

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50016326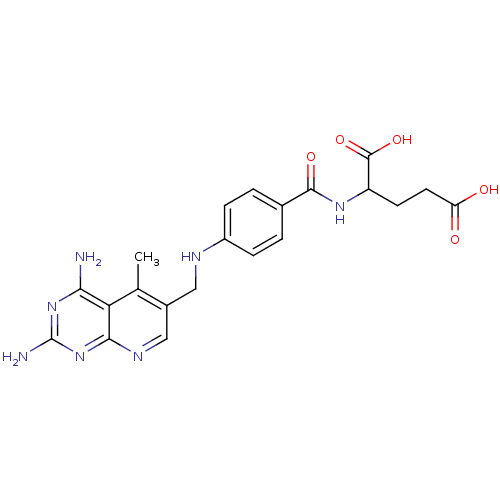 (2-{4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells | J Med Chem 31: 1209-15 (1988) BindingDB Entry DOI: 10.7270/Q2930S6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM66082 ((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells | J Med Chem 31: 1209-15 (1988) BindingDB Entry DOI: 10.7270/Q2930S6N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50023681 (2-{4-[(2,4-Diamino-5,7-dimethyl-pyrido[2,3-d]pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells | J Med Chem 31: 1209-15 (1988) BindingDB Entry DOI: 10.7270/Q2930S6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50023680 (2-{4-[(2,4-Diamino-7-phenyl-pyrido[2,3-d]pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells | J Med Chem 31: 1209-15 (1988) BindingDB Entry DOI: 10.7270/Q2930S6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50023682 (2-{4-[(2,4-Diamino-5-methyl-7-phenyl-pyrido[2,3-d]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells | J Med Chem 31: 1209-15 (1988) BindingDB Entry DOI: 10.7270/Q2930S6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50023683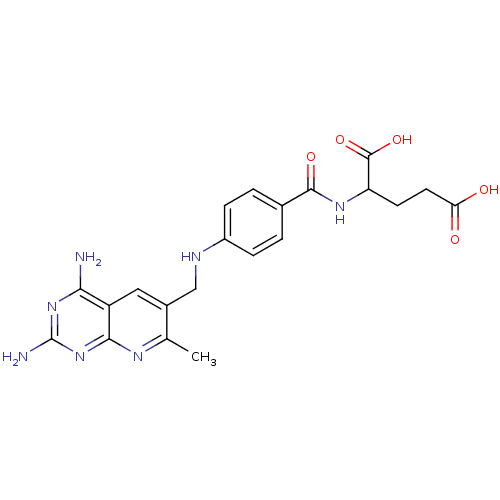 (2-{4-[(2,4-Diamino-7-methyl-pyrido[2,3-d]pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells | J Med Chem 31: 1209-15 (1988) BindingDB Entry DOI: 10.7270/Q2930S6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50023684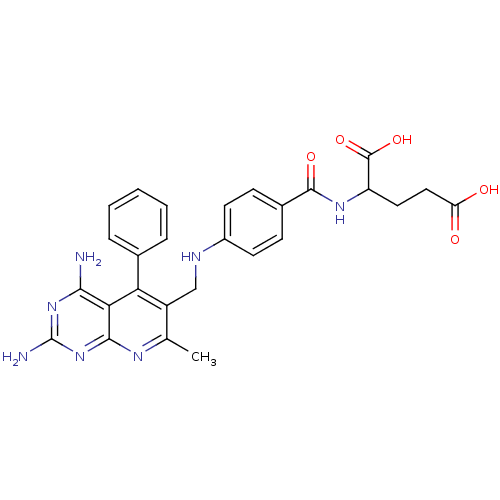 (2-{4-[(2,4-Diamino-7-methyl-5-phenyl-pyrido[2,3-d]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells | J Med Chem 31: 1209-15 (1988) BindingDB Entry DOI: 10.7270/Q2930S6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50474150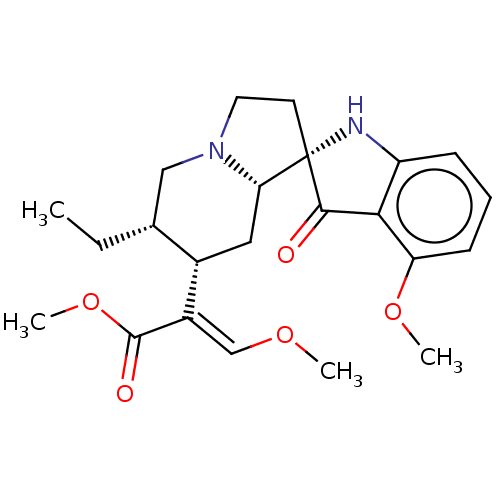 (CHEMBL58362) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor mu 1 using [3H]- DAMGO as radioligand | J Med Chem 45: 1949-56 (2002) Article DOI: 10.1021/jm010576e BindingDB Entry DOI: 10.7270/Q2WS8X0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50491838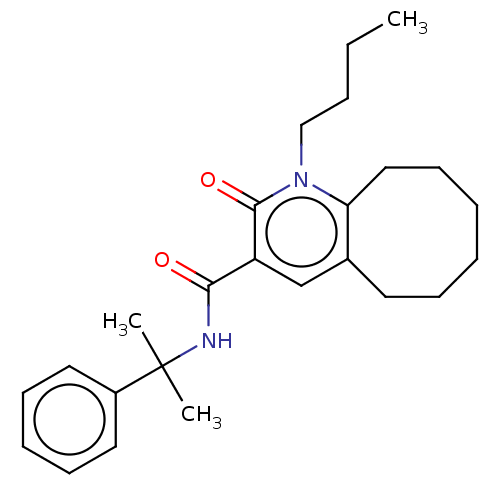 (CHEMBL2387185) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from