 Found 38291 hits with Last Name = 'shi' and Initial = 'k'
Found 38291 hits with Last Name = 'shi' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50423789
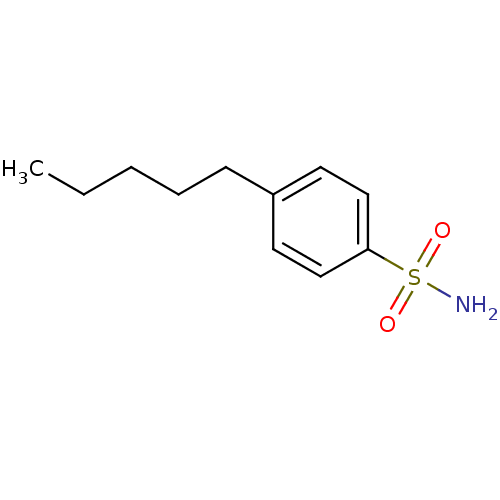
(4-Pentyl-Benzenesulfonamide | 4-Pentylbenzenesulfo...)Show InChI InChI=1S/C11H17NO2S/c1-2-3-4-5-10-6-8-11(9-7-10)15(12,13)14/h6-9H,2-5H2,1H3,(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.000800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 2 |
Bioorg Med Chem Lett 21: 141-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.050
BindingDB Entry DOI: 10.7270/Q2K938T6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50423789

(4-Pentyl-Benzenesulfonamide | 4-Pentylbenzenesulfo...)Show InChI InChI=1S/C11H17NO2S/c1-2-3-4-5-10-6-8-11(9-7-10)15(12,13)14/h6-9H,2-5H2,1H3,(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.000800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 2 |
Bioorg Med Chem Lett 21: 141-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.050
BindingDB Entry DOI: 10.7270/Q2K938T6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50423788
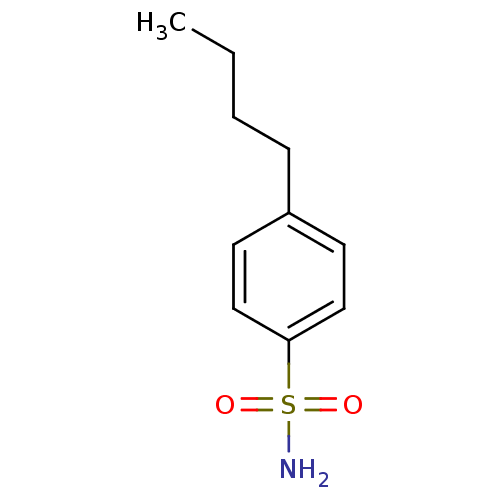
(4-Butyl-Benzenesulfonamide | 4-Butylbenzenesulfona...)Show InChI InChI=1S/C10H15NO2S/c1-2-3-4-9-5-7-10(8-6-9)14(11,12)13/h5-8H,2-4H2,1H3,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 2 |
Bioorg Med Chem Lett 21: 141-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.050
BindingDB Entry DOI: 10.7270/Q2K938T6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50423788

(4-Butyl-Benzenesulfonamide | 4-Butylbenzenesulfona...)Show InChI InChI=1S/C10H15NO2S/c1-2-3-4-9-5-7-10(8-6-9)14(11,12)13/h5-8H,2-4H2,1H3,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 2 |
Bioorg Med Chem Lett 21: 141-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.050
BindingDB Entry DOI: 10.7270/Q2K938T6 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50180655

(A-157378-0 | A-157378.0 | ABT-378 | CHEBI:31781 | ...)Show SMILES CC(C)[C@H](N1CCCNC1=O)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)Cc1ccccc1 Show InChI InChI=1S/C37H48N4O5/c1-25(2)34(41-20-12-19-38-37(41)45)36(44)39-30(21-28-15-7-5-8-16-28)23-32(42)31(22-29-17-9-6-10-18-29)40-33(43)24-46-35-26(3)13-11-14-27(35)4/h5-11,13-18,25,30-32,34,42H,12,19-24H2,1-4H3,(H,38,45)(H,39,44)(H,40,43)/t30-,31-,32-,34-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem 16: 1299-308 (2008)
Article DOI: 10.1016/j.bmc.2007.10.062
BindingDB Entry DOI: 10.7270/Q2RJ4N8X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50194487
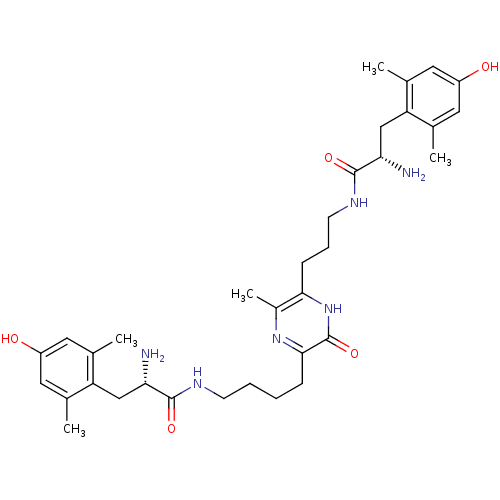
(3-[4'-(H-Dmt)-aminobutyl]-6-[3'-(H-Dmt)-aminopropy...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)NCCCCc1nc(C)c(CCCNC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)[nH]c1=O Show InChI InChI=1S/C34H48N6O5/c1-19-13-24(41)14-20(2)26(19)17-28(35)32(43)37-11-7-6-9-31-34(45)40-30(23(5)39-31)10-8-12-38-33(44)29(36)18-27-21(3)15-25(42)16-22(27)4/h13-16,28-29,41-42H,6-12,17-18,35-36H2,1-5H3,(H,37,43)(H,38,44)(H,40,45)/t28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat synaptosomes P2 fraction |
Bioorg Med Chem Lett 16: 5793-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.079
BindingDB Entry DOI: 10.7270/Q26Q1WWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10597
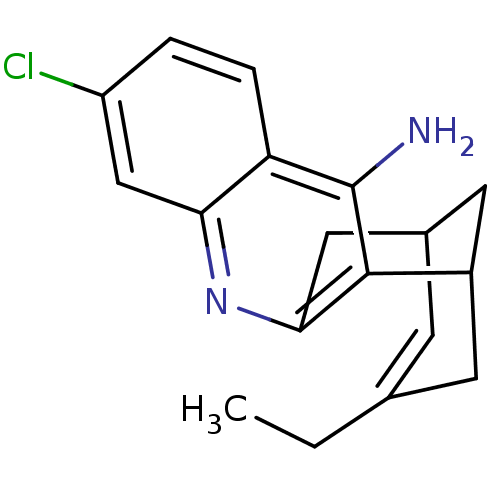
((1S)-7-chloro-15-ethyl-10-azatetracyclo[11.3.1.0^{...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1N |t:2,TLB:11:9:5:3.2.7,19:8:5:3.2.7| Show InChI InChI=1S/C18H19ClN2/c1-2-10-5-11-7-12(6-10)17-16(8-11)21-15-9-13(19)3-4-14(15)18(17)20/h3-5,9,11-12H,2,6-8H2,1H3,(H2,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0260 | -60.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mayo Clinic College of Medicine
| Assay Description
Assays were conducted at 25 C in 20 mM sodium phosphate buffer (pH 7.0) and 0.01% bovine serum albumin unless otherwise noted. AChE concentrations we... |
Biochemistry 52: 7486-99 (2013)
Article DOI: 10.1021/bi401043w
BindingDB Entry DOI: 10.7270/Q24X56GT |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50045514
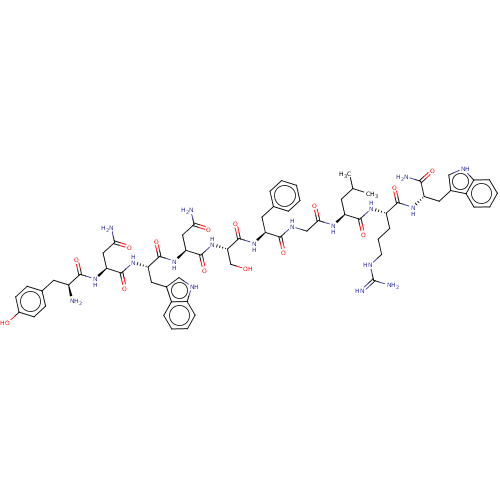
