1-(4-{3-[4-(Hydroxy-diphenyl-methyl)-piperidin-1-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Tritiated [3H]- mepyramine binding to histamine H1 receptor in guinea pig cerebral cortex | J Med Chem 32: 105-18 (1989) BindingDB Entry DOI: 10.7270/Q2WM1CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50317607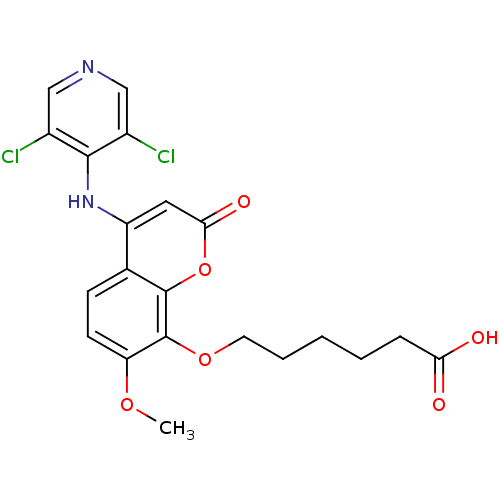 (6-(4-(3,5-dichloropyridin-4-ylamino)-7-methoxy-2-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. Curated by ChEMBL | Assay Description Inhibition of human His6-tagged PDE4B (152-487) expressed in Escherichia coli BL21 (DE3) after 1 hr | Bioorg Med Chem Lett 20: 2928-32 (2010) Article DOI: 10.1016/j.bmcl.2010.03.023 BindingDB Entry DOI: 10.7270/Q2VD6ZMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50317612 (4-(3,5-dichloropyridin-4-ylamino)-8-(4-(dimethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. Curated by ChEMBL | Assay Description Inhibition of human His6-tagged PDE4B (152-487) expressed in Escherichia coli BL21 (DE3) after 1 hr | Bioorg Med Chem Lett 20: 2928-32 (2010) Article DOI: 10.1016/j.bmcl.2010.03.023 BindingDB Entry DOI: 10.7270/Q2VD6ZMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50017372 (4-(3-{4-[Bis-(4-fluoro-phenyl)-hydroxy-methyl]-pip...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Tritiated [3H]- mepyramine binding to histamine H1 receptor in guinea pig cerebral cortex | J Med Chem 32: 105-18 (1989) BindingDB Entry DOI: 10.7270/Q2WM1CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50317606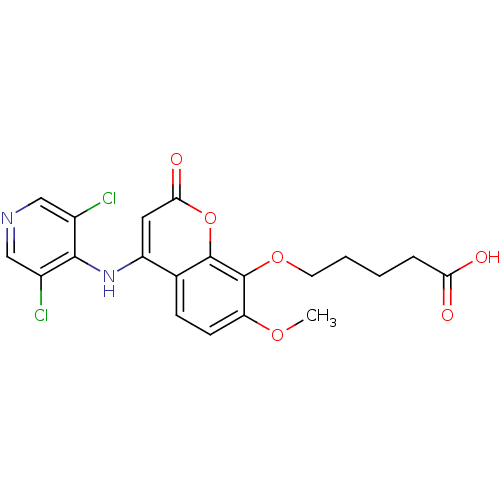 (5-(4-(3,5-dichloropyridin-4-ylamino)-7-methoxy-2-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. Curated by ChEMBL | Assay Description Inhibition of human His6-tagged