

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394718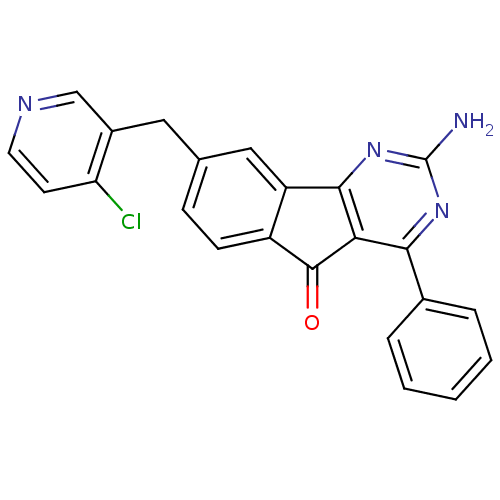 (CHEMBL2165801) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394722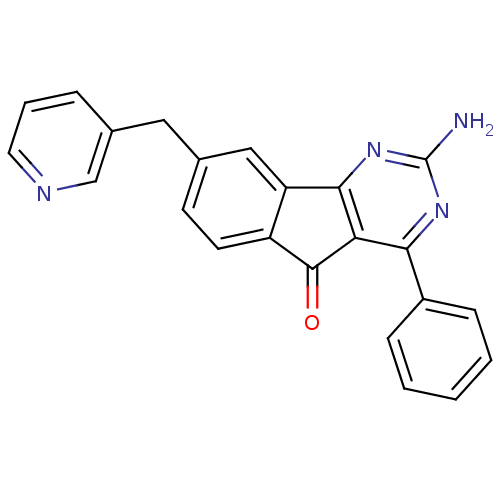 (CHEMBL2165807) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394717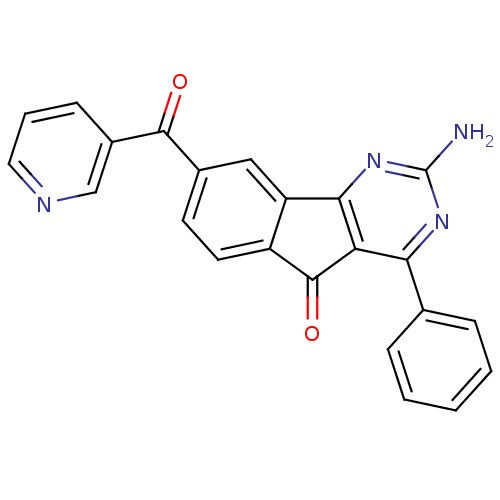 (CHEMBL2165802) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394718 (CHEMBL2165801) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394719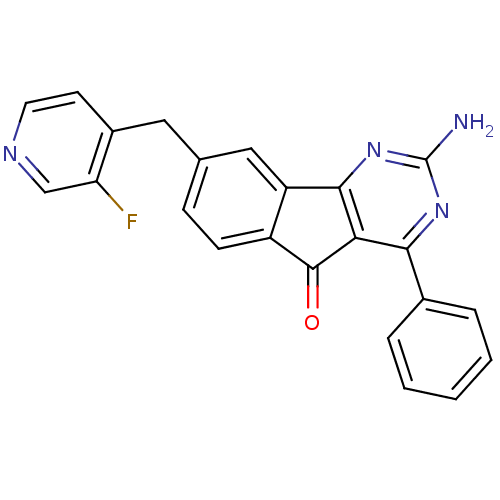 (CHEMBL2165800) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394720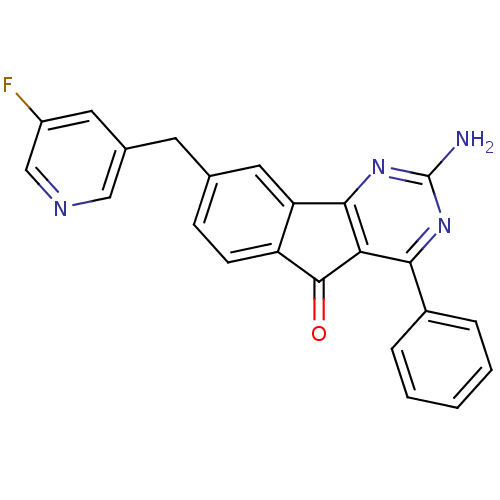 (CHEMBL2165799) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394721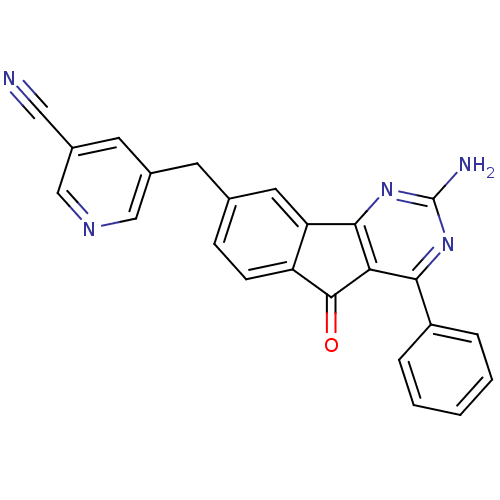 (CHEMBL2165808) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394716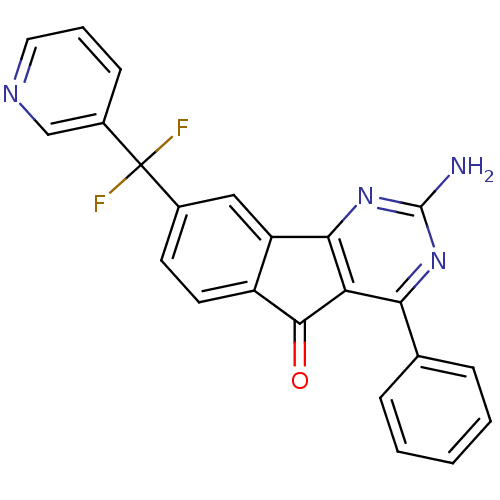 (CHEMBL2165803) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394717 (CHEMBL2165802) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394715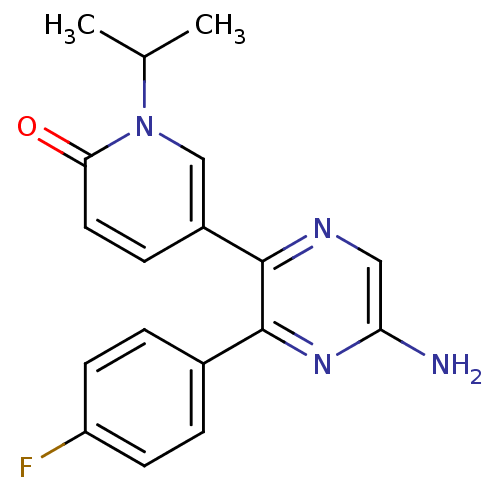 (CHEMBL2165804) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394722 (CHEMBL2165807) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394719 (CHEMBL2165800) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394723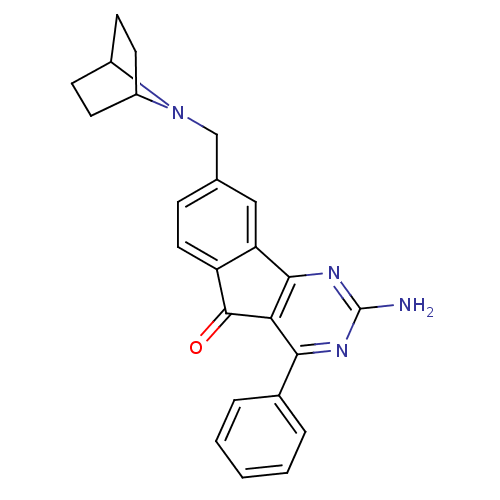 (CHEMBL2165806) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50330987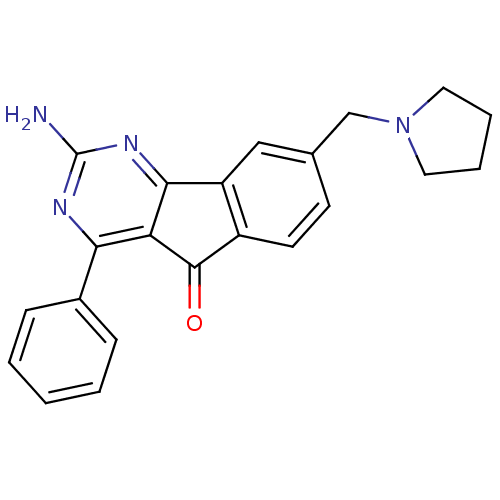 (2-amino-4-phenyl-8-(pyrrolidin-1-ylmethyl)-5H-inde...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50330987 (2-amino-4-phenyl-8-(pyrrolidin-1-ylmethyl)-5H-inde...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at adenosine A2A receptor | J Med Chem 53: 8104-15 (2010) Checked by Author Article DOI: 10.1021/jm100971t BindingDB Entry DOI: 10.7270/Q2VT1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394721 (CHEMBL2165808) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394720 (CHEMBL2165799) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316887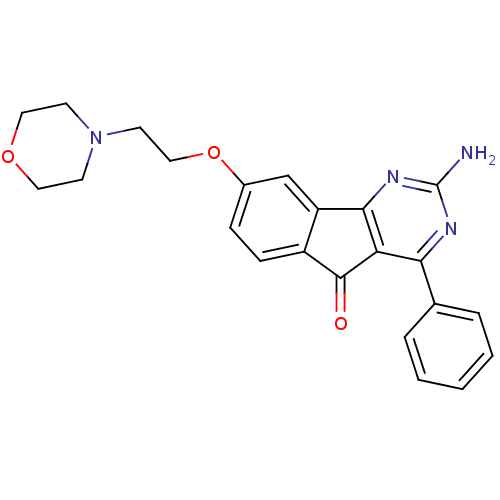 (2-amino-8-(2-morpholinoethoxy)-4-phenyl-5H-indeno[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316876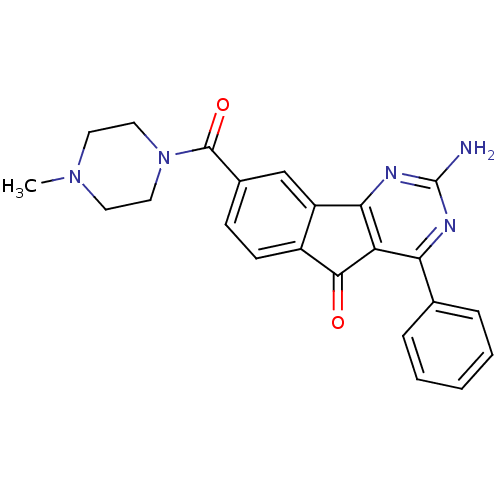 (2-amino-8-(4-methylpiperazine-1-carbonyl)-4-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394715 (CHEMBL2165804) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394724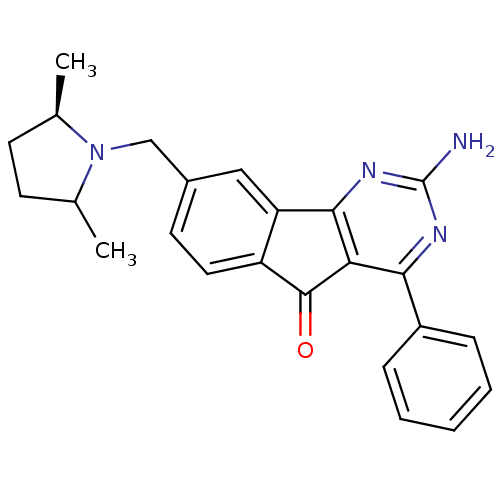 (CHEMBL2165805) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50330987 (2-amino-4-phenyl-8-(pyrrolidin-1-ylmethyl)-5H-inde...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at adenosine A1 receptor | J Med Chem 53: 8104-15 (2010) Checked by Author Article DOI: 10.1021/jm100971t BindingDB Entry DOI: 10.7270/Q2VT1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50330987 (2-amino-4-phenyl-8-(pyrrolidin-1-ylmethyl)-5H-inde...