(CHEMBL3315315)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human KISS1R transfected in CHO cells |
J Med Chem 57: 6105-15 (2014)
Article DOI: 10.1021/jm5005489
BindingDB Entry DOI: 10.7270/Q2SF2XSG |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50045510
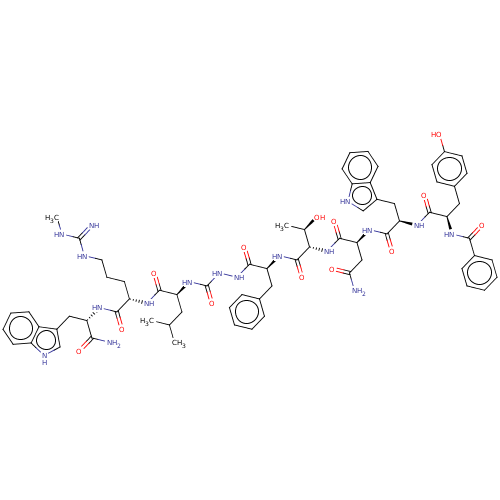
(CHEMBL3314227)Show SMILES CNC(=N)NCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](Cc1ccc(O)cc1)NC(=O)c1ccccc1)[C@@H](C)O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human KISS1R transfected in CHO cells |
J Med Chem 57: 6105-15 (2014)
Article DOI: 10.1021/jm5005489
BindingDB Entry DOI: 10.7270/Q2SF2XSG |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50045500
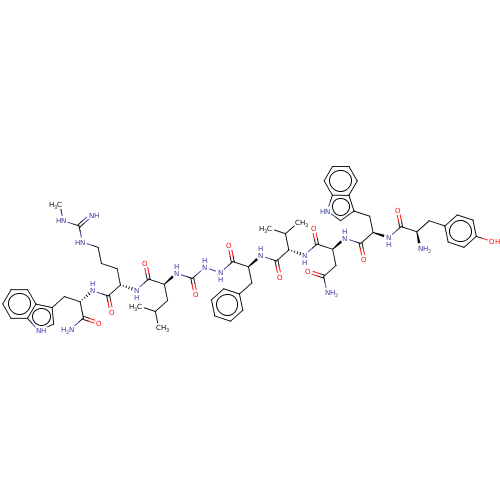
(CHEMBL3314217)Show SMILES CNC(=N)NCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human KISS1R transfected in CHO cells |
J Med Chem 57: 6105-15 (2014)
Article DOI: 10.1021/jm5005489
BindingDB Entry DOI: 10.7270/Q2SF2XSG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50423787
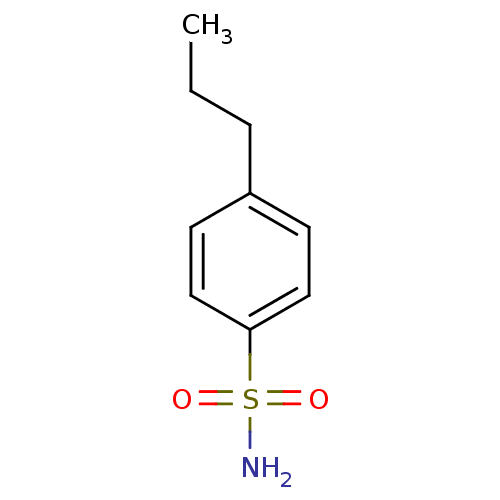
(4-Propyl-Benzenesulfonamide | 4-Propylbenzenesulfo...)Show InChI InChI=1S/C9H13NO2S/c1-2-3-8-4-6-9(7-5-8)13(10,11)12/h4-7H,2-3H2,1H3,(H2,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 2 |
Bioorg Med Chem Lett 21: 141-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.050
BindingDB Entry DOI: 10.7270/Q2K938T6 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50143784
((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-2-butyl-7-[2-((...)Show SMILES CCCCc1ccc2[C@@H]([C@H]([C@@H](c2n1)c1ccc(OC)cc1C[C@H](C)C(O)=O)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C31H33NO7/c1-4-5-6-20-8-10-23-26(18-7-12-24-25(15-18)39-16-38-24)28(31(35)36)27(29(23)32-20)22-11-9-21(37-3)14-19(22)13-17(2)30(33)34/h7-12,14-15,17,26-28H,4-6,13,16H2,1-3H3,(H,33,34)(H,35,36)/t17-,26-,27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 1262-70 (1999)
BindingDB Entry DOI: 10.7270/Q2Q52N5Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50423787

(4-Propyl-Benzenesulfonamide | 4-Propylbenzenesulfo...)Show InChI InChI=1S/C9H13NO2S/c1-2-3-8-4-6-9(7-5-8)13(10,11)12/h4-7H,2-3H2,1H3,(H2,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 2 |
Bioorg Med Chem Lett 21: 141-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.050
BindingDB Entry DOI: 10.7270/Q2K938T6 |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50442965
(CHEMBL3086282)Show SMILES CNC(N)=NCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,w:4.4| Show InChI InChI=1S/C61H79N15O12/c1-35(2)27-46(55(83)68-44(19-12-26-67-60(65)66-3)54(82)69-45(52(64)80)30-36-13-6-4-7-14-36)74-61(88)76-75-59(87)48(31-37-15-8-5-9-16-37)71-58(86)50(34-77)73-57(85)49(33-51(63)79)72-56(84)47(32-39-20-23-40-17-10-11-18-41(40)28-39)70-53(81)43(62)29-38-21-24-42(78)25-22-38/h4-11,13-18,20-25,28,35,43-50,77-78H,12,19,26-27,29-34,62H2,1-3H3,(H2,63,79)(H2,64,80)(H,68,83)(H,69,82)(H,70,81)(H,71,86)(H,72,84)(H,73,85)(H,75,87)(H3,65,66,67)(H2,74,76,88)/t43-,44+,45+,46+,47+,48+,49+,50+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human KISS1R expressed in CHO cell membranes |
J Med Chem 56: 8298-307 (2013)
Article DOI: 10.1021/jm401056w
BindingDB Entry DOI: 10.7270/Q25M675S |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50442966
(CHEMBL3087927)Show SMILES CNC(N)=NCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](Cc1cnc[nH]1)NC(=O)[C@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,w:4.4| Show InChI InChI=1S/C65H85N19O13/c1-36(2)25-48(58(91)75-46(19-12-24-72-64(69)70-3)57(90)76-47(55(68)88)27-37-13-6-4-7-14-37)82-65(97)84-83-63(96)49(28-38-15-8-5-9-16-38)78-62(95)53(34-85)81-61(94)52(31-54(67)87)80-59(92)50(29-40-32-73-45-18-11-10-17-43(40)45)79-60(93)51(30-41-33-71-35-74-41)77-56(89)44(66)26-39-20-22-42(86)23-21-39/h4-11,13-18,20-23,32-33,35-36,44,46-53,73,85-86H,12,19,24-31,34,66H2,1-3H3,(H2,67,87)(H2,68,88)(H,71,74)(H,75,91)(H,76,90)(H,77,89)(H,78,95)(H,79,93)(H,80,92)(H,81,94)(H,83,96)(H3,69,70,72)(H2,82,84,97)/t44-,46+,47+,48+,49+,50+,51-,52+,53+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human KISS1R expressed in CHO cell membranes |
