

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50269559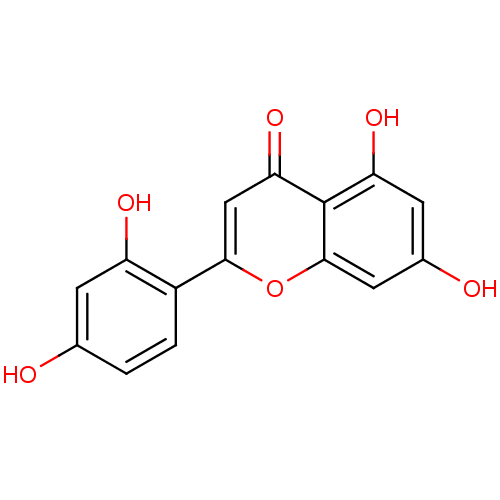 (2-(2,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.610 | -53.5 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
Gyeongsang National University | Assay Description Mushroom tyrosinase using either L-DOPA or L-tyrosine as substrate. In spectrophotometric experiments, enzyme activity was monitored by dopachrome f... | J Enzyme Inhib Med Chem 23: 922-30 (2008) Article DOI: 10.1080/14756360701810207 BindingDB Entry DOI: 10.7270/Q2P849G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134709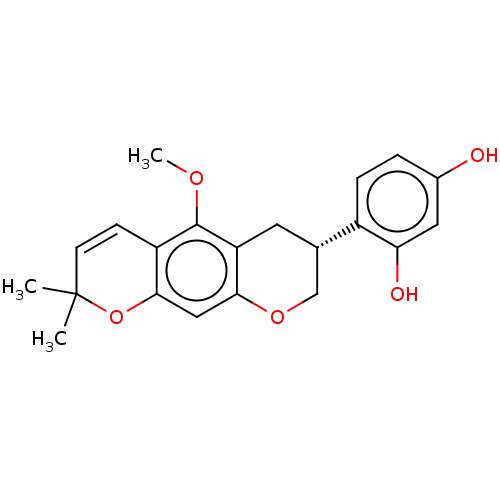 (CHEMBL3747057) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Time dependent inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate measured every 30 secs by Morrison and Walsh... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134709 (CHEMBL3747057) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate preincubated for 5 to 60 mins by Lineweaver-Bur... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50343137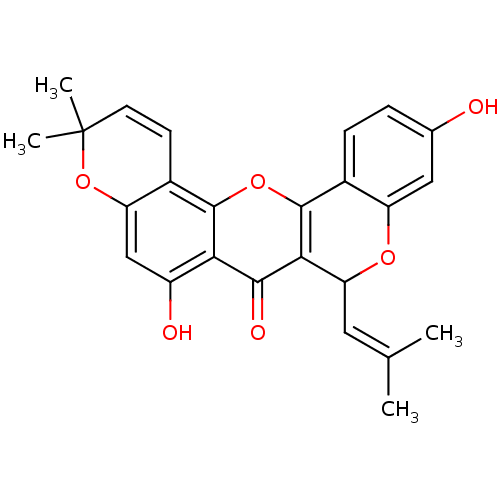 (CHEMBL1770313 | Cyclomorusin | Cycolmorusin, 2) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 46.4 | -42.6 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
Gyeongsang National University | Assay Description Mushroom tyrosinase using either L-DOPA or L-tyrosine as substrate. In spectrophotometric experiments, enzyme activity was monitored by dopachrome f... | J Enzyme Inhib Med Chem 23: 922-30 (2008) Article DOI: 10.1080/14756360701810207 BindingDB Entry DOI: 10.7270/Q2P849G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254430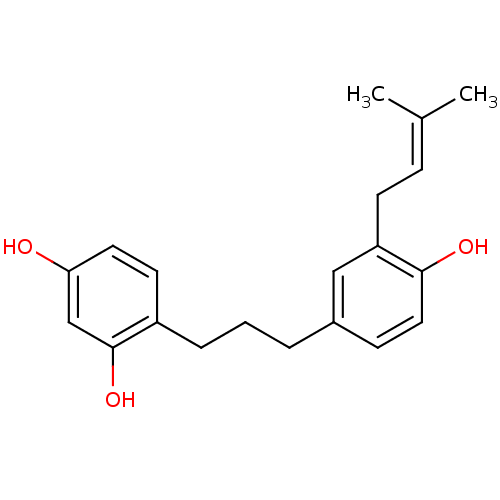 (CHEMBL468906 | broussonin C) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | 48.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase by kinetic based assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM91591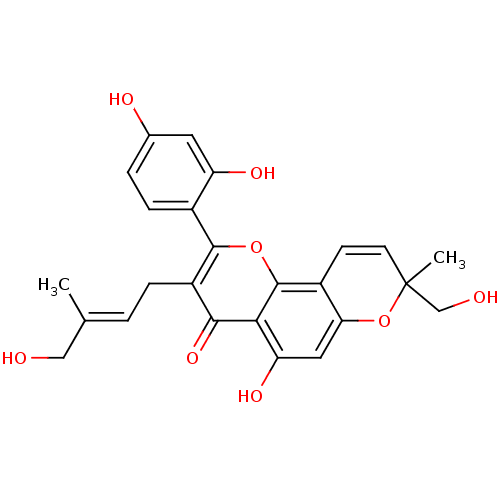 (Mormin, 1) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 49.2 | -42.4 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