PDE4B (152-487) expressed in Escherichia coli BL21 (DE3) after 1 hr | Bioorg Med Chem Lett 20: 2928-32 (2010) Article DOI: 10.1016/j.bmcl.2010.03.023 BindingDB Entry DOI: 10.7270/Q2VD6ZMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50002461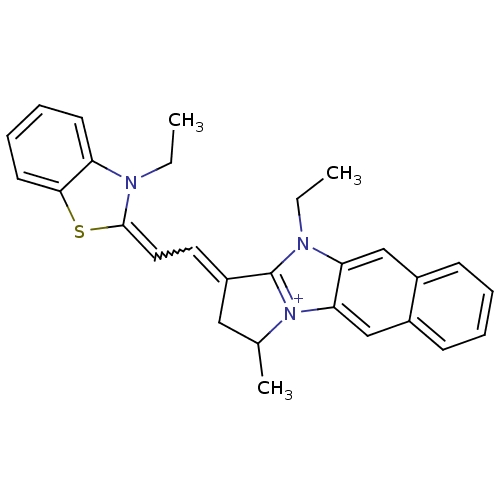 (10-Ethyl-1-[2-(3-ethyl-3H-benzothiazol-2-ylidene)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Inhibition of Tachykinin receptor 1 of rat forebrain tissue | J Med Chem 35: 1273-9 (1992) BindingDB Entry DOI: 10.7270/Q2222SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50017376 ((+/-)1-(4-tert-butylphenyl)-4-(4-(hydroxydiphenylm...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 295 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Tritiated [3H]- mepyramine binding to histamine H1 receptor in guinea pig cerebral cortex | J Med Chem 32: 105-18 (1989) BindingDB Entry DOI: 10.7270/Q2WM1CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50002464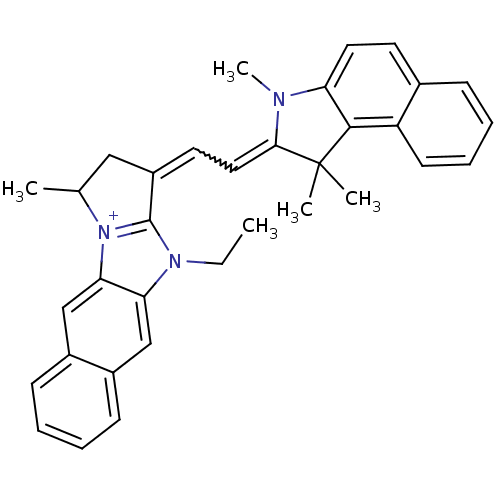 (10-Ethyl-1-[2-(1,1,3-trimethyl-1,3-dihydro-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Inhibition of Tachykinin receptor 1 of rat forebrain tissue | J Med Chem 35: 1273-9 (1992) BindingDB Entry DOI: 10.7270/Q2222SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50002460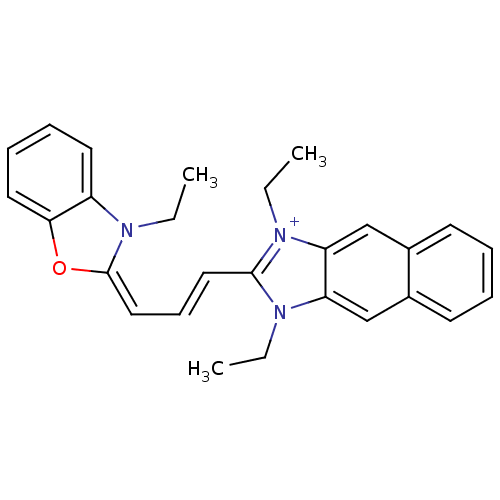 (1,3-Diethyl-2-[3-(3-ethyl-3H-benzooxazol-2-ylidene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Inhibition of Tachykinin receptor 1 of rat forebrain tissue | J Med Chem 35: 1273-9 (1992) BindingDB Entry DOI: 10.7270/Q2222SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50002456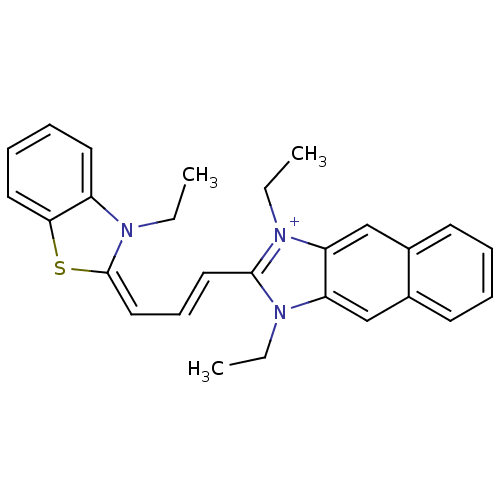 (1,3-Diethyl-2-[3-(3-ethyl-3H-benzothiazol-2-yliden...