J Med Chem 56: 8298-307 (2013)
Article DOI: 10.1021/jm401056w
BindingDB Entry DOI: 10.7270/Q25M675S |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50045509
(CHEMBL3314226)Show SMILES CNC(=N)NCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](Cc1ccc(O)cc1)NC(=O)C1CC1)[C@@H](C)O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human KISS1R transfected in CHO cells |
J Med Chem 57: 6105-15 (2014)
Article DOI: 10.1021/jm5005489
BindingDB Entry DOI: 10.7270/Q2SF2XSG |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50045513
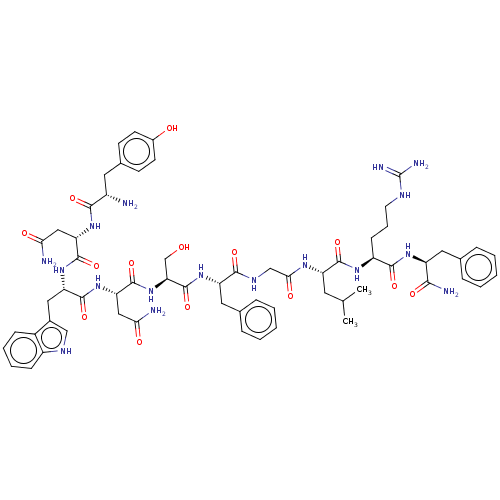
(Kisspeptin-10)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human KISS1R transfected in CHO cells |
J Med Chem 57: 6105-15 (2014)
Article DOI: 10.1021/jm5005489
BindingDB Entry DOI: 10.7270/Q2SF2XSG |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM580
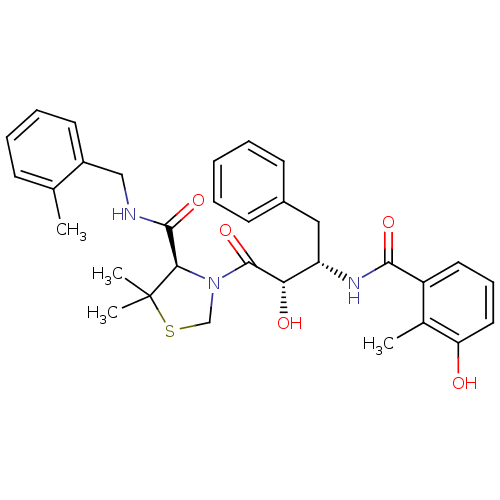
((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...)Show SMILES Cc1ccccc1CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(O)c1C |r| Show InChI InChI=1S/C32H37N3O5S/c1-20-11-8-9-14-23(20)18-33-30(39)28-32(3,4)41-19-35(28)31(40)27(37)25(17-22-12-6-5-7-13-22)34-29(38)24-15-10-16-26(36)21(24)2/h5-16,25,27-28,36-37H,17-19H2,1-4H3,(H,33,39)(H,34,38)/t25-,27-,28+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem 16: 1299-308 (2008)
Article DOI: 10.1016/j.bmc.2007.10.062
BindingDB Entry DOI: 10.7270/Q2RJ4N8X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50045507
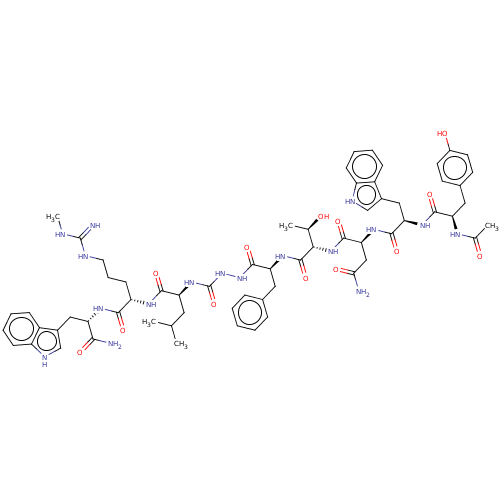
(CHEMBL3314224)Show SMILES CNC(=N)NCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](Cc1ccc(O)cc1)NC(C)=O)[C@@H](C)O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human KISS1R transfected in CHO cells |
J Med Chem 57: 6105-15 (2014)
Article DOI: 10.1021/jm5005489
BindingDB Entry DOI: 10.7270/Q2SF2XSG |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50045499
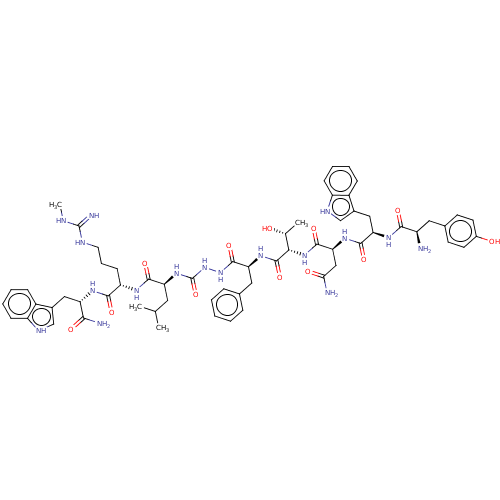
(CHEMBL3314216)Show SMILES CNC(=N)NCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](N)Cc1ccc(O)cc1)[C@@H](C)O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human KISS1R transfected in CHO cells |
J Med Chem 57: 6105-15 (2014)
Article DOI: 10.1021/jm5005489
BindingDB Entry DOI: 10.7270/Q2SF2XSG |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50045498
(CHEMBL3314215)Show SMILES CNC(=N)NCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human KISS1R transfected in CHO cells |
J Med Chem 57: 6105-15 (2014)
Article DOI: 10.1021/jm5005489
BindingDB Entry DOI: 10.7270/Q2SF2XSG |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50045512
(CHEMBL3314229)Show SMILES CNC(=N)NCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](Cc1ccc(O)cc1)NC(C)=O)[C@@H](C)O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human KISS1R transfected in CHO cells |
J Med Chem 57: 6105-15 (2014)
Article DOI: 10.1021/jm5005489
BindingDB Entry DOI: 10.7270/Q2SF2XSG |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM26349

((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-amino-3-(4-hydroxyp...)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:83.86,72.75,12.20,29.34,37.50,51.58,59.62,wD:4.4,23.26,(23.93,-21.81,;22.46,-22.29,;22.15,-23.79,;21.33,-21.26,;21.65,-19.76,;20.51,-18.74,;19.04,-19.2,;18.73,-20.72,;17.9,-18.17,;16.44,-18.65,;15.29,-17.62,;15.61,-16.12,;13.83,-18.1,;12.69,-17.06,;13.01,-15.57,;11.86,-14.54,;12.18,-13.03,;13.65,-12.55,;14.79,-13.59,;14.47,-15.08,;13.51,-19.6,;12.05,-20.08,;10.9,-19.05,;11.73,-21.57,;12.87,-22.61,;14.34,-22.12,;10.27,-22.05,;9.94,-23.55,;11.09,-24.58,;8.49,-24.03,;7.34,-23,;7.67,-21.49,;6.52,-20.46,;9.12,-21.02,;8.16,-25.53,;6.7,-26.01,;5.56,-24.98,;6.38,-27.51,;4.92,-27.99,;4.6,-29.49,;5.63,-30.63,;4.86,-31.97,;3.35,-31.65,;2.11,-32.56,;.71,-31.92,;.55,-30.4,;1.79,-29.49,;3.2,-30.12,;7.53,-28.54,;8.98,-28.07,;9.31,-26.56,;10.13,-29.1,;9.81,-30.6,;8.35,-31.08,;8.02,-32.58,;7.2,-30.05,;11.59,-28.62,;12.74,-29.65,;12.42,-31.16,;14.21,-29.18,;14.52,-27.67,;15.34,-30.2,;16.8,-29.73,;17.12,-28.22,;18.58,-27.75,;19.72,-28.77,;21.19,-28.3,;19.41,-30.28,;17.94,-30.75,;23.11,-19.28,;23.42,-17.77,;24.24,-20.31,;25.71,-19.83,;26.03,-18.32,;27.49,-17.85,;27.81,-16.34,;29.28,-15.87,;29.59,-14.36,;28.45,-13.34,;31.06,-13.9,;26.85,-20.86,;26.53,-22.37,;28.32,-20.39,;29.46,-21.42,;30.93,-20.94,;32.07,-21.97,;33.53,-21.49,;34.68,-22.52,;34.35,-24.03,;32.89,-24.5,;31.75,-23.47,;29.14,-22.92,;27.68,-23.4,;30.28,-23.95,)| Show