human CB2 receptor expressed in CHO cell membranes | Bioorg Med Chem 21: 3154-63 (2013) Article DOI: 10.1016/j.bmc.2013.03.030 BindingDB Entry DOI: 10.7270/Q22R3VKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50505569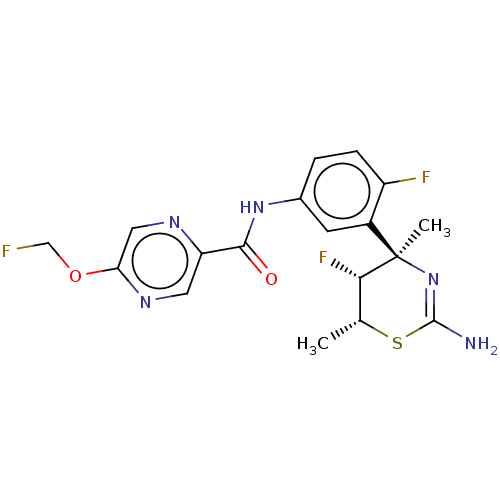 (CHEMBL4557670) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] JNJ-962 from BACE2 (unknown origin) expressed in HEK293 cell membranes assessed as inhibition constant at pH 6.2 by scintillatio... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00359 BindingDB Entry DOI: 10.7270/Q2WW7NJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM210070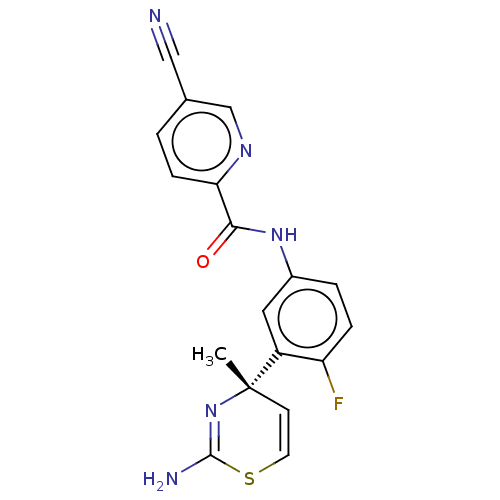 (US9270353, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] JNJ-962 from BACE1 (unknown origin) expressed in HEK293 cell membranes assessed as inhibition constant at pH 6.2 by scintillatio... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00359 BindingDB Entry DOI: 10.7270/Q2WW7NJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50491839 (CHEMBL2387183) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from human CB2 receptor expressed in CHO cell membranes | Bioorg Med Chem 21: 3154-63 (2013) Article DOI: 10.1016/j.bmc.2013.03.030 BindingDB Entry DOI: 10.7270/Q22R3VKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50579806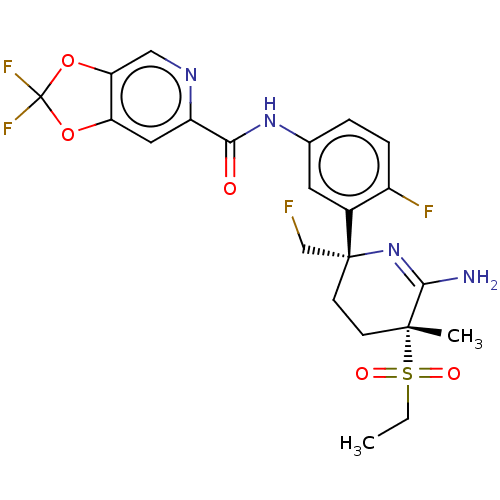 (CHEMBL5092328) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-JNJ962 from BACE1 (unknown origin) expressed in HEK293 cell membrane assessed as inhibition constant by scintillation counting a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00935 BindingDB Entry DOI: 10.7270/Q2V69PFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50580217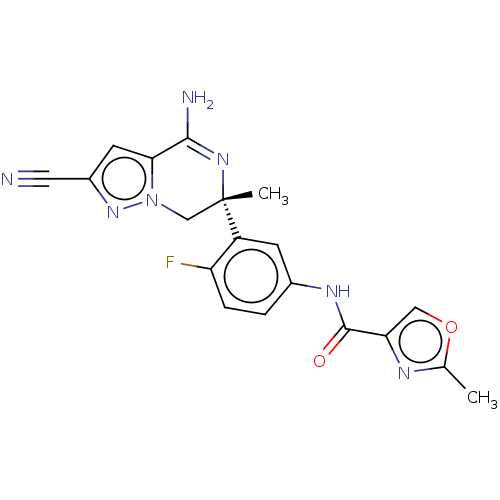 (CHEMBL5093195) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to BACE2 (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00359 BindingDB Entry DOI: 10.7270/Q2WW7NJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50505569 (CHEMBL4557670) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] JNJ-962 from BACE1 (unknown origin) expressed in HEK293 cell membranes assessed as inhibition constant at pH 6.2 by scintillatio... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00359 BindingDB Entry DOI: 10.7270/Q2WW7NJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50580216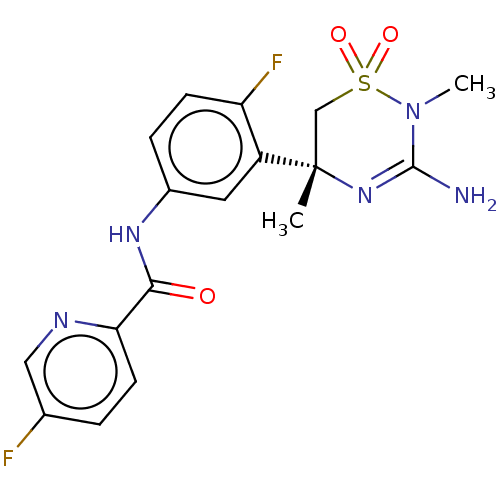 (MK-8931 | SCH 900931 | SCH-900931 | SCH900931 | VE...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] JNJ-962 from BACE1 (unknown origin) expressed in HEK293 cell membranes assessed as inhibition constant at pH 6.2 by scintillatio... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00359 BindingDB Entry DOI: 10.7270/Q2WW7NJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM186927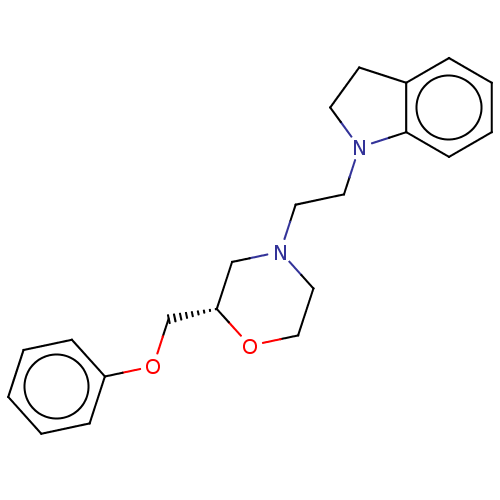 (US9079895, 19s) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Dundee US Patent | Assay Description The detailed experimental protocols for the radioligand and functional receptor assays are available on the NIMH PDSP website at http://pdsp.med.un... | US Patent US9079895 (2015) BindingDB Entry DOI: 10.7270/Q2D7996K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50580217 (CHEMBL5093195) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to BACE1 (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00359 BindingDB Entry DOI: 10.7270/Q2WW7NJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50579805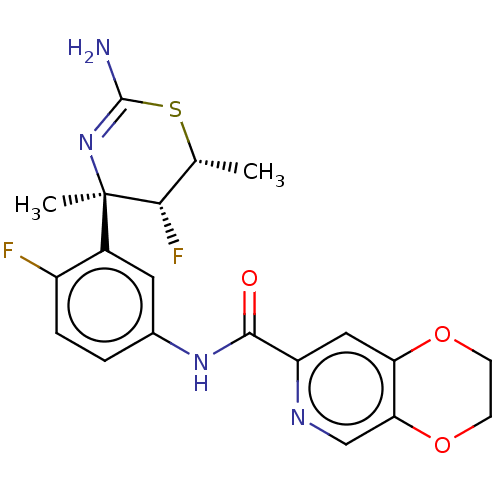 (CHEMBL5075689) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] JNJ-962 from BACE1 (unknown origin) expressed in HEK293 cell membranes assessed as inhibition constant at pH 6.2 by scintillatio... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00359 BindingDB Entry DOI: 10.7270/Q2WW7NJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50491833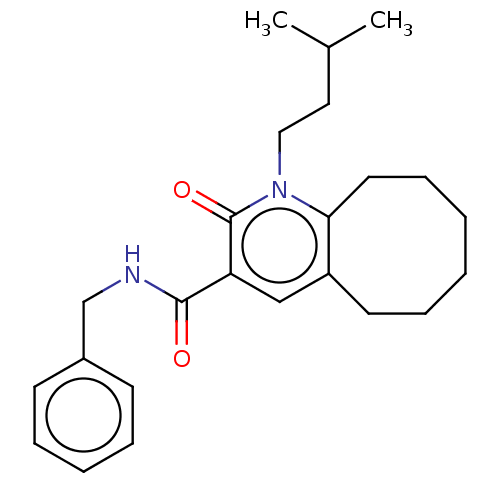 (CHEMBL2387178) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from human CB2 receptor expressed in CHO cell membranes | Bioorg Med Chem 21: 3154-63 (2013) Article DOI: 10.1016/j.bmc.2013.03.030 BindingDB Entry DOI: 10.7270/Q22R3VKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50491838 (CHEMBL2387185) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from human CB1 receptor expressed in CHO cell membranes | Bioorg Med Chem 21: 3154-63 (2013) Article DOI: 10.1016/j.bmc.2013.03.030 BindingDB Entry DOI: 10.7270/Q22R3VKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alcohol dehydrogenase E/S chain (Equus caballus) | BDBM50368629 (CHEMBL2368671) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University Curated by ChEMBL | Assay Description Inhibition of horse liver alcohol dehydrogenase enzyme by Non-competitive inhibition | J Med Chem 36: 1855-9 (1993) BindingDB Entry DOI: 10.7270/Q2X63NM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50491842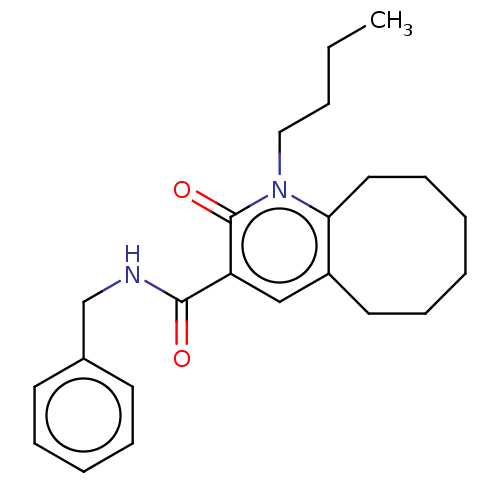 (CHEMBL2387078) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from human CB2 receptor expressed in CHO cell membranes | Bioorg Med Chem 21: 3154-63 (2013) Article DOI: 10.1016/j.bmc.2013.03.030 BindingDB Entry DOI: 10.7270/Q22R3VKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50491840 (CHEMBL2387186) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from human CB2 receptor expressed in CHO cell membranes | Bioorg Med Chem 21: 3154-63 (2013) Article DOI: 10.1016/j.bmc.2013.03.030 BindingDB Entry DOI: 10.7270/Q22R3VKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501808 (CHEMBL4465534) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-JNJ-962 from BACE1 (unknown origin) expressed in HEK293 cell membranes by scintillation counting method | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alcohol dehydrogenase 1A (Homo sapiens (Human)) | BDBM50368954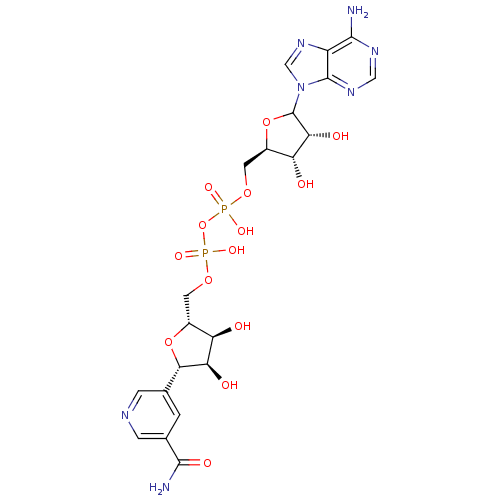 (CHEMBL610377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Medical Center Curated by ChEMBL | Assay Description Apparent inhibition constant against mammalian liver alcohol dehydrogenase (ADH) | J Med Chem 37: 392-9 (1994) BindingDB Entry DOI: 10.7270/Q2RN38H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor | Bioorg Med Chem Lett 17: 4030-4 (2007) Article DOI: 10.1016/j.bmcl.2007.04.093 BindingDB Entry DOI: 10.7270/Q2P55N60 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50474150 (CHEMBL58362) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor delta 1 using [3H]DPDPE as radioligand | J Med Chem 45: 1949-56 (2002) Article DOI: 10.1021/jm010576e