Gyeongsang National University | Assay Description Mushroom tyrosinase using either L-DOPA or L-tyrosine as substrate. In spectrophotometric experiments, enzyme activity was monitored by dopachrome f... | J Enzyme Inhib Med Chem 23: 922-30 (2008) Article DOI: 10.1080/14756360701810207 BindingDB Entry DOI: 10.7270/Q2P849G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50278443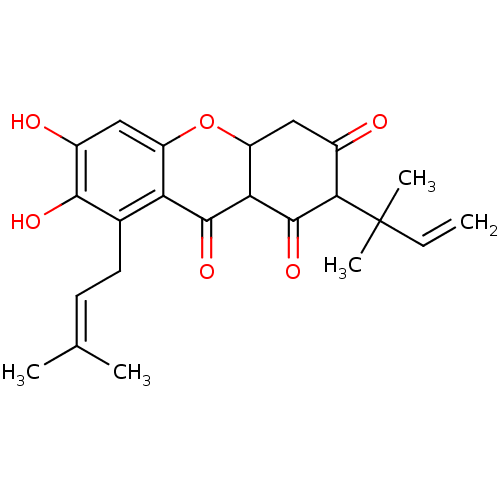 (1,3,6,7-tetrahydroxy-2-(3-methylbut-2-enyl)-8-(2-m...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium perfringens neuraminidase by Lineweaver-Burke plot and Dixon plot | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50242015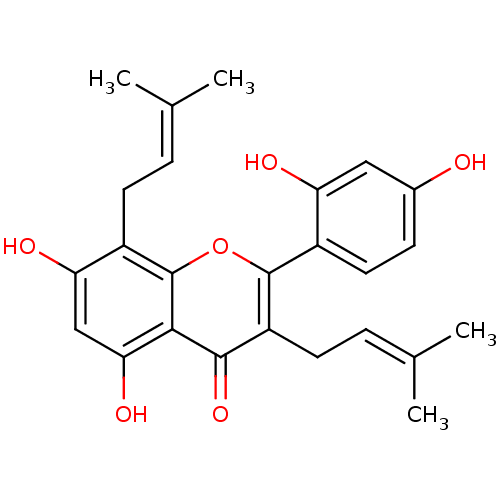 (CHEMBL518543 | Kuwanon C, 4 | kuwanon C) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 80.5 | -41.2 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
Gyeongsang National University | Assay Description Mushroom tyrosinase using either L-DOPA or L-tyrosine as substrate. In spectrophotometric experiments, enzyme activity was monitored by dopachrome f... | J Enzyme Inhib Med Chem 23: 922-30 (2008) Article DOI: 10.1080/14756360701810207 BindingDB Entry DOI: 10.7270/Q2P849G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50175018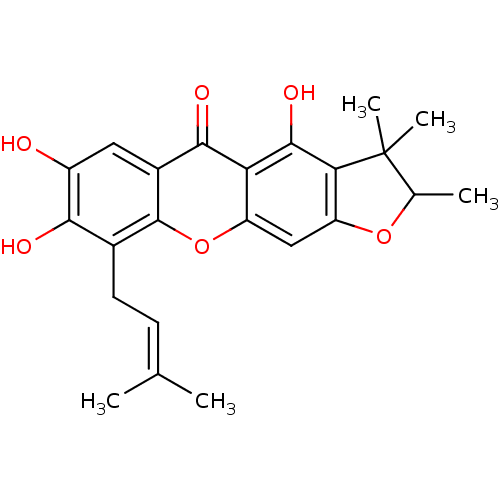 (4,7,8-trihydroxy-2,3,3-trimethyl-9-(3-methylbut-2-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium perfringens neuraminidase by Lineweaver-Burke plot and Dixon plot | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50378020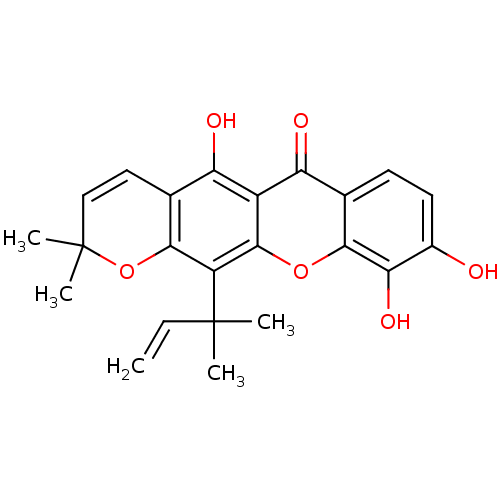 (MACLURAXANTHONE) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium perfringens neuraminidase by Lineweaver-Burke plot and Dixon plot | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50355214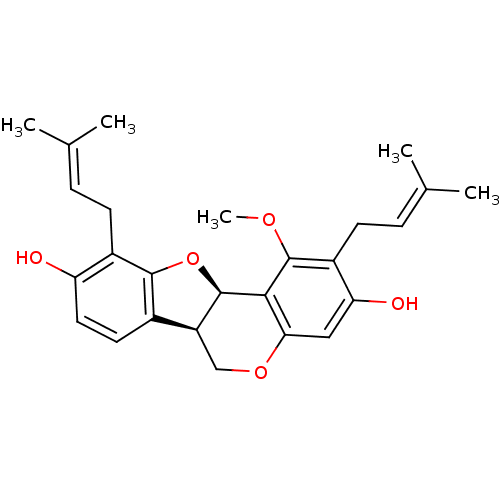 (CHEMBL401566) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate by Linewe... | Bioorg Med Chem Lett 21: 6100-3 (2011) Article DOI: 10.1016/j.bmcl.2011.08.046 BindingDB Entry DOI: 10.7270/Q2C53M8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50278443 (1,3,6,7-tetrahydroxy-2-(3-methylbut-2-enyl)-8-(2-m...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Apparent binding affinity at Clostridium perfringens neuraminidase by fluorimetry | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50442400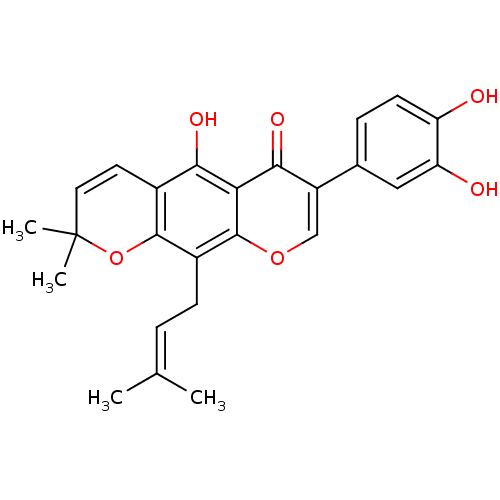 (AURICULASIN) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134709 (CHEMBL3747057) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate preincubated for 5 to 60 mins by Lineweaver-Burk and ... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50278346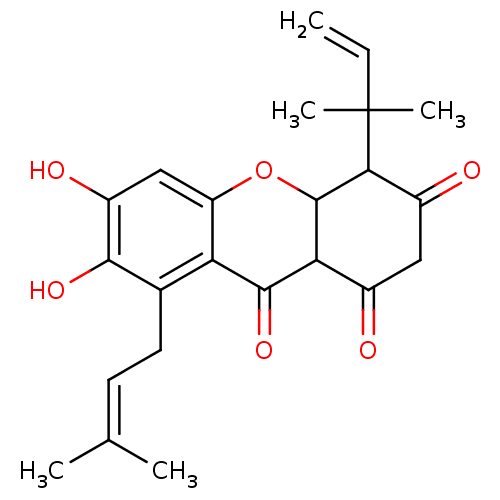 (CHEMBL470844 | Cudratricusxanthone) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium perfringens neuraminidase by Lineweaver-Burke plot and Dixon plot | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50175013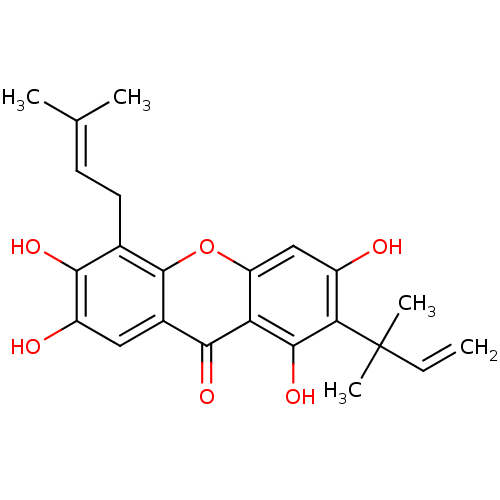 (1,3,6,7-tetrahydroxy-5-(3-methylbut-2-enyl)-2-(2-m...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium perfringens neuraminidase by Lineweaver-Burke plot and Dixon plot | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50175019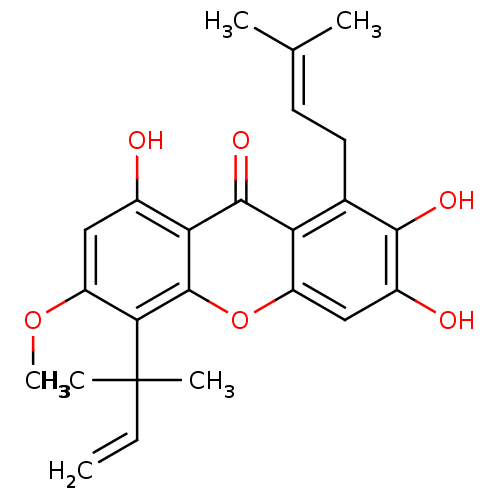 (2,3,8-trihydroxy-6-methoxy-1-(3-methylbut-2-enyl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium perfringens neuraminidase by Lineweaver-Burke plot and Dixon plot | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50325675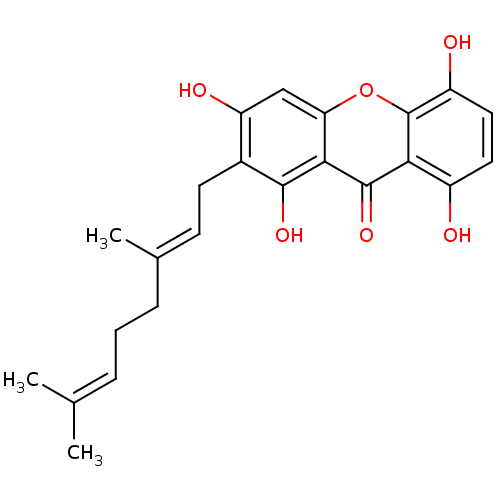 (CHEMBL480158 | smeathxanthone A) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium perfringens neuraminidase by fluorometry | Bioorg Med Chem 18: 6258-64 (2010) Article DOI: 10.1016/j.bmc.2010.07.033 BindingDB Entry DOI: 10.7270/Q2BK1CK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134710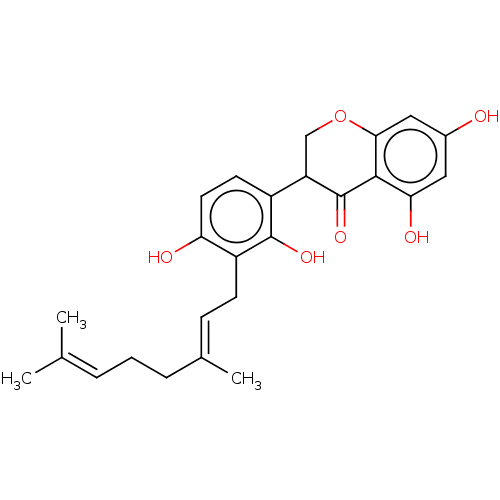 (CHEMBL3745886) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Time dependent inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate measured every 30 secs by Morrison and Walsh... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50355212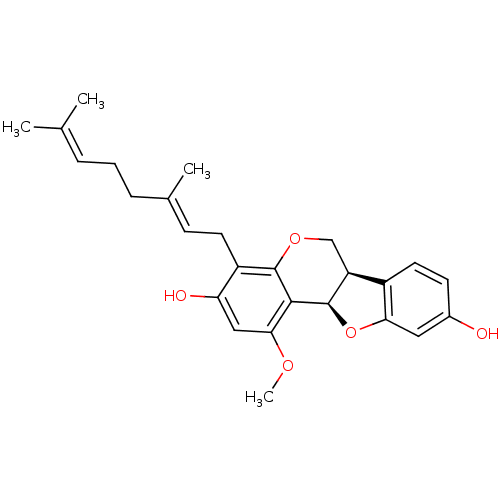 (CHEMBL1835717) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate by Linewe... | Bioorg Med Chem Lett 21: 6100-3 (2011) Article DOI: 10.1016/j.bmcl.2011.08.046 BindingDB Entry DOI: 10.7270/Q2C53M8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50355210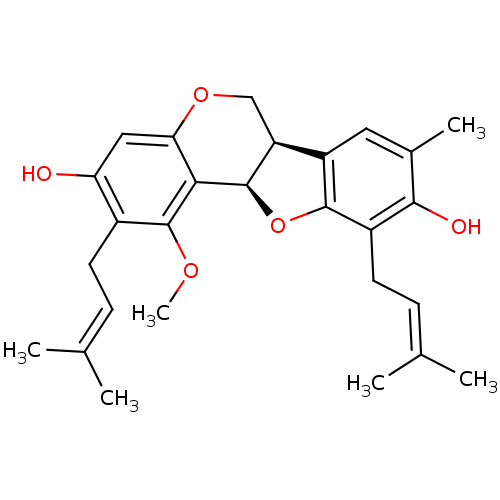 (CHEMBL1835715) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate by Linewe... | Bioorg Med Chem Lett 21: 6100-3 (2011) Article DOI: 10.1016/j.bmcl.2011.08.046 BindingDB Entry DOI: 10.7270/Q2C53M8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50251001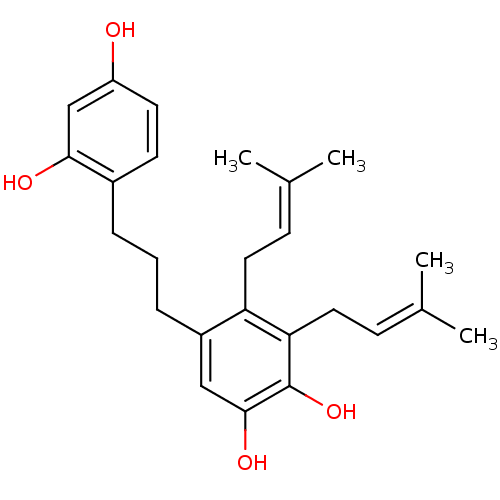 (CHEMBL457677 | Kazinol F) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase by kinetic based assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50366237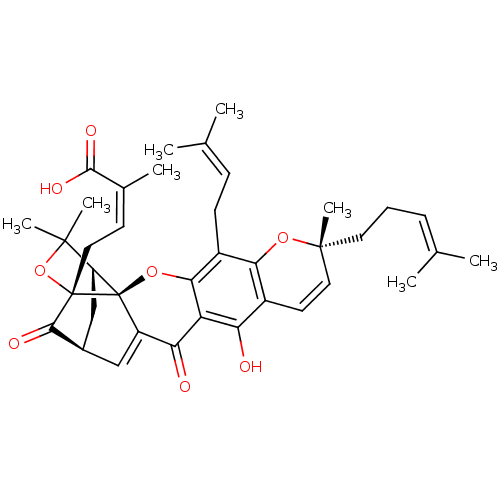 (GAMBOGIC ACID) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate by Dixon plot analysis | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50278346 (CHEMBL470844 | Cudratricusxanthone) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Apparent binding affinity at Clostridium perfringens neuraminidase by