InChI InChI=1S/C63H83N17O14/c1-34(2)24-45(58(90)74-43(18-11-23-70-63(68)69)57(89)75-44(54(67)86)26-35-12-5-3-6-13-35)73-53(85)32-72-56(88)46(27-36-14-7-4-8-15-36)77-62(94)50(33-81)80-61(93)49(30-52(66)84)79-59(91)47(28-38-31-71-42-17-10-9-16-40(38)42)78-60(92)48(29-51(65)83)76-55(87)41(64)25-37-19-21-39(82)22-20-37/h3-10,12-17,19-22,31,34,41,43-50,71,81-82H,11,18,23-30,32-33,64H2,1-2H3,(H2,65,83)(H2,66,84)(H2,67,86)(H,72,88)(H,73,85)(H,74,90)(H,75,89)(H,76,87)(H,77,94)(H,78,92)(H,79,91)(H,80,93)(H4,68,69,70)/t41-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human KISS1R expressed in CHO cell membranes |
J Med Chem 56: 8298-307 (2013)
Article DOI: 10.1021/jm401056w
BindingDB Entry DOI: 10.7270/Q25M675S |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50045506
(CHEMBL3314223)Show SMILES CNC(=N)NCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)CCc1ccc(O)cc1)[C@@H](C)O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human KISS1R transfected in CHO cells |
J Med Chem 57: 6105-15 (2014)
Article DOI: 10.1021/jm5005489
BindingDB Entry DOI: 10.7270/Q2SF2XSG |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17428
(1,2,4-Triazole Compound, 86 | N-[5-(benzylsulfanyl...)Show InChI InChI=1S/C14H13N5S/c1-2-5-11(6-3-1)10-20-14-17-13(18-19-14)16-12-7-4-8-15-9-12/h1-9H,10H2,(H2,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17355
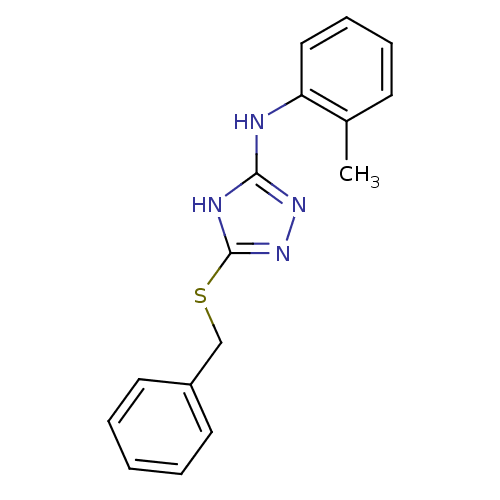
(1,2,4-Triazole Compound, 13 | 5-(benzylsulfanyl)-N...)Show InChI InChI=1S/C16H16N4S/c1-12-7-5-6-10-14(12)17-15-18-16(20-19-15)21-11-13-8-3-2-4-9-13/h2-10H,11H2,1H3,(H2,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Rattus norvegicus) | BDBM50442968

(CHEMBL3087793)Show SMILES CC(C)C[C@H](NC(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:59.62,37.50,23.26,72.75,wD:51.58,29.34,12.20,4.4,83.86,(40.93,-34.78,;40.93,-33.25,;42.27,-32.48,;39.59,-32.48,;39.59,-30.94,;38.26,-30.17,;36.93,-30.95,;36.93,-32.48,;35.6,-30.18,;34.27,-30.95,;32.94,-30.18,;32.94,-28.64,;31.6,-30.95,;31.6,-32.49,;32.94,-33.25,;34.27,-32.49,;35.6,-33.25,;35.61,-34.79,;34.27,-35.56,;32.94,-34.8,;30.27,-30.18,;28.93,-30.95,;28.93,-32.49,;27.6,-30.19,;27.6,-28.64,;28.93,-27.88,;26.27,-30.96,;24.94,-30.19,;24.94,-28.65,;23.6,-30.96,;23.6,-32.5,;24.94,-33.27,;26.27,-32.5,;24.94,-34.8,;22.28,-30.2,;20.94,-30.96,;20.94,-32.5,;19.61,-30.19,;19.61,-28.66,;20.94,-27.88,;22.34,-28.51,;23.37,-27.36,;22.6,-26.03,;23.08,-24.56,;22.04,-23.42,;20.53,-23.75,;20.06,-25.21,;21.09,-26.35,;18.27,-30.97,;16.94,-30.2,;16.94,-28.66,;15.6,-30.97,;15.6,-32.51,;16.94,-33.28,;18.27,-32.51,;16.94,-34.81,;14.28,-30.2,;12.94,-30.97,;12.94,-32.51,;11.61,-30.2,;10.27,-30.97,;11.61,-28.67,;12.94,-27.9,;14.27,-28.66,;15.61,-27.89,;15.6,-26.35,;16.93,-25.58,;14.27,-25.58,;12.93,-26.35,;40.93,-30.17,;40.93,-28.63,;42.27,-30.94,;43.6,-30.16,;43.6,-28.62,;44.93,-27.85,;44.93,-26.32,;46.26,-25.54,;46.26,-24.01,;44.92,-23.23,;47.59,-23.24,;44.93,-30.93,;44.93,-32.47,;46.26,-30.16,;47.59,-30.93,;47.59,-32.47,;48.93,-33.24,;50.26,-32.46,;51.6,-33.23,;51.6,-34.77,;50.27,-35.54,;48.94,-34.77,;48.93,-30.16,;50.26,-30.93,;48.93,-28.62,)| Show InChI InChI=1S/C62H82N18O14/c1-33(2)24-44(55(88)71-42(18-11-23-69-61(67)68)54(87)72-43(52(66)85)26-34-12-5-3-6-13-34)78-62(94)80-79-60(93)45(27-35-14-7-4-8-15-35)74-59(92)49(32-81)77-58(91)48(30-51(65)84)76-56(89)46(28-37-31-70-41-17-10-9-16-39(37)41)75-57(90)47(29-50(64)83)73-53(86)40(63)25-36-19-21-38(82)22-20-36/h3-10,12-17,19-22,31,33,40,42-49,70,81-82H,11,18,23-30,32,63H2,1-2H3,(H2,64,83)(H2,65,84)(H2,66,85)(H,71,88)(H,72,87)(H,73,86)(H,74,92)(H,75,90)(H,76,89)(H,77,91)(H,79,93)(H4,67,68,69)(H2,78,80,94)/t40-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to rat KISS1R |
J Med Chem 56: 8298-307 (2013)
Article DOI: 10.1021/jm401056w