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Inhibition of Tachykinin receptor 1 of rat forebrain tissue | J Med Chem 35: 1273-9 (1992) BindingDB Entry DOI: 10.7270/Q2222SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50009225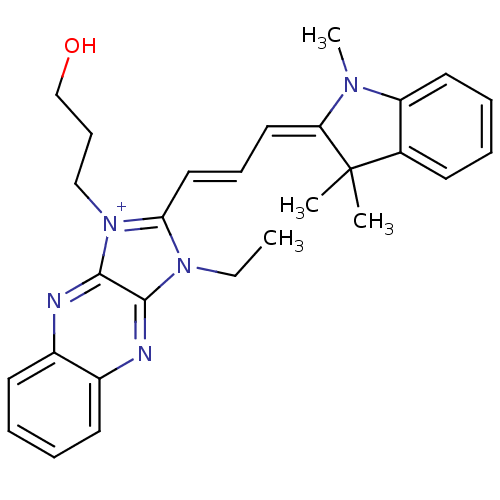 (3-Ethyl-1-(3-hydroxy-propyl)-2-[3-(1,3,3-trimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for tachykinin receptor 1 from rat forebrain tissue, [125I]-BH-SP as radioligand | J Med Chem 34: 1751-3 (1991) BindingDB Entry DOI: 10.7270/Q2JQ11MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50002459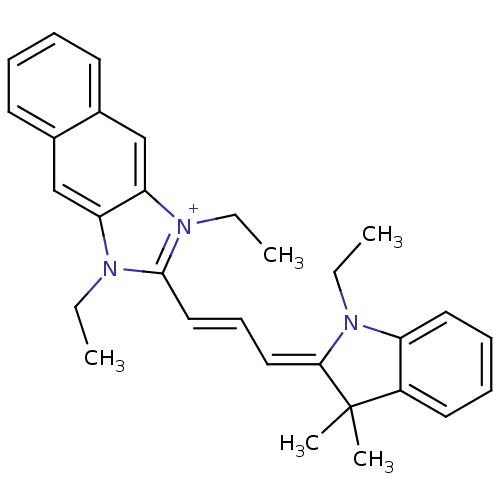 (1,3-Diethyl-2-[3-(1-ethyl-3,3-dimethyl-1,3-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Inhibition of Tachykinin receptor 1 of rat forebrain tissue | J Med Chem 35: 1273-9 (1992) BindingDB Entry DOI: 10.7270/Q2222SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50009226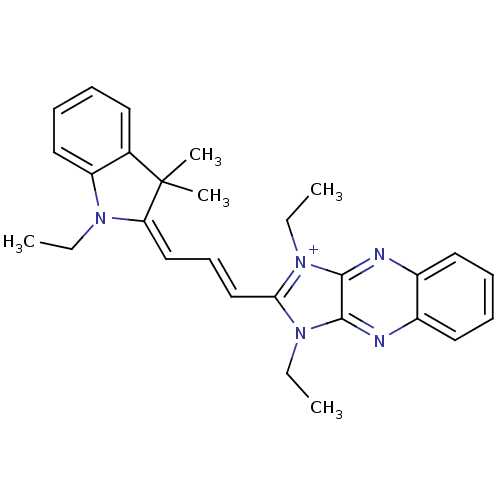 (1,3-Diethyl-2-[3-(1-ethyl-3,3-dimethyl-1,3-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for tachykinin receptor 1 from rat forebrain tissue, [125I]-BH-SP as radioligand | J Med Chem 34: 1751-3 (1991) BindingDB Entry DOI: 10.7270/Q2JQ11MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50002458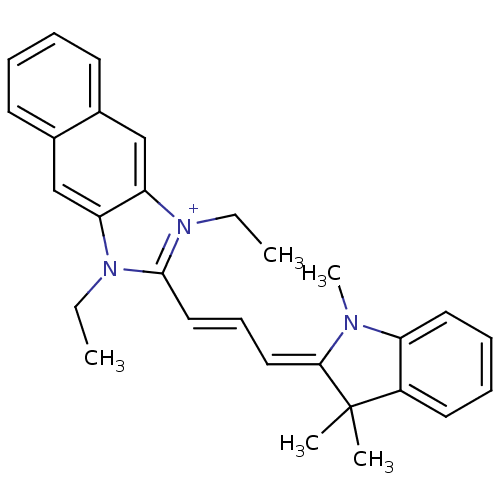 (1,3-Diethyl-2-[3-(1,3,3-trimethyl-1,3-dihydro-indo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Inhibition of Tachykinin receptor 1 of rat forebrain tissue | J Med Chem 35: 1273-9 (1992) BindingDB Entry DOI: 10.7270/Q2222SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50092350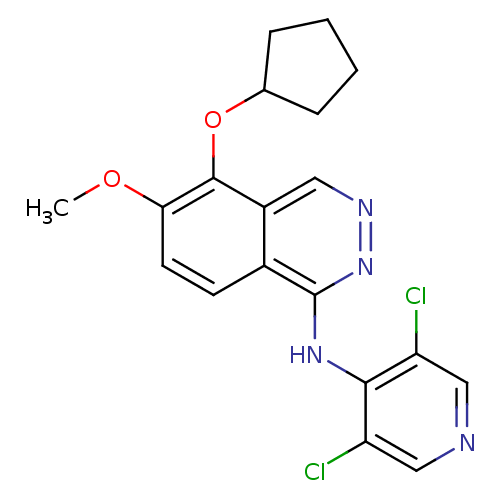 ((5-Cyclopentyloxy-6-methoxy-phthalazin-1-yl)-(3,5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. Curated by ChEMBL | Assay Description