fluorimetry | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254430 (CHEMBL468906 | broussonin C) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate by Lineweaver-Burk plot based kinetic assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254430 (CHEMBL468906 | broussonin C) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using as L-DOPA substrate by Lineweaver-Burk plot based kinetic assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256793 (CHEMBL4095800) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate by Dixon plot analysis | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256807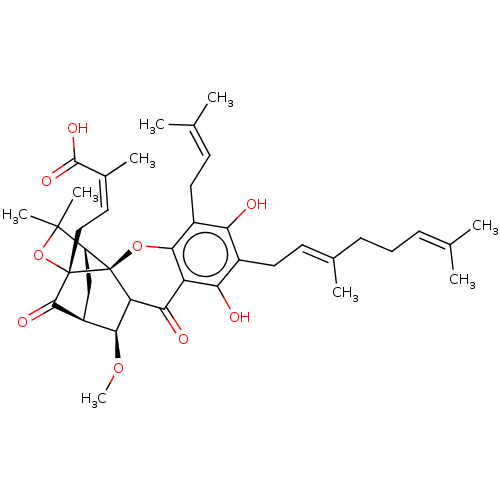 (CHEMBL4069859) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 60 mins followed by ... | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trans-sialidase (Trypanosoma cruzi) | BDBM84698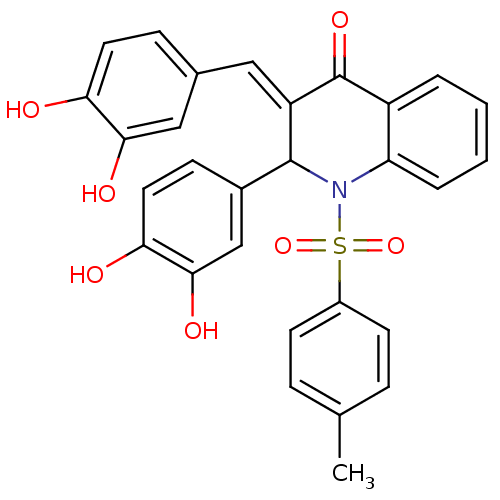 (Quinolinone derivative, 9) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 400 | -37.7 | 600 | n/a | n/a | n/a | n/a | 7.6 | 35 |
University of British Columbia | Assay Description Inhibition assay using trans-sialidase, a membrane-associated protein. | Chembiochem 10: 2475-9 (2009) Article DOI: 10.1002/cbic.200900108 BindingDB Entry DOI: 10.7270/Q2MG7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50251001 (CHEMBL457677 | Kazinol F) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate by Lineweaver-Burk plot based kinetic assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50175019 (2,3,8-trihydroxy-6-methoxy-1-(3-methylbut-2-enyl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 436 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Apparent binding affinity at Clostridium perfringens neuraminidase by fluorimetry | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50378020 (MACLURAXANTHONE) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | 477 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Apparent binding affinity at Clostridium perfringens neuraminidase by fluorimetry | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495308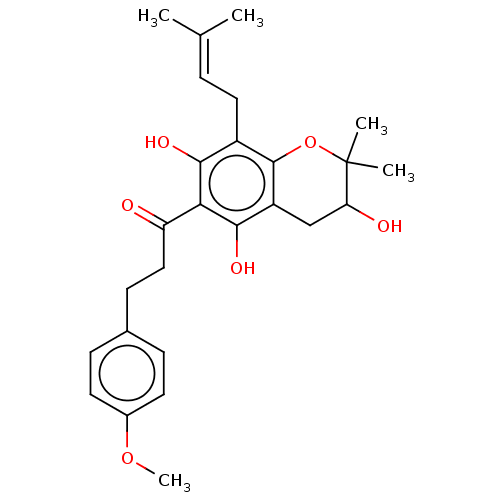 (CHEMBL3103546) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins with substrate followed by enzyme addition measu... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50366237 (GAMBOGIC ACID) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 499 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 60 mins followed by ... | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50175018 (4,7,8-trihydroxy-2,3,3-trimethyl-9-(3-methylbut-2-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Apparent binding affinity at Clostridium perfringens neuraminidase by fluorimetry | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50175013 (1,3,6,7-tetrahydroxy-5-(3-methylbut-2-enyl)-2-(2-m...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 568 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Apparent binding affinity at Clostridium perfringens neuraminidase by fluorimetry | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495305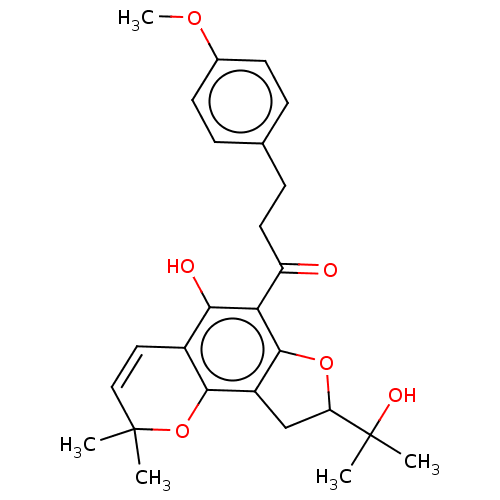 (CHEMBL3103544) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins with substrate followed by enzyme addition measu... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50442881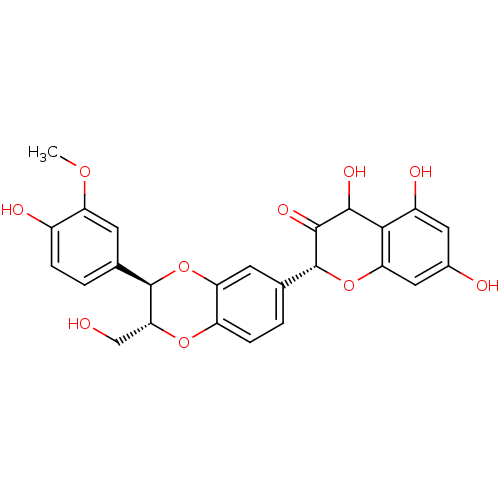 (SILYBIN A) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Mixed type inhibition of monophenolase activity of mushroom tyrosinase assessed as reduction in dopachrome formation using L-Tyrosine substrate by UV... | Bioorg Med Chem 27: 2499-2507 (2019) Article DOI: 10.1016/j.bmc.2019.03.013 BindingDB Entry DOI: 