BindingDB Entry DOI: 10.7270/Q25M675S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM221048
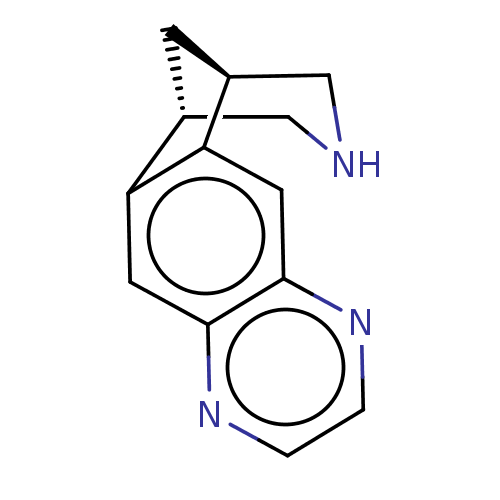
(US9284322, varenicline | US9303017, Varenicline)Show InChI InChI=1S/C13H13N3/c1-2-16-13-5-11-9-3-8(6-14-7-9)10(11)4-12(13)15-1/h1-2,4-5,8-9,14H,3,6-7H2/t8-,9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
KiSS-1 receptor
(Rattus norvegicus) | BDBM50442967
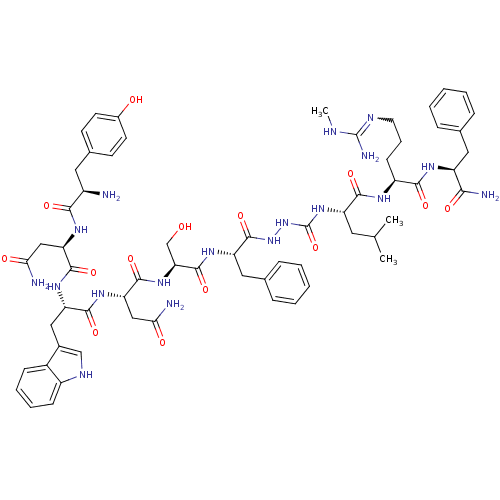
(CHEMBL3087925)Show SMILES CNC(N)=NCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,w:4.4| Show InChI InChI=1S/C63H84N18O14/c1-34(2)25-45(56(89)72-43(19-12-24-70-62(68)69-3)55(88)73-44(53(67)86)27-35-13-6-4-7-14-35)79-63(95)81-80-61(94)46(28-36-15-8-5-9-16-36)75-60(93)50(33-82)78-59(92)49(31-52(66)85)77-57(90)47(29-38-32-71-42-18-11-10-17-40(38)42)76-58(91)48(30-51(65)84)74-54(87)41(64)26-37-20-22-39(83)23-21-37/h4-11,13-18,20-23,32,34,41,43-50,71,82-83H,12,19,24-31,33,64H2,1-3H3,(H2,65,84)(H2,66,85)(H2,67,86)(H,72,89)(H,73,88)(H,74,87)(H,75,93)(H,76,91)(H,77,90)(H,78,92)(H,80,94)(H3,68,69,70)(H2,79,81,95)/t41-,43+,44+,45+,46+,47+,48-,49+,50+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to rat KISS1R |
J Med Chem 56: 8298-307 (2013)
Article DOI: 10.1021/jm401056w
BindingDB Entry DOI: 10.7270/Q25M675S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50221787
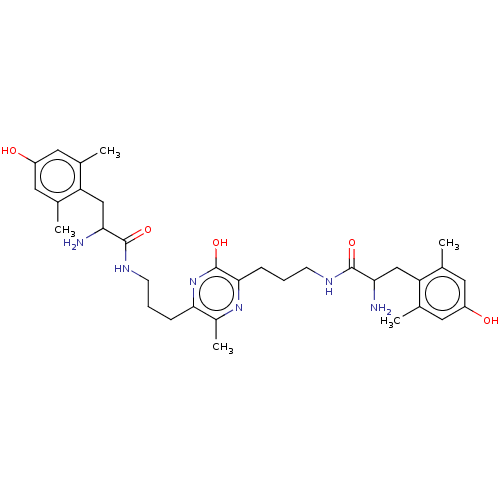
(CHEMBL3216418)Show SMILES Cl.Cl.Cc1cc(O)cc(C)c1CC(N)C(=O)NCCCc1nc(O)c(CCCNC(=O)C(N)Cc2c(C)cc(O)cc2C)nc1C Show InChI InChI=1S/C33H46N6O5.2ClH/c1-18-12-23(40)13-19(2)25(18)16-27(34)31(42)36-10-6-8-29-22(5)38-30(33(44)39-29)9-7-11-37-32(43)28(35)17-26-20(3)14-24(41)15-21(26)4;;/h12-15,27-28,40-41H,6-11,16-17,34-35H2,1-5H3,(H,36,42)(H,37,43)(H,39,44);2*1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Environmental Health Sciences
Curated by ChEMBL
| Assay Description
Compound was tested for Opioid receptor mu 1 agonism in isolated tissues from guinea pig ileum |
J Med Chem 47: 2599-610 (2004)
Article DOI: 10.1021/jm0304616
BindingDB Entry DOI: 10.7270/Q2RB755V |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Rattus norvegicus) | BDBM50442966

(CHEMBL3087927)Show SMILES CNC(N)=NCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](Cc1cnc[nH]1)NC(=O)[C@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,w:4.4| Show InChI InChI=1S/C65H85N19O13/c1-36(2)25-48(58(91)75-46(19-12-24-72-64(69)70-3)57(90)76-47(55(68)88)27-37-13-6-4-7-14-37)82-65(97)84-83-63(96)49(28-38-15-8-5-9-16-38)78-62(95)53(34-85)81-61(94)52(31-54(67)87)80-59(92)50(29-40-32-73-45-18-11-10-17-43(40)45)79-60(93)51(30-41-33-71-35-74-41)77-56(89)44(66)26-39-20-22-42(86)23-21-39/h4-11,13-18,20-23,32-33,35-36,44,46-53,73,85-86H,12,19,24-31,34,66H2,1-3H3,(H2,67,87)(H2,68,88)(H,71,74)(H,75,91)(H,76,90)(H,77,89)(H,78,95)(H,79,93)(H,80,92)(H,81,94)(H,83,96)(H3,69,70,72)(H2,82,84,97)/t44-,46+,47+,48+,49+,50+,51-,52+,53+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to rat KISS1R |
J Med Chem 56: 8298-307 (2013)
Article DOI: 10.1021/jm401056w
BindingDB Entry DOI: 10.7270/Q25M675S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50212613
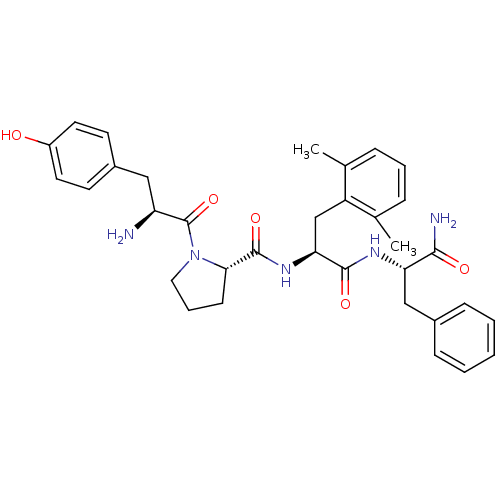
(CHEMBL266122 | Tyr-Pro-Dmp-Phe-NH2)Show SMILES Cc1cccc(C)c1C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C34H41N5O5/c1-21-8-6-9-22(2)26(21)20-29(32(42)37-28(31(36)41)19-23-10-4-3-5-11-23)38-33(43)30-12-7-17-39(30)34(44)27(35)18-24-13-15-25(40)16-14-24/h3-6,8-11,13-16,27-30,40H,7,12,17-20,35H2,1-2H3,(H2,36,41)(H,37,42)(H,38,43)/t27-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosome membrane |
J Med Chem 50: 2753-66 (2007)
Article DOI: 10.1021/jm061238m
BindingDB Entry DOI: 10.7270/Q25B0264 |
More data for this
Ligand-Target Pair | |
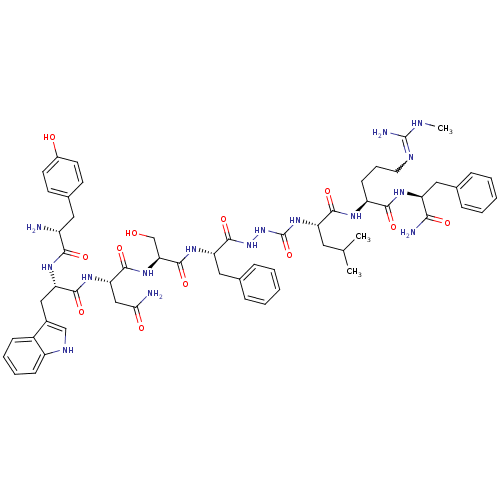
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50442964
(CHEMBL3085809)Show SMILES CNC(N)=NCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,w:4.4| Show InChI InChI=1S/C59H78N16O12/c1-33(2)25-44(53(82)67-42(19-12-24-65-58(63)64-3)52(81)68-43(50(62)79)27-34-13-6-4-7-14-34)73-59(87)75-74-57(86)45(28-35-15-8-5-9-16-35)70-56(85)48(32-76)72-55(84)47(30-49(61)78)71-54(83)46(29-37-31-66-41-18-11-10-17-39(37)41)69-51(80)40(60)26-36-20-22-38(77)23-21-36/h4-11,13-18,20-23,31,33,40,42-48,66,76-77H,12,19,24-30,32,60H2,1-3H3,(H2,61,78)(H2,62,79)(H,67,82)(H,68,81)(H,69,80)(H,70,85)(H,71,83)(H,72,84)(H,74,86)(H3,63,64,65)(H2,73,75,87)/t40-,42+,43+,44+,45+,46+,47+,48+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human KISS1R expressed in CHO cell membranes |