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394716 (CHEMBL2165803) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394724 (CHEMBL2165805) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50316887 (2-amino-8-(2-morpholinoethoxy)-4-phenyl-5H-indeno[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 48.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50316876 (2-amino-8-(4-methylpiperazine-1-carbonyl)-4-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 58.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394723 (CHEMBL2165806) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248583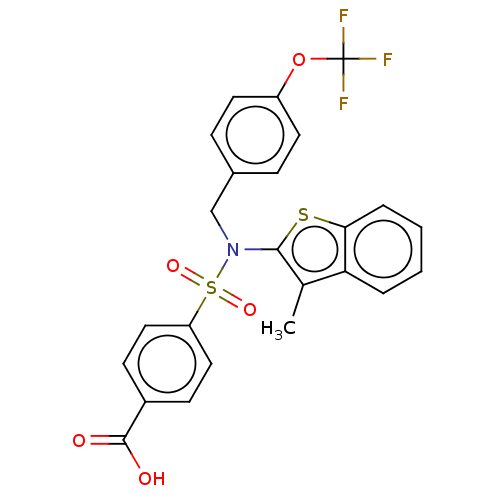 (US9434711, 496) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.183 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica, N.V. US Patent | Assay Description For patch clamp experiments, HEK293 cells are stably transfected with canine TRPM8 and cultured in DMEM supplemented with 10% fetal bovine serum, 100... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM50036555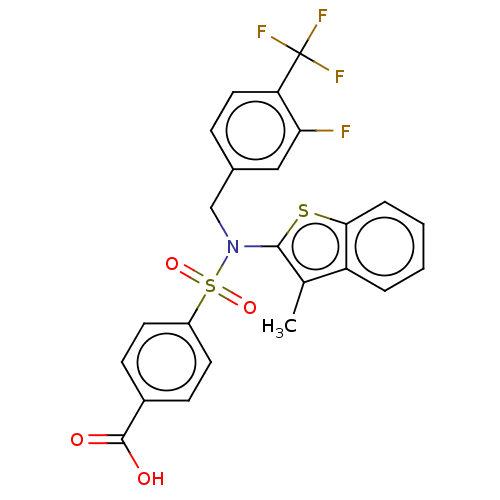 (CHEMBL3353610 | US9434711, 497) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248583 (US9434711, 496) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.554 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica, N.V. US Patent | Assay Description For patch clamp experiments, HEK293 cells are stably transfected with canine TRPM8 and cultured in DMEM supplemented with 10% fetal bovine serum, 100... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248576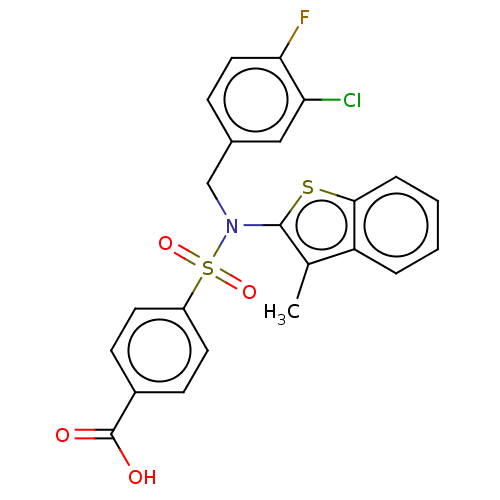 (US9434711, 489) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248370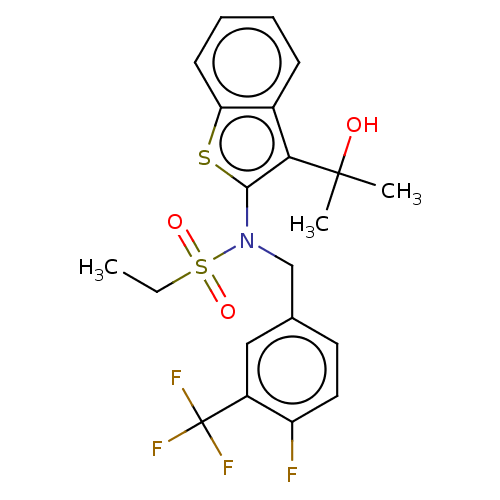 (US9434711, 227) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248838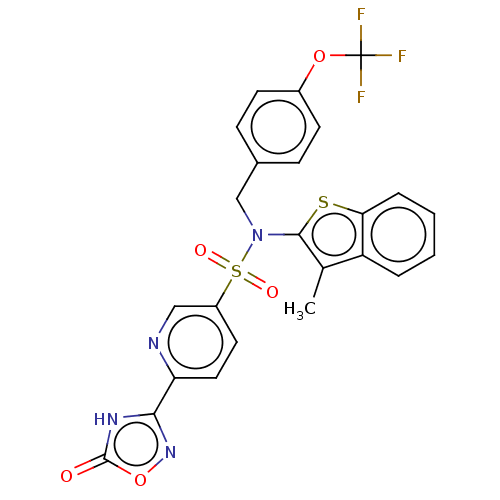 (US9434711, 808) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248599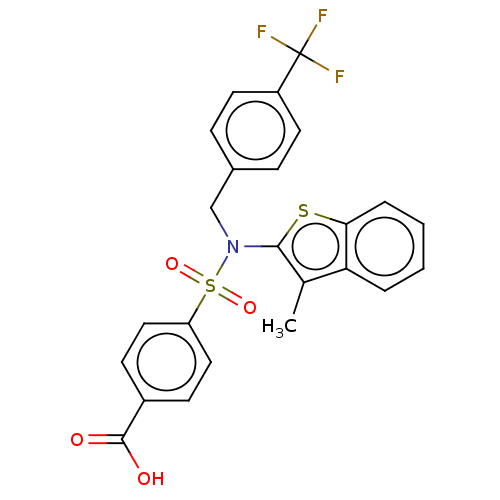 (US9434711, 518) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248480 (US9434711, 361) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248426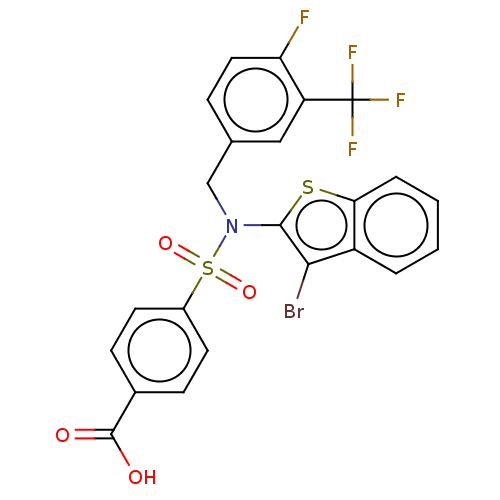 (US9434711, 292) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248580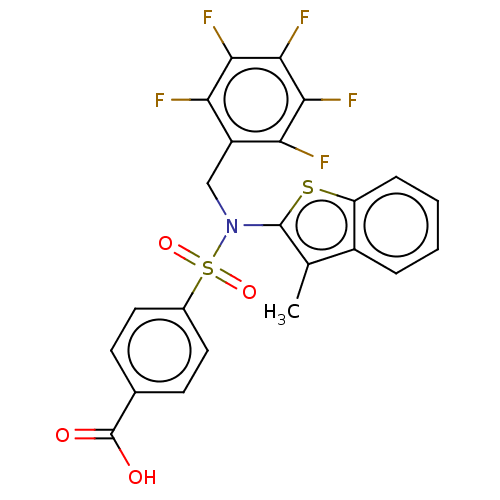 (US9434711, 493) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM50426573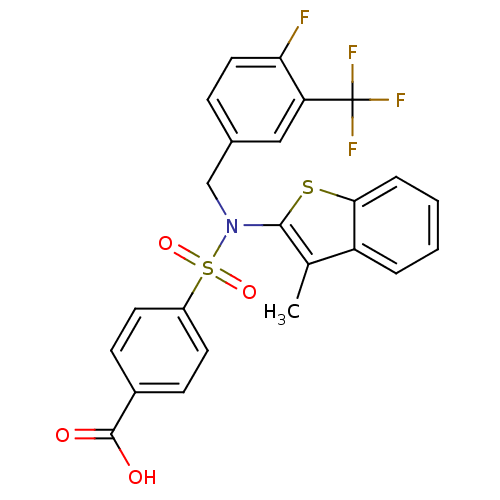 (CHEMBL2324349 | US9434711, 306) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248583 (US9434711, 496) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248581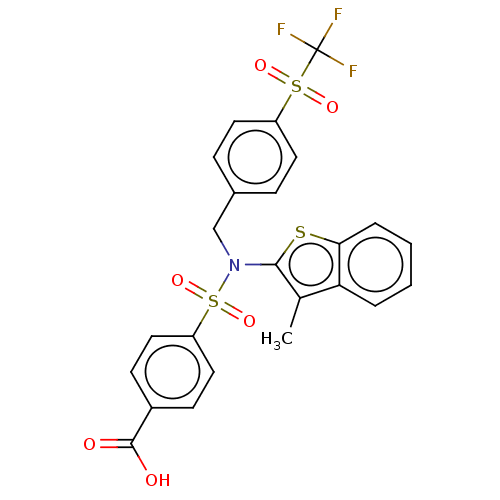 (US9434711, 494) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248789 (US9434711, 755) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248389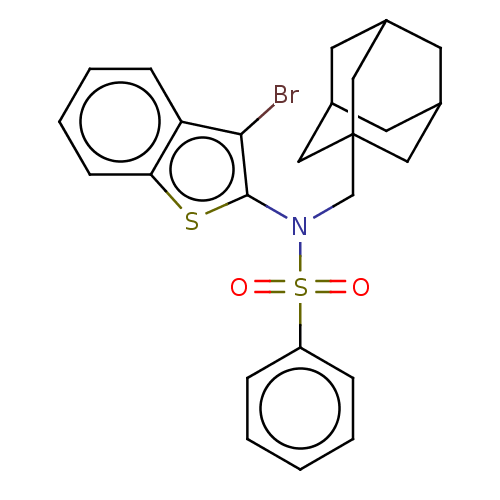 (US9434711, 249) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248367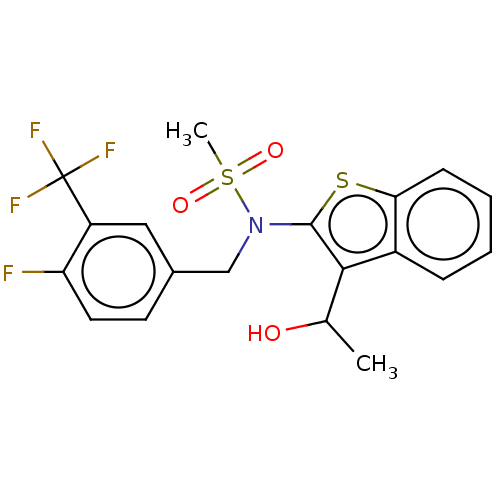 (US9434711, 224) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248458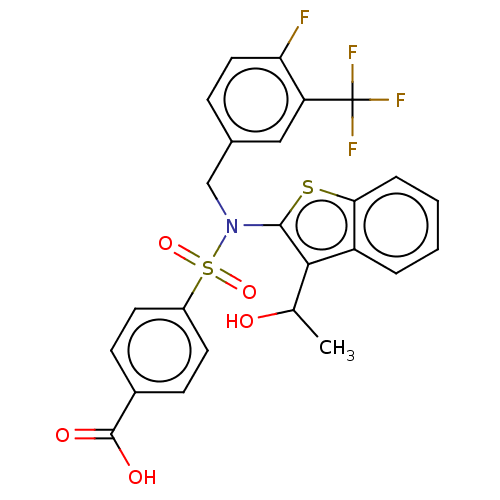 (US9434711, 335) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248840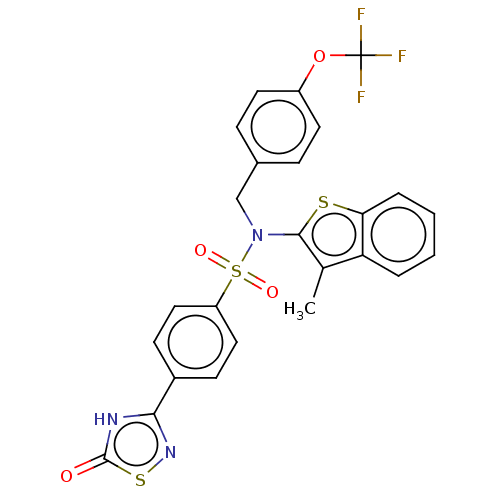 (US9434711, 810) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248414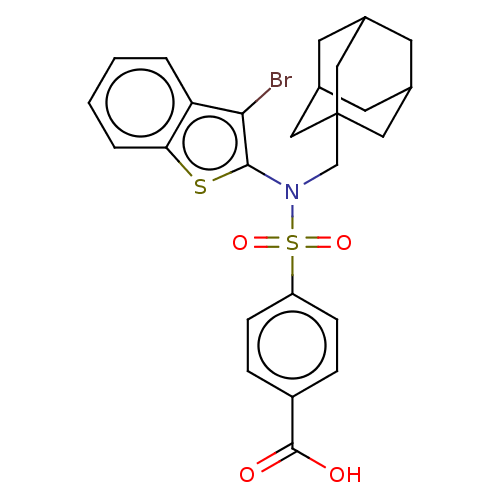 (US9434711, 278) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248578 (US9434711, 491) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248613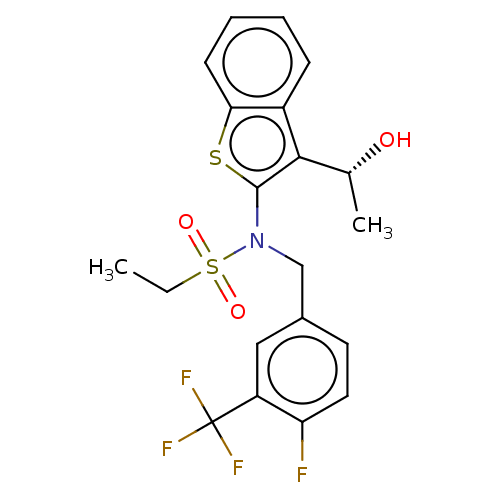 (US9434711, 535) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248607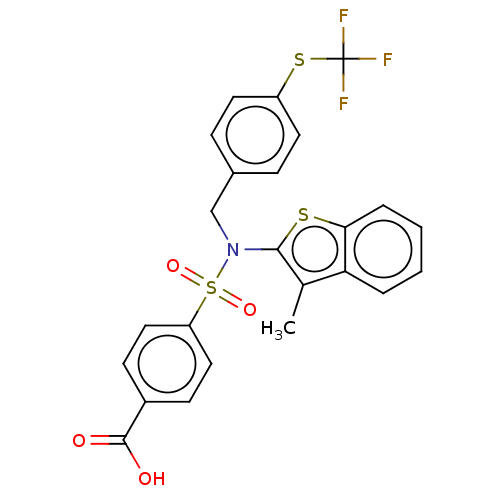 (US9434711, 526) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 737 total ) | Next | Last >> |