Inhibition of human His6-tagged PDE4B (152-487) expressed in Escherichia coli BL21 (DE3) after 1 hr | Bioorg Med Chem Lett 20: 2928-32 (2010) Article DOI: 10.1016/j.bmcl.2010.03.023 BindingDB Entry DOI: 10.7270/Q2VD6ZMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50009234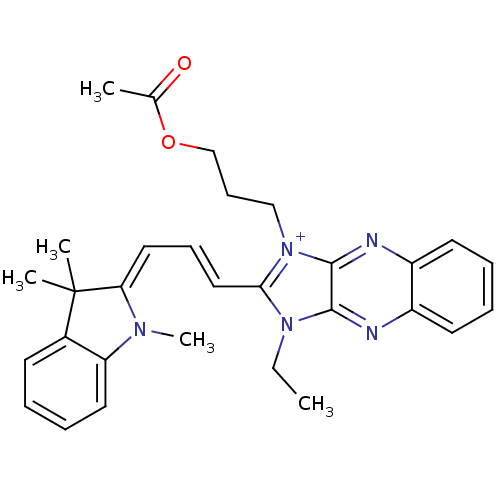 (1-(3-Acetoxy-propyl)-3-ethyl-2-[3-(1,3,3-trimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for tachykinin receptor 1 from rat forebrain tissue, [125I]-BH-SP as radioligand | J Med Chem 34: 1751-3 (1991) BindingDB Entry DOI: 10.7270/Q2JQ11MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50002457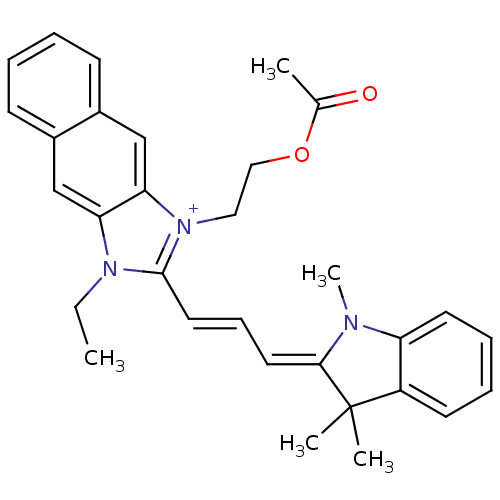 (1-(2-Acetoxy-ethyl)-3-ethyl-2-[3-(1,3,3-trimethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Inhibition of Tachykinin receptor 1 of rat forebrain tissue | J Med Chem 35: 1273-9 (1992) BindingDB Entry DOI: 10.7270/Q2222SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50002452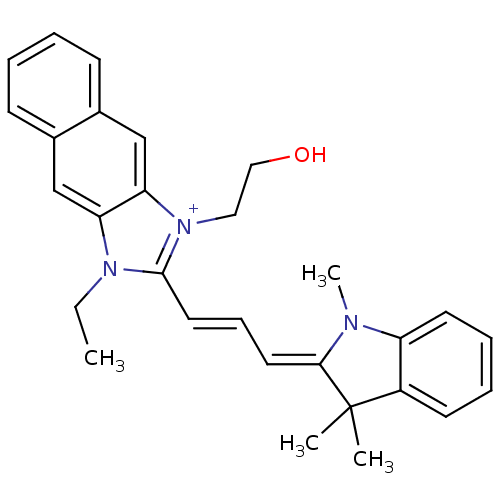 (3-Ethyl-1-(2-hydroxy-ethyl)-2-[3-(1,3,3-trimethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Inhibition of Tachykinin receptor 1 of rat forebrain tissue | J Med Chem 35: 1273-9 (1992) BindingDB Entry DOI: 10.7270/Q2222SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50002454 (CHEMBL386306 | D-Lys(Nicotinoyl)-Pro(3-Pyridyl)-Al...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for tachykinin receptor 1 from rat forebrain tissue, [125I]-BH-SP as radioligand | J Med Chem 34: 1751-3 (1991) BindingDB Entry DOI: 10.7270/Q2JQ11MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50002454 (CHEMBL386306 | D-Lys(Nicotinoyl)-Pro(3-Pyridyl)-Al...