J Med Chem 56: 8298-307 (2013)
Article DOI: 10.1021/jm401056w
BindingDB Entry DOI: 10.7270/Q25M675S |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50442968

(CHEMBL3087793)Show SMILES CC(C)C[C@H](NC(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:59.62,37.50,23.26,72.75,wD:51.58,29.34,12.20,4.4,83.86,(40.93,-34.78,;40.93,-33.25,;42.27,-32.48,;39.59,-32.48,;39.59,-30.94,;38.26,-30.17,;36.93,-30.95,;36.93,-32.48,;35.6,-30.18,;34.27,-30.95,;32.94,-30.18,;32.94,-28.64,;31.6,-30.95,;31.6,-32.49,;32.94,-33.25,;34.27,-32.49,;35.6,-33.25,;35.61,-34.79,;34.27,-35.56,;32.94,-34.8,;30.27,-30.18,;28.93,-30.95,;28.93,-32.49,;27.6,-30.19,;27.6,-28.64,;28.93,-27.88,;26.27,-30.96,;24.94,-30.19,;24.94,-28.65,;23.6,-30.96,;23.6,-32.5,;24.94,-33.27,;26.27,-32.5,;24.94,-34.8,;22.28,-30.2,;20.94,-30.96,;20.94,-32.5,;19.61,-30.19,;19.61,-28.66,;20.94,-27.88,;22.34,-28.51,;23.37,-27.36,;22.6,-26.03,;23.08,-24.56,;22.04,-23.42,;20.53,-23.75,;20.06,-25.21,;21.09,-26.35,;18.27,-30.97,;16.94,-30.2,;16.94,-28.66,;15.6,-30.97,;15.6,-32.51,;16.94,-33.28,;18.27,-32.51,;16.94,-34.81,;14.28,-30.2,;12.94,-30.97,;12.94,-32.51,;11.61,-30.2,;10.27,-30.97,;11.61,-28.67,;12.94,-27.9,;14.27,-28.66,;15.61,-27.89,;15.6,-26.35,;16.93,-25.58,;14.27,-25.58,;12.93,-26.35,;40.93,-30.17,;40.93,-28.63,;42.27,-30.94,;43.6,-30.16,;43.6,-28.62,;44.93,-27.85,;44.93,-26.32,;46.26,-25.54,;46.26,-24.01,;44.92,-23.23,;47.59,-23.24,;44.93,-30.93,;44.93,-32.47,;46.26,-30.16,;47.59,-30.93,;47.59,-32.47,;48.93,-33.24,;50.26,-32.46,;51.6,-33.23,;51.6,-34.77,;50.27,-35.54,;48.94,-34.77,;48.93,-30.16,;50.26,-30.93,;48.93,-28.62,)| Show InChI InChI=1S/C62H82N18O14/c1-33(2)24-44(55(88)71-42(18-11-23-69-61(67)68)54(87)72-43(52(66)85)26-34-12-5-3-6-13-34)78-62(94)80-79-60(93)45(27-35-14-7-4-8-15-35)74-59(92)49(32-81)77-58(91)48(30-51(65)84)76-56(89)46(28-37-31-70-41-17-10-9-16-39(37)41)75-57(90)47(29-50(64)83)73-53(86)40(63)25-36-19-21-38(82)22-20-36/h3-10,12-17,19-22,31,33,40,42-49,70,81-82H,11,18,23-30,32,63H2,1-2H3,(H2,64,83)(H2,65,84)(H2,66,85)(H,71,88)(H,72,87)(H,73,86)(H,74,92)(H,75,90)(H,76,89)(H,77,91)(H,79,93)(H4,67,68,69)(H2,78,80,94)/t40-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human KISS1R expressed in CHO cell membranes |
J Med Chem 56: 8298-307 (2013)
Article DOI: 10.1021/jm401056w
BindingDB Entry DOI: 10.7270/Q25M675S |
More data for this
Ligand-Target Pair | |
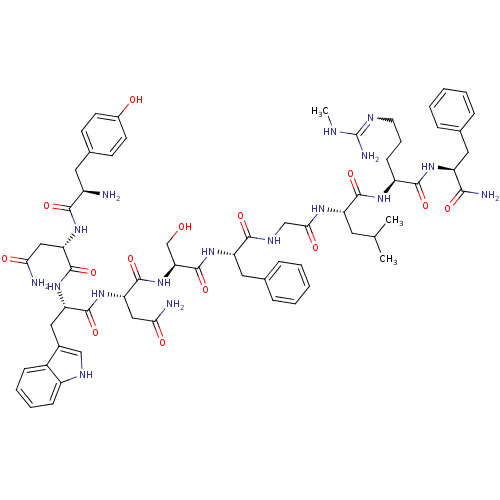
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50392401
(CHEMBL2151642)Show SMILES CNC(N)=NCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,w:4.4| Show InChI InChI=1S/C64H85N17O14/c1-35(2)25-46(59(91)75-44(19-12-24-71-64(69)70-3)58(90)76-45(55(68)87)27-36-13-6-4-7-14-36)74-54(86)33-73-57(89)47(28-37-15-8-5-9-16-37)78-63(95)51(34-82)81-62(94)50(31-53(67)85)80-60(92)48(29-39-32-72-43-18-11-10-17-41(39)43)79-61(93)49(30-52(66)84)77-56(88)42(65)26-38-20-22-40(83)23-21-38/h4-11,13-18,20-23,32,35,42,44-51,72,82-83H,12,19,24-31,33-34,65H2,1-3H3,(H2,66,84)(H2,67,85)(H2,68,87)(H,73,89)(H,74,86)(H,75,91)(H,76,90)(H,77,88)(H,78,95)(H,79,93)(H,80,92)(H,81,94)(H3,69,70,71)/t42-,44+,45+,46+,47+,48+,49+,50+,51+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human KISS1R expressed in CHO cell membranes |
J Med Chem 56: 8298-307 (2013)
Article DOI: 10.1021/jm401056w
BindingDB Entry DOI: 10.7270/Q25M675S |
More data for this
Ligand-Target Pair | |
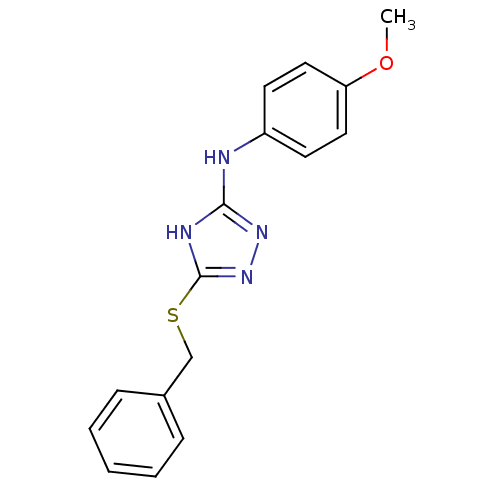
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17388
(1,2,4-Triazole Compound, 46 | 5-(benzylsulfanyl)-N...)Show InChI InChI=1S/C16H16N4OS/c1-21-14-9-7-13(8-10-14)17-15-18-16(20-19-15)22-11-12-5-3-2-4-6-12/h2-10H,11H2,1H3,(H2,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409020
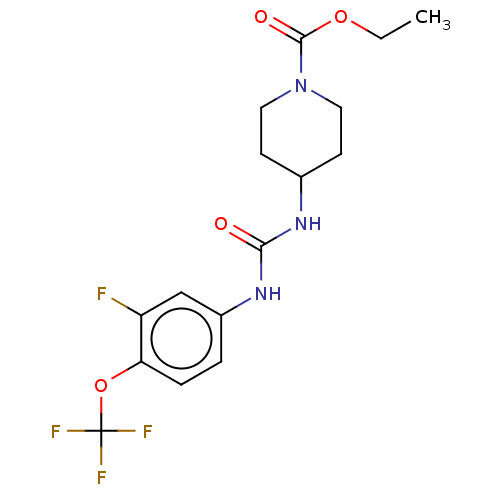