10.7270/Q2PV6PTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50442881 (SILYBIN A) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase assessed as inhibition constant for free enzyme-inhibitor complex by measuring reduction ... | Bioorg Med Chem 27: 2499-2507 (2019) Article DOI: 10.1016/j.bmc.2019.03.013 BindingDB Entry DOI: 10.7270/Q2PV6PTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50251001 (CHEMBL457677 | Kazinol F) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using as L-DOPA substrate by Lineweaver-Burk plot based kinetic assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50481948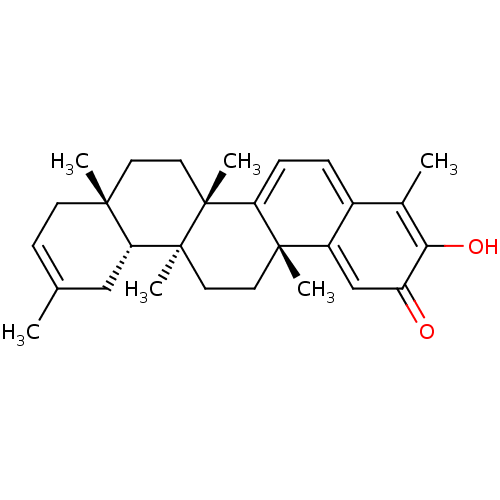 (Iguesterin | acs.jmedchem.1c00409_ST.224) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of 3C-like protease of SARS coronavirus assessed as concentration of FRET peptide for 60 mins by dixon plot | Bioorg Med Chem Lett 20: 1873-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.152 BindingDB Entry DOI: 10.7270/Q28P63CF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50241453 (1,3,6,7-tetrahydroxy-2,8-bis(3-methylbut-2-enyl)-9...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium perfringens neuraminidase by fluorometry | Bioorg Med Chem 18: 6258-64 (2010) Article DOI: 10.1016/j.bmc.2010.07.033 BindingDB Entry DOI: 10.7270/Q2BK1CK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495309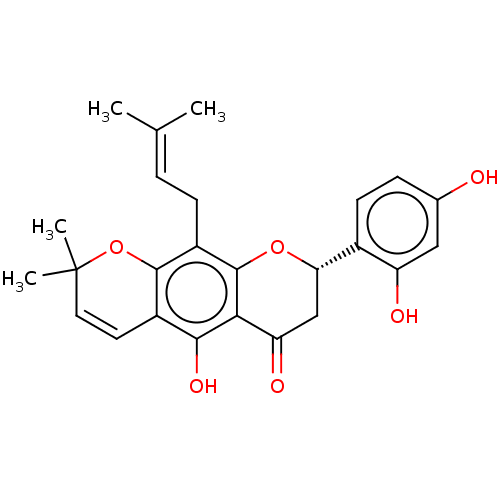 (Flemichin D) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins with substrate followed by enzyme addition measu... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254431 (5'-(2-methylbut-3-en-2-yl)-6''-(3-methylbut-2-enyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase by kinetic based assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50278394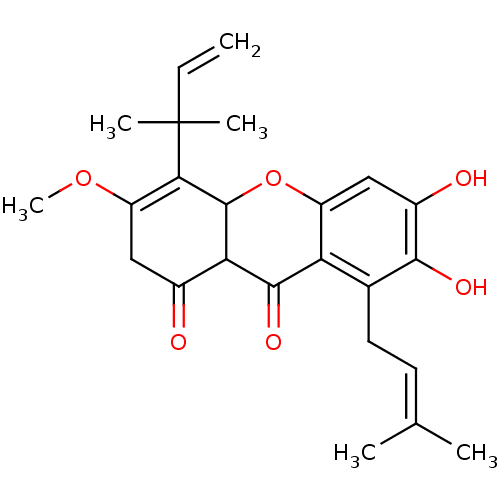 (CHEMBL469813 | Cudratricusxanthone F) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium perfringens neuraminidase by Lineweaver-Burke plot and Dixon plot | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50442401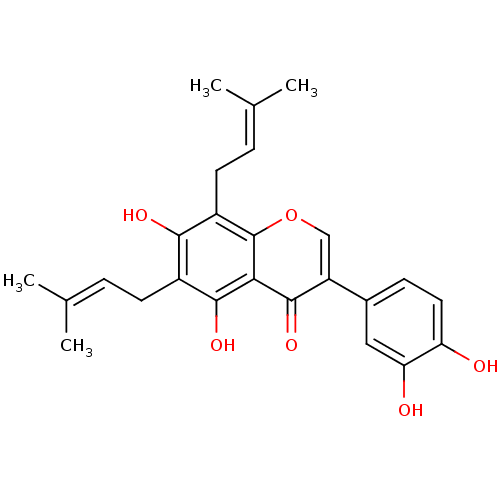 (CHEMBL2442947) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50526629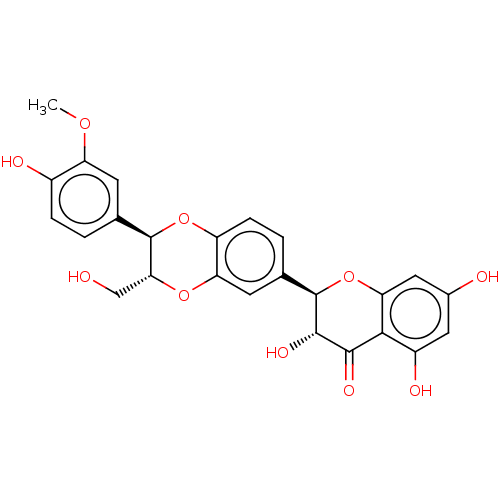 (ISOSILYBIN A) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase assessed as inhibition constant for free enzyme-inhibitor complex by measuring reduction ... | Bioorg Med Chem 27: 2499-2507 (2019) Article DOI: 10.1016/j.bmc.2019.03.013 BindingDB Entry DOI: 10.7270/Q2PV6PTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50526629 (ISOSILYBIN A) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Mixed type inhibition of monophenolase activity of mushroom tyrosinase assessed as reduction in dopachrome formation using L-Tyrosine substrate by UV... | Bioorg Med Chem 27: 2499-2507 (2019) Article DOI: 10.1016/j.bmc.2019.03.013 BindingDB Entry DOI: 10.7270/Q2PV6PTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50380205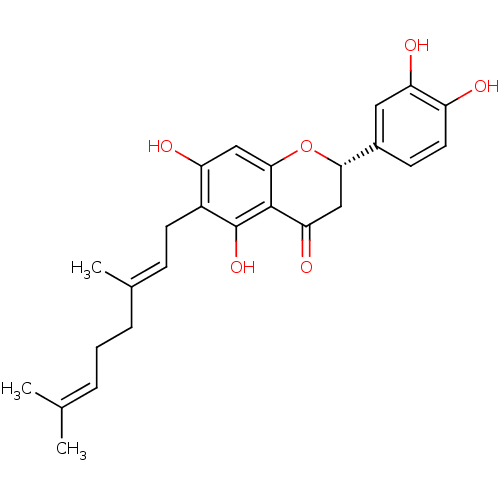 (NYMPHAEOL A) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Mixed type inhibition of equine BChE using butyrylthiocholine iodide as substrate by Lineweaver-Burk double-reciprocal-plot and dixon plot analysis | Bioorg Med Chem 20: 2595-602 (2012) Article DOI: 10.1016/j.bmc.2012.02.044 BindingDB Entry DOI: 10.7270/Q22808MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50355211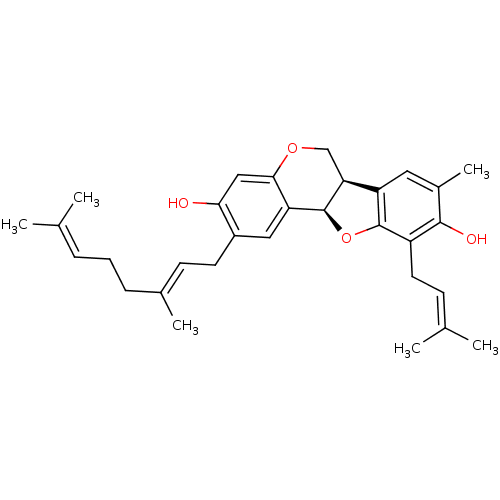 (CHEMBL1835716) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate by Linewe... | Bioorg Med Chem Lett 21: 6100-3 (2011) Article DOI: 10.1016/j.bmcl.2011.08.046 BindingDB Entry DOI: 10.7270/Q2C53M8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 611 total ) | Next | Last >> |