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Inhibition of Tachykinin receptor 1 of rat forebrain tissue | J Med Chem 35: 1273-9 (1992) BindingDB Entry DOI: 10.7270/Q2222SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50317599 (5-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. Curated by ChEMBL | Assay Description Inhibition of human His6-tagged PDE4B (152-487) expressed in Escherichia coli BL21 (DE3) after 1 hr | Bioorg Med Chem Lett 20: 2928-32 (2010) Article DOI: 10.1016/j.bmcl.2010.03.023 BindingDB Entry DOI: 10.7270/Q2VD6ZMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50002462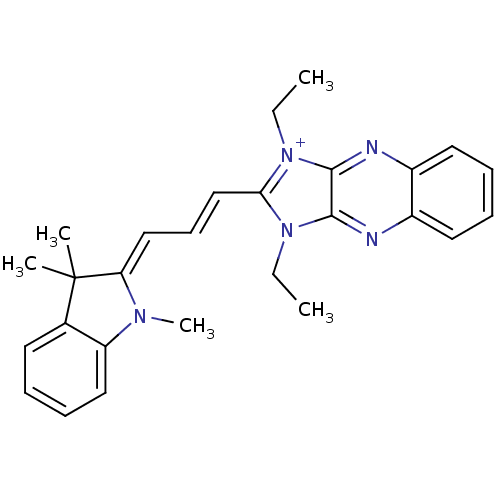 (1,3-Diethyl-2-[3-(1,3,3-trimethyl-1,3-dihydro-indo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Inhibition of Tachykinin receptor 1 of rat forebrain tissue | J Med Chem 35: 1273-9 (1992) BindingDB Entry DOI: 10.7270/Q2222SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50002462 (1,3-Diethyl-2-[3-(1,3,3-trimethyl-1,3-dihydro-indo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for tachykinin receptor 1 from rat forebrain tissue, [125I]-BH-SP as radioligand | J Med Chem 34: 1751-3 (1991) BindingDB Entry DOI: 10.7270/Q2JQ11MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50009235 (1,3-Diethyl-2-[3-(3-methyl-3H-benzothiazol-2-ylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for tachykinin receptor 1 from rat forebrain tissue, [125I]-BH-SP as radioligand | J Med Chem 34: 1751-3 (1991) BindingDB Entry DOI: 10.7270/Q2JQ11MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50002455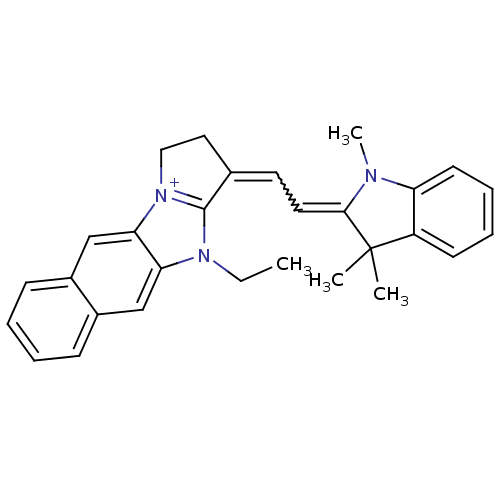 (10-Ethyl-1-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Inhibition of Tachykinin receptor 1 of rat forebrain tissue | J Med Chem 35: 1273-9 (1992) BindingDB Entry DOI: 10.7270/Q2222SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50009233 (1,3-Diethyl-2-[3-(1-methyl-2a,5-dihydro-1H-benzo[c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for tachykinin receptor 1 from rat forebrain tissue, [125I]-BH-SP as radioligand | J Med Chem 34: 1751-3 (1991) BindingDB Entry DOI: 10.7270/Q2JQ11MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50009227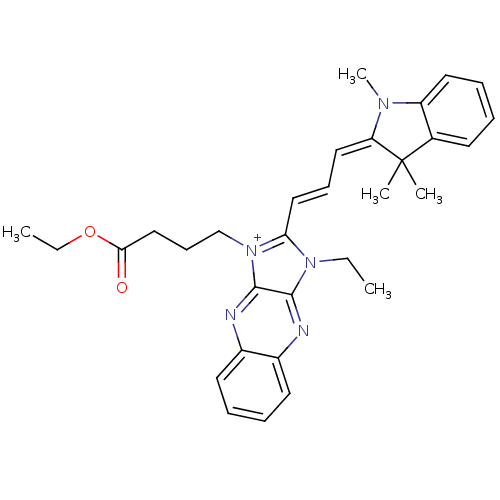 (1-(3-Ethoxycarbonyl-propyl)-3-ethyl-2-[3-(1,3,3-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for tachykinin receptor 1 from rat forebrain tissue, [125I]-BH-SP as radioligand | J Med Chem 34: 1751-3 (1991) BindingDB Entry DOI: 10.7270/Q2JQ11MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50017373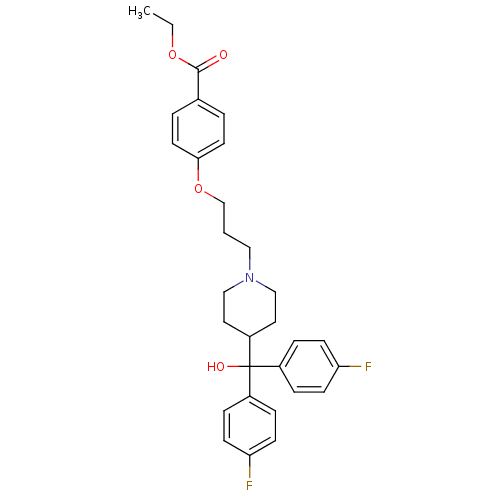 (4-(3-{4-[Bis-(4-fluoro-phenyl)-hydroxy-methyl]-pip...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 956 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Tritiated [3H]- mepyramine binding to histamine H1 receptor in guinea pig cerebral cortex | J Med Chem 32: 105-18 (1989) BindingDB Entry DOI: 10.7270/Q2WM1CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50009237 (1,3-Diethyl-2-{3-[1-(2-hydroxy-ethyl)-3,3-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for tachykinin receptor 1 from rat forebrain tissue, [125I]-BH-SP as radioligand | J Med Chem 34: 1751-3 (1991) BindingDB Entry DOI: 10.7270/Q2JQ11MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50009231 (6,7-Dichloro-1,3-diethyl-2-[3-(1,3,3-trimethyl-1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for tachykinin receptor 1 from rat forebrain tissue, [125I]-BH-SP as radioligand | J Med Chem 34: 1751-3 (1991) BindingDB Entry DOI: 10.7270/Q2JQ11MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50317605 (4-(4-(3,5-dichloropyridin-4-ylamino)-7-methoxy-2-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. Curated by ChEMBL | Assay Description Inhibition of human His6-tagged PDE4B (152-487) expressed in Escherichia coli BL21 (DE3) after 1 hr | Bioorg Med Chem Lett 20: 2928-32 (2010) Article DOI: 10.1016/j.bmcl.2010.03.023 BindingDB Entry DOI: 10.7270/Q2VD6ZMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50002451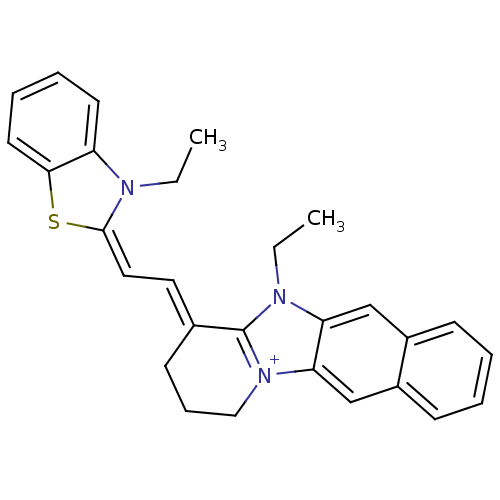 (5-Ethyl-4-[2-(3-ethyl-3H-benzothiazol-2-ylidene)-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Inhibition of Tachykinin receptor 1 of rat forebrain tissue | J Med Chem 35: 1273-9 (1992) BindingDB Entry DOI: 10.7270/Q2222SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50009236 (1-(3-Dimethylamino-propyl)-3-ethyl-2-[3-(1,3,3-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for tachykinin receptor 1 from rat forebrain tissue, [125I]-BH-SP as radioligand | J Med Chem 34: 1751-3 (1991) BindingDB Entry DOI: 10.7270/Q2JQ11MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50009229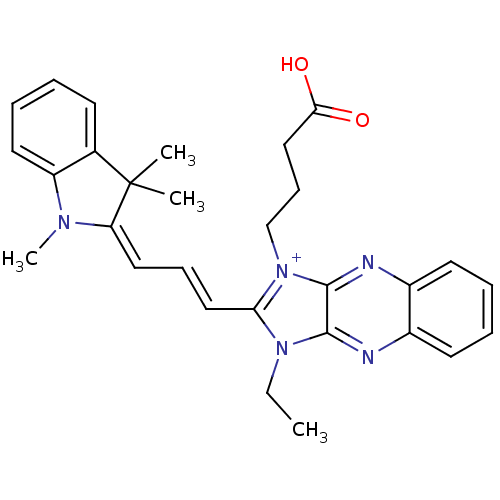 (1-(3-Carboxy-propyl)-3-ethyl-2-[3-(1,3,3-trimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for tachykinin receptor 1 from rat forebrain tissue, [125I]-BH-SP as radioligand | J Med Chem 34: 1751-3 (1991) BindingDB Entry DOI: 10.7270/Q2JQ11MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50009230 (1,3-Diethyl-2-[3-(3-methyl-thiazolidin-2-ylidene)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for tachykinin receptor 1 from rat forebrain tissue, [125I]-BH-SP as radioligand | J Med Chem 34: 1751-3 (1991) BindingDB Entry DOI: 10.7270/Q2JQ11MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 64 total ) | Next | Last >> |