(US10377744, Compound No. 40 | US11123311, Compound...)Show SMILES CCOC(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)c(F)c1 Show InChI InChI=1S/C16H19F4N3O4/c1-2-26-15(25)23-7-5-10(6-8-23)21-14(24)22-11-3-4-13(12(17)9-11)27-16(18,19)20/h3-4,9-10H,2,5-8H2,1H3,(H2,21,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81H7M |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50415863
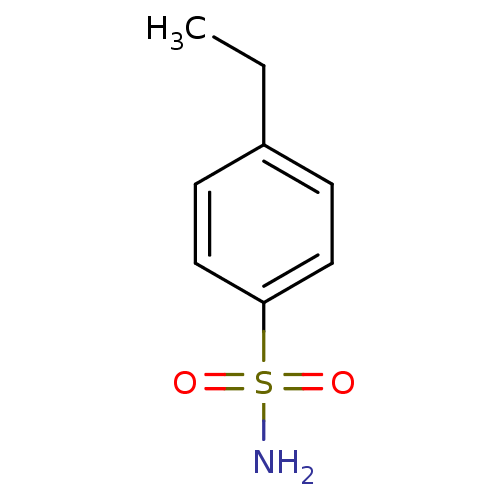
(CHEMBL358263)Show InChI InChI=1S/C8H11NO2S/c1-2-7-3-5-8(6-4-7)12(9,10)11/h3-6H,2H2,1H3,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 2 |
Bioorg Med Chem Lett 21: 141-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.050
BindingDB Entry DOI: 10.7270/Q2K938T6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50415863

(CHEMBL358263)Show InChI InChI=1S/C8H11NO2S/c1-2-7-3-5-8(6-4-7)12(9,10)11/h3-6H,2H2,1H3,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 2 |
Bioorg Med Chem Lett 21: 141-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.050
BindingDB Entry DOI: 10.7270/Q2K938T6 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409009
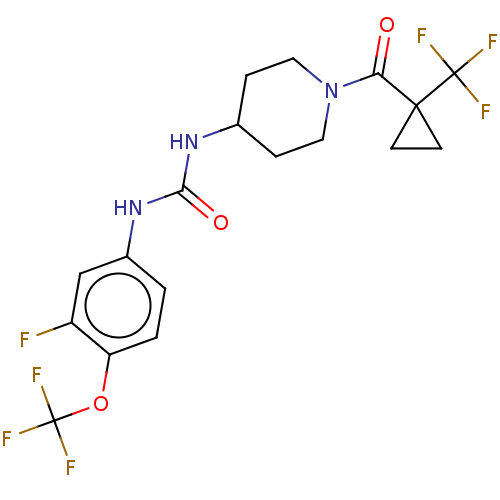
(US10377744, Compound No. 30 | US11123311, Compound...)Show SMILES Fc1cc(NC(=O)NC2CCN(CC2)C(=O)C2(CC2)C(F)(F)F)ccc1OC(F)(F)F Show InChI InChI=1S/C18H18F7N3O3/c19-12-9-11(1-2-13(12)31-18(23,24)25)27-15(30)26-10-3-7-28(8-4-10)14(29)16(5-6-16)17(20,21)22/h1-2,9-10H,3-8H2,(H2,26,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81H7M |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM517699
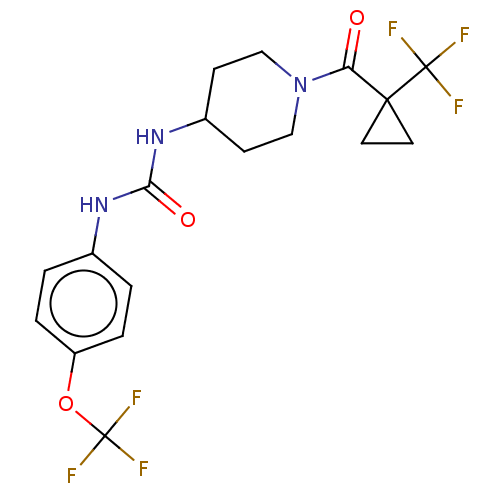
(US11123311, Compound 28)Show SMILES FC(F)(F)Oc1ccc(NC(=O)NC2CCN(CC2)C(=O)C2(CC2)C(F)(F)F)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81H7M |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409005
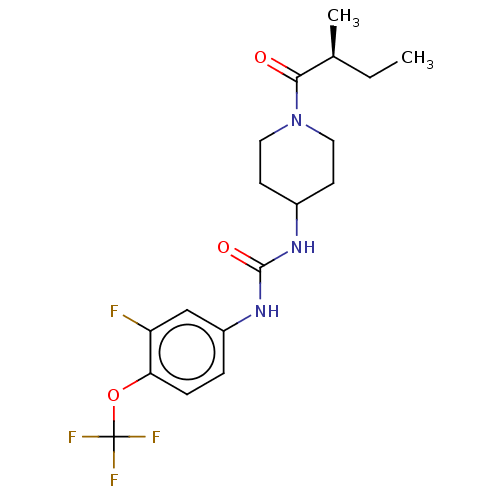
(US10377744, Compound No. 26 | US11123311, Compound...)Show SMILES CC[C@H](C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)c(F)c1 |r| Show InChI InChI=1S/C18H23F4N3O3/c1-3-11(2)16(26)25-8-6-12(7-9-25)23-17(27)24-13-4-5-15(14(19)10-13)28-18(20,21)22/h4-5,10-12H,3,6-9H2,1-2H3,(H2,23,24,27)/t11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81H7M |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM13934

(Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)C(C)(C)C |r| Show InChI InChI=1S/C38H52N6O7/c1-37(2,3)31(41-35(48)50-7)33(46)40-29(22-25-14-10-9-11-15-25)30(45)24-44(43-34(47)32(38(4,5)6)42-36(49)51-8)23-26-17-19-27(20-18-26)28-16-12-13-21-39-28/h9-21,29-32,45H,22-24H2,1-8H3,(H,40,46)(H,41,48)(H,42,49)(H,43,47)/t29-,30-,31+,32+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem 16: 1299-308 (2008)
Article DOI: 10.1016/j.bmc.2007.10.062
BindingDB Entry DOI: 10.7270/Q2RJ4N8X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50457085

(CHEMBL4203542)Show SMILES Cc1nc2cc(N3CCN(CCn4c5nc(N)n6nc(nc6c5n(C)c4=O)-c4ccco4)CC3)c(F)cc2o1 Show InChI InChI=1S/C25H25FN10O3/c1-14-28-16-13-17(15(26)12-19(16)39-14)34-8-5-33(6-9-34)7-10-35-22-20(32(2)25(35)37)23-29-21(18-4-3-11-38-18)31-36(23)24(27)30-22/h3-4,11-13H,5-10H2,1-2H3,(H2,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at rat A2A receptor assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 mins followed by CGS-21680 addition m... |
ACS Med Chem Lett 8: 835-840 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00175
BindingDB Entry DOI: 10.7270/Q2571FMD |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Rattus norvegicus) | BDBM50442965

(CHEMBL3086282)Show SMILES CNC(N)=NCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,w:4.4| Show InChI InChI=1S/C61H79N15O12/c1-35(2)27-46(55(83)68-44(19-12-26-67-60(65)66-3)54(82)69-45(52(64)80)30-36-13-6-4-7-14-36)74-61(88)76-75-59(87)48(31-37-15-8-5-9-16-37)71-58(86)50(34-77)73-57(85)49(33-51(63)79)72-56(84)47(32-39-20-23-40-17-10-11-18-41(40)28-39)70-53(81)43(62)29-38-21-24-42(78)25-22-38/h4-11,13-18,20-25,28,35,43-50,77-78H,12,19,26-27,29-34,62H2,1-3H3,(H2,63,79)(H2,64,80)(H,68,83)(H,69,82)(H,70,81)(H,71,86)(H,72,84)(H,73,85)(H,75,87)(H3,65,66,67)(H2,74,76,88)/t43-,44+,45+,46+,47+,48+,49+,50+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to rat KISS1R |