BindingDB Entry DOI: 10.7270/Q2WS8X0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50570460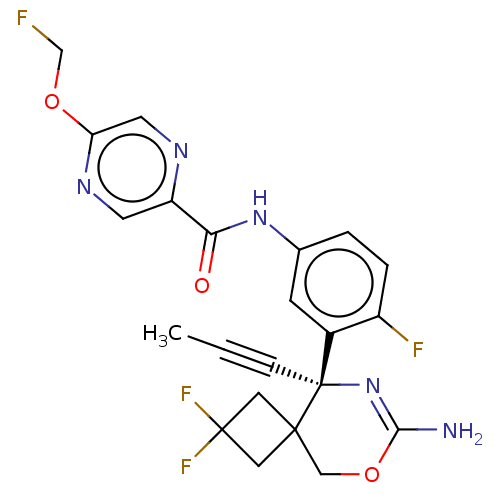 (CHEMBL4854629) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-JNJ962 from BACE1 (unknown origin) expressed in HEK293 cell membrane assessed as inhibition constant by scintillation counting a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01858 BindingDB Entry DOI: 10.7270/Q2GH9NQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor mu 1 using [3H]- DAMGO as radioligand | J Med Chem 45: 1949-56 (2002) Article DOI: 10.1021/jm010576e BindingDB Entry DOI: 10.7270/Q2WS8X0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501810 (CHEMBL4443968) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-JNJ-962 from BACE1 (unknown origin) expressed in HEK293 cell membranes by scintillation counting method | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alcohol dehydrogenase 1A (Homo sapiens (Human)) | BDBM50368954 (CHEMBL610377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Medical Center Curated by ChEMBL | Assay Description Inhibition constant against mammalian liver alcohol dehydrogenase (ADH) | J Med Chem 37: 392-9 (1994) BindingDB Entry DOI: 10.7270/Q2RN38H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50491831 (CHEMBL2387182) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from human CB2 receptor expressed in CHO cell membranes | Bioorg Med Chem 21: 3154-63 (2013) Article DOI: 10.1016/j.bmc.2013.03.030 BindingDB Entry DOI: 10.7270/Q22R3VKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM19264 ((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Codon Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 2 (IMPDH type II isoform); Range is 6-10 nM | J Med Chem 41: 618-22 (1998) Article DOI: 10.1021/jm970705k BindingDB Entry DOI: 10.7270/Q20C4WGZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from mouse CB2 receptor | Bioorg Med Chem Lett 17: 4030-4 (2007) Article DOI: 10.1016/j.bmcl.2007.04.093 BindingDB Entry DOI: 10.7270/Q2P55N60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501818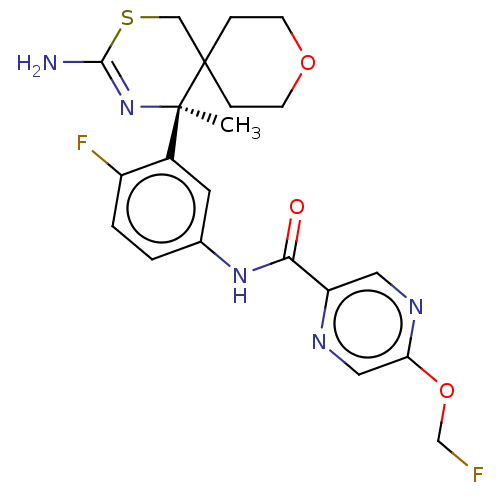 (CHEMBL4583340) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-JNJ-962 from BACE1 (unknown origin) expressed in HEK293 cell membranes by scintillation counting method | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from mouse CB1 receptor | Bioorg Med Chem Lett 17: 4030-4 (2007) Article DOI: 10.1016/j.bmcl.2007.04.093 BindingDB Entry DOI: 10.7270/Q2P55N60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50474152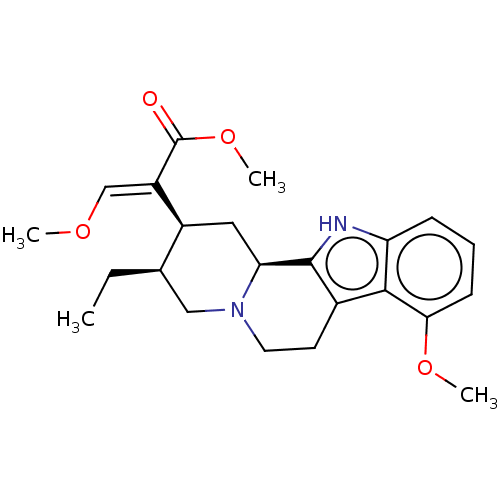 (CHEBI:6956 | CHEMBL299031) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor mu 1 using [3H]- DAMGO as radioligand | J Med Chem 45: 1949-56 (2002) Article DOI: 10.1021/jm010576e BindingDB Entry DOI: 10.7270/Q2WS8X0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50491841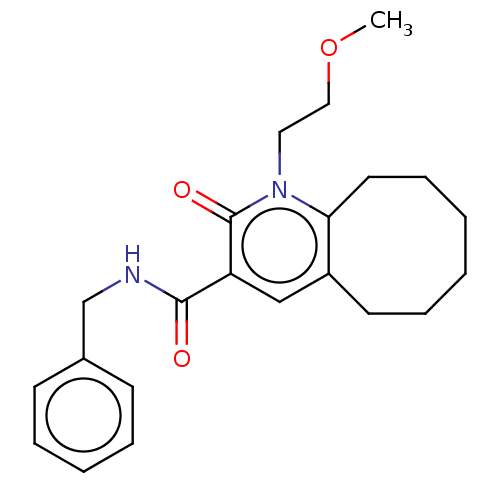 (CHEMBL2387179) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from human CB2 receptor expressed in CHO cell membranes | Bioorg Med Chem 21: 3154-63 (2013) Article DOI: 10.1016/j.bmc.2013.03.030 BindingDB Entry DOI: 10.7270/Q22R3VKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50213617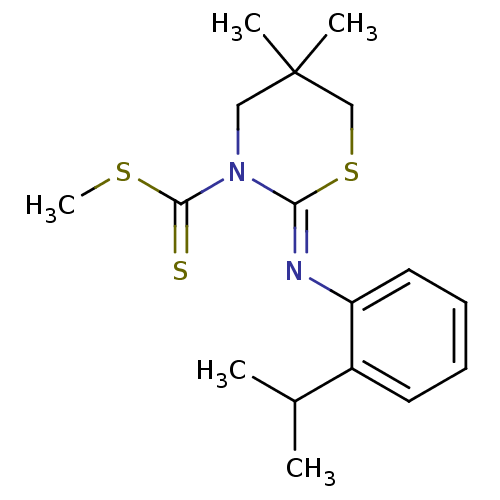 ((Z)-methyl 2-(2-isopropylphenylimino)-5,5-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor | Bioorg Med Chem Lett 17: 4030-4 (2007) Article DOI: 10.1016/j.bmcl.2007.04.093 BindingDB Entry DOI: 10.7270/Q2P55N60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50213617 ((Z)-methyl 2-(2-isopropylphenylimino)-5,5-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from mouse CB2 receptor | Bioorg Med Chem Lett 17: 4030-4 (2007) Article DOI: 10.1016/j.bmcl.2007.04.093 BindingDB Entry DOI: 10.7270/Q2P55N60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50491833 (CHEMBL2387178) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from human CB1 receptor expressed in CHO cell membranes | Bioorg Med Chem 21: 3154-63 (2013) Article DOI: 10.1016/j.bmc.2013.03.030 BindingDB Entry DOI: 10.7270/Q22R3VKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50579805 (CHEMBL5075689) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] JNJ-962 from BACE2 (unknown origin) expressed in HEK293 cell membranes assessed as inhibition constant at pH 6.2 by scintillatio... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00359 BindingDB Entry DOI: 10.7270/Q2WW7NJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM19264 ((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 2 (IMPDH type II) | J Med Chem 45: 703-12 (2002) BindingDB Entry DOI: 10.7270/Q29S1RS4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM19264 ((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 2 (IMPDH type II) | J Med Chem 45: 703-12 (2002) BindingDB Entry DOI: 10.7270/Q29S1RS4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50580216 (MK-8931 | SCH 900931 | SCH-900931 | SCH900931 | VE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] JNJ-962 from BACE2 (unknown origin) expressed in HEK293 cell membranes assessed as inhibition constant at pH 6.2 by scintillatio... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00359 BindingDB Entry DOI: 10.7270/Q2WW7NJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50474151 (CHEMBL292521) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor mu 1 using [3H]- DAMGO as radioligand | J Med Chem 45: 1949-56 (2002) Article DOI: 10.1021/jm010576e BindingDB Entry DOI: 10.7270/Q2WS8X0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50431426 (CHEMBL2348280) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Displacement of [H3]-CP-55940 from human recombinant CB2 receptor expressed in CHO cells | Bioorg Med Chem 21: 2045-55 (2013) Article DOI: 10.1016/j.bmc.2013.01.006 BindingDB Entry DOI: 10.7270/Q2PG1T2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50491839 (CHEMBL2387183) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from mouse brain CB1 receptor expressed in CHO cell membranes | Bioorg Med Chem 21: 3154-63 (2013) Article DOI: 10.1016/j.bmc.2013.03.030 BindingDB Entry DOI: 10.7270/Q22R3VKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50474153 (CHEMBL61630) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor mu 1 using [3H]- DAMGO as radioligand | J Med Chem 45: 1949-56 (2002) Article DOI: 10.1021/jm010576e BindingDB Entry DOI: 10.7270/Q2WS8X0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4154 total ) | Next | Last >> |