J Med Chem 56: 8298-307 (2013)
Article DOI: 10.1021/jm401056w
BindingDB Entry DOI: 10.7270/Q25M675S |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50392405
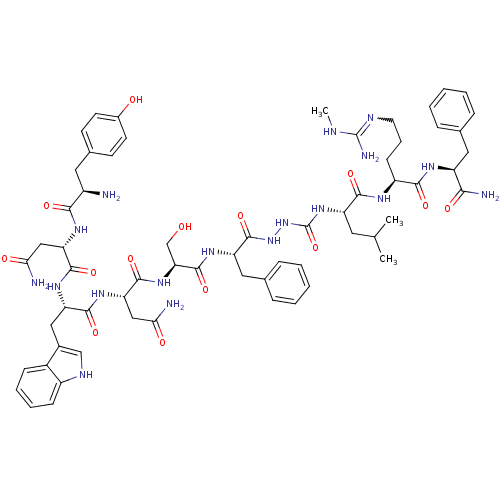
(CHEMBL2151646)Show SMILES CNC(N)=NCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,w:4.4| Show InChI InChI=1S/C63H84N18O14/c1-34(2)25-45(56(89)72-43(19-12-24-70-62(68)69-3)55(88)73-44(53(67)86)27-35-13-6-4-7-14-35)79-63(95)81-80-61(94)46(28-36-15-8-5-9-16-36)75-60(93)50(33-82)78-59(92)49(31-52(66)85)77-57(90)47(29-38-32-71-42-18-11-10-17-40(38)42)76-58(91)48(30-51(65)84)74-54(87)41(64)26-37-20-22-39(83)23-21-37/h4-11,13-18,20-23,32,34,41,43-50,71,82-83H,12,19,24-31,33,64H2,1-3H3,(H2,65,84)(H2,66,85)(H2,67,86)(H,72,89)(H,73,88)(H,74,87)(H,75,93)(H,76,91)(H,77,90)(H,78,92)(H,80,94)(H3,68,69,70)(H2,79,81,95)/t41-,43+,44+,45+,46+,47+,48+,49+,50+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human KISS1R expressed in CHO cell membranes |
J Med Chem 56: 8298-307 (2013)
Article DOI: 10.1021/jm401056w
BindingDB Entry DOI: 10.7270/Q25M675S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50194488

(6-[4'-(H-Dmt)-aminobutyl]-3-[3'-(H-Dmt)-aminopropy...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)NCCCCc1[nH]c(=O)c(CCCNC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)nc1C Show InChI InChI=1S/C34H48N6O5/c1-19-13-24(41)14-20(2)26(19)17-28(35)32(43)37-11-7-6-9-30-23(5)39-31(34(45)40-30)10-8-12-38-33(44)29(36)18-27-21(3)15-25(42)16-22(27)4/h13-16,28-29,41-42H,6-12,17-18,35-36H2,1-5H3,(H,37,43)(H,38,44)(H,40,45)/t28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat synaptosomes P2 fraction |
Bioorg Med Chem Lett 16: 5793-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.079
BindingDB Entry DOI: 10.7270/Q26Q1WWH |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50442963
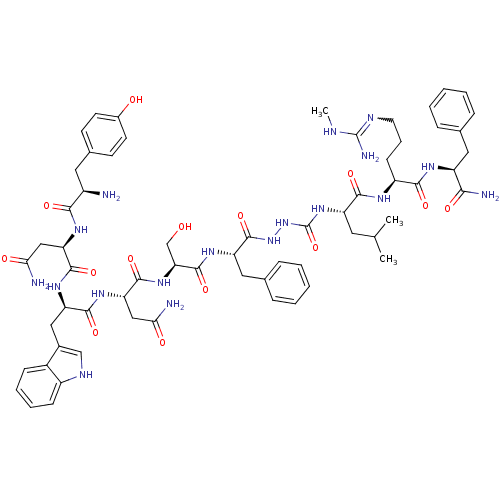
(CHEMBL3085804)Show SMILES CNC(N)=NCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,w:4.4| Show InChI InChI=1S/C63H84N18O14/c1-34(2)25-45(56(89)72-43(19-12-24-70-62(68)69-3)55(88)73-44(53(67)86)27-35-13-6-4-7-14-35)79-63(95)81-80-61(94)46(28-36-15-8-5-9-16-36)75-60(93)50(33-82)78-59(92)49(31-52(66)85)77-57(90)47(29-38-32-71-42-18-11-10-17-40(38)42)76-58(91)48(30-51(65)84)74-54(87)41(64)26-37-20-22-39(83)23-21-37/h4-11,13-18,20-23,32,34,41,43-50,71,82-83H,12,19,24-31,33,64H2,1-3H3,(H2,65,84)(H2,66,85)(H2,67,86)(H,72,89)(H,73,88)(H,74,87)(H,75,93)(H,76,91)(H,77,90)(H,78,92)(H,80,94)(H3,68,69,70)(H2,79,81,95)/t41-,43+,44+,45+,46+,47-,48-,49+,50+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human KISS1R expressed in CHO cell membranes |
J Med Chem 56: 8298-307 (2013)
Article DOI: 10.1021/jm401056w
BindingDB Entry DOI: 10.7270/Q25M675S |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50045517

(CHEMBL3314209)Show SMILES CNC(=N)NCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](Cc1ccncc1)NC(=O)[C@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human KISS1R transfected in CHO cells |
J Med Chem 57: 6105-15 (2014)
Article DOI: 10.1021/jm5005489
BindingDB Entry DOI: 10.7270/Q2SF2XSG |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50442962
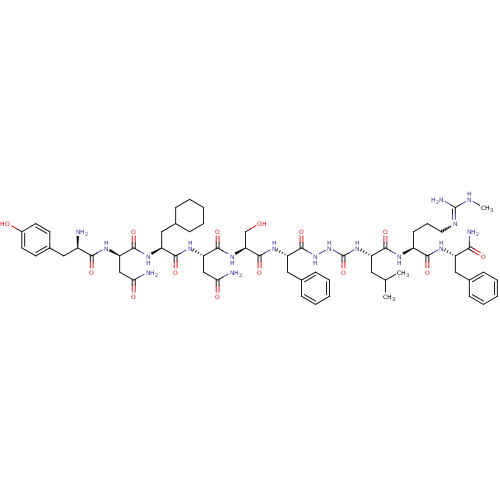
(CHEMBL3087929)Show SMILES CNC(N)=NCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,w:4.4| Show InChI InChI=1S/C61H89N17O14/c1-34(2)26-43(54(86)69-41(20-13-25-68-60(66)67-3)53(85)70-42(51(65)83)28-35-14-7-4-8-15-35)76-61(92)78-77-59(91)45(30-37-18-11-6-12-19-37)73-58(90)48(33-79)75-57(89)47(32-50(64)82)74-55(87)44(29-36-16-9-5-10-17-36)72-56(88)46(31-49(63)81)71-52(84)40(62)27-38-21-23-39(80)24-22-38/h4,6-8,11-12,14-15,18-19,21-24,34,36,40-48,79-80H,5,9-10,13,16-17,20,25-33,62H2,1-3H3,(H2,63,81)(H2,64,82)(H2,65,83)(H,69,86)(H,70,85)(H,71,84)(H,72,88)(H,73,90)(H,74,87)(H,75,89)(H,77,91)(H3,66,67,68)(H2,76,78,92)/t40-,41+,42+,43+,44+,45+,46-,47+,48+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human KISS1R expressed in CHO cell membranes |
J Med Chem 56: 8298-307 (2013)
Article DOI: 10.1021/jm401056w
BindingDB Entry DOI: 10.7270/Q25M675S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data