

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50483329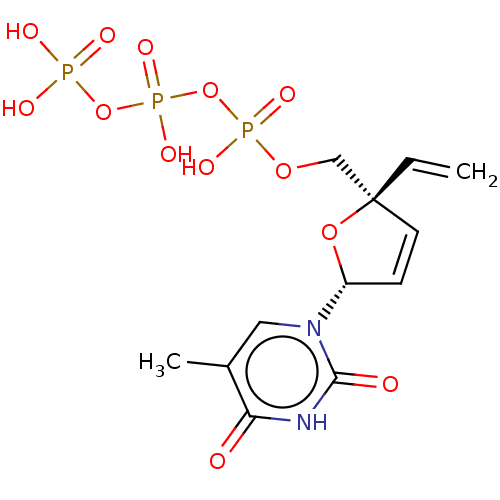 (CHEMBL1650290) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of HIV1 Reverse transcriptase P119S/T165A mutant | Antimicrob Agents Chemother 53: 4640-6 (2009) Article DOI: 10.1128/AAC.00686-09 BindingDB Entry DOI: 10.7270/Q2MC92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50483329 (CHEMBL1650290) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 Reverse transcriptase | Antimicrob Agents Chemother 53: 4640-6 (2009) Article DOI: 10.1128/AAC.00686-09 BindingDB Entry DOI: 10.7270/Q2MC92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50483329 (CHEMBL1650290) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of HIV1 Reverse transcriptase T165A mutant | Antimicrob Agents Chemother 53: 4640-6 (2009) Article DOI: 10.1128/AAC.00686-09 BindingDB Entry DOI: 10.7270/Q2MC92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50065258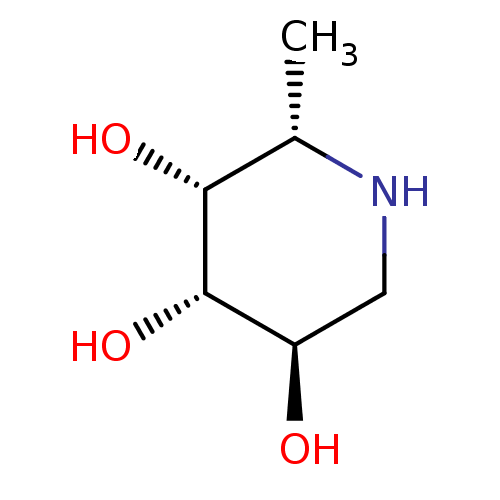 ((2S,3R,4S,5R)-2-Methyl-piperidine-3,4,5-triol | (2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibitory activity measured against alpha-L-fucosidase of bovine epididymis by colorimetric assay using the D-glucose oxidase-peroxidase method | J Med Chem 41: 2565-71 (1998) Article DOI: 10.1021/jm970836l BindingDB Entry DOI: 10.7270/Q25D8SHK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50065257 ((2R,3R,4R,5R,6S)-2-Hydroxymethyl-6-methyl-piperidi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibitory activity measured against alpha-L-fucosidase of bovine epididymis by colorimetric assay using the D-glucose oxidase-peroxidase method | J Med Chem 41: 2565-71 (1998) Article DOI: 10.1021/jm970836l BindingDB Entry DOI: 10.7270/Q25D8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50483329 (CHEMBL1650290) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of HIV1 Reverse transcriptase P119S mutant | Antimicrob Agents Chemother 53: 4640-6 (2009) Article DOI: 10.1128/AAC.00686-09 BindingDB Entry DOI: 10.7270/Q2MC92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50483329 (CHEMBL1650290) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of HIV1 Reverse transcriptase M184V mutant | Antimicrob Agents Chemother 53: 4640-6 (2009) Article DOI: 10.1128/AAC.00686-09 BindingDB Entry DOI: 10.7270/Q2MC92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50370655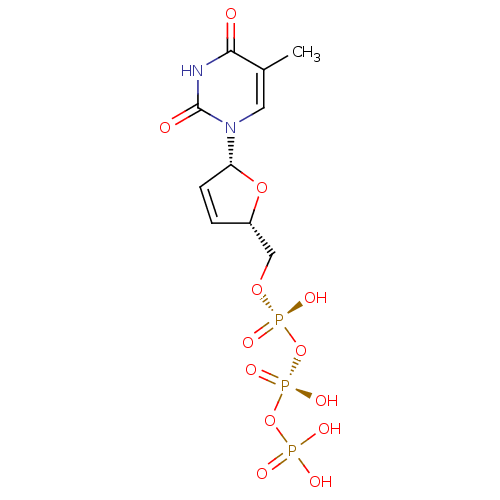 (CHEMBL485652) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of HIV1 Reverse transcriptase T165A mutant | Antimicrob Agents Chemother 53: 4640-6 (2009) Article DOI: 10.1128/AAC.00686-09 BindingDB Entry DOI: 10.7270/Q2MC92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50370655 (CHEMBL485652) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of HIV1 Reverse transcriptase P119S/T165A mutant | Antimicrob Agents Chemother 53: 4640-6 (2009) Article DOI: 10.1128/AAC.00686-09 BindingDB Entry DOI: 10.7270/Q2MC92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50408432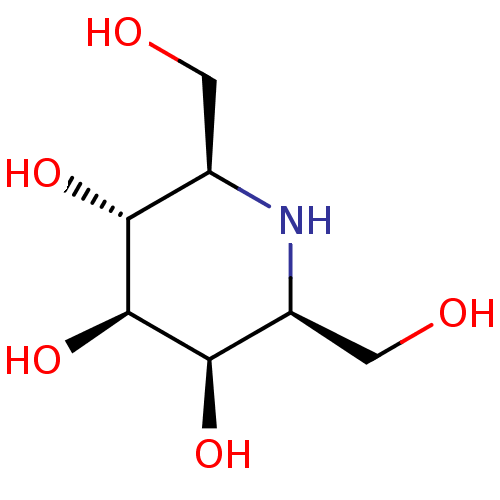 (CHEMBL2115215) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Compound was tested for binding affinity against alpha-glucosidase | J Med Chem 41: 2565-71 (1998) Article DOI: 10.1021/jm970836l BindingDB Entry DOI: 10.7270/Q25D8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50370655 (CHEMBL485652) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of HIV1 Reverse transcriptase P119S/M184V mutant | Antimicrob Agents Chemother 53: 4640-6 (2009) Article DOI: 10.1128/AAC.00686-09 BindingDB Entry DOI: 10.7270/Q2MC92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50370655 (CHEMBL485652) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 Reverse transcriptase | Antimicrob Agents Chemother 53: 4640-6 (2009) Article DOI: 10.1128/AAC.00686-09 BindingDB Entry DOI: 10.7270/Q2MC92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50483329 (CHEMBL1650290) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of HIV1 Reverse transcriptase P119S/M184V mutant | Antimicrob Agents Chemother 53: 4640-6 (2009) Article DOI: 10.1128/AAC.00686-09 BindingDB Entry DOI: 10.7270/Q2MC92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50370655 (CHEMBL485652) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of HIV1 Reverse transcriptase P119S mutant | Antimicrob Agents Chemother 53: 4640-6 (2009) Article DOI: 10.1128/AAC.00686-09 BindingDB Entry DOI: 10.7270/Q2MC92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50483329 (CHEMBL1650290) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of HIV1 Reverse transcriptase P119S/T165A/M184V mutant | Antimicrob Agents Chemother 53: 4640-6 (2009) Article DOI: 10.1128/AAC.00686-09 BindingDB Entry DOI: 10.7270/Q2MC92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50370655 (CHEMBL485652) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of HIV1 Reverse transcriptase M184V mutant | Antimicrob Agents Chemother 53: 4640-6 (2009) Article DOI: 10.1128/AAC.00686-09 BindingDB Entry DOI: 10.7270/Q2MC92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50370655 (CHEMBL485652) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of HIV1 Reverse transcriptase P119S/T165A/M184V mutant | Antimicrob Agents Chemother 53: 4640-6 (2009) Article DOI: 10.1128/AAC.00686-09 BindingDB Entry DOI: 10.7270/Q2MC92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50065259 ((2R,3R,4R,5R)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibitory activity measured against alpha-L-fucosidase of bovine epididymis by colorimetric assay using the D-glucose oxidase-peroxidase method | J Med Chem 41: 2565-71 (1998) Article DOI: 10.1021/jm970836l BindingDB Entry DOI: 10.7270/Q25D8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50408431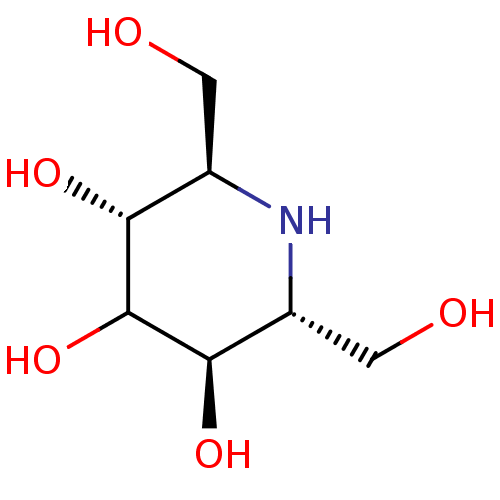 (CHEMBL2114210) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Compound was tested for binding affinity against alpha-glucosidase | J Med Chem 41: 2565-71 (1998) Article DOI: 10.1021/jm970836l BindingDB Entry DOI: 10.7270/Q25D8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50065255 ((R)-2,6-Bis-hydroxymethyl-piperidine-3,4,5-triol |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Compound was tested for binding affinity against alpha-glucosidase | J Med Chem 41: 2565-71 (1998) Article DOI: 10.1021/jm970836l BindingDB Entry DOI: 10.7270/Q25D8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50108112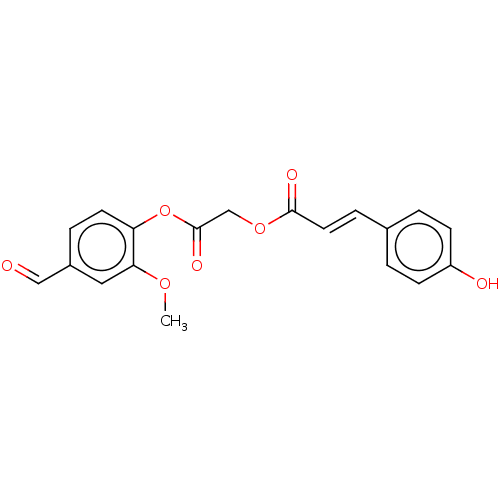 (CHEMBL3601648) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kongju National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as enzyme-inhibitor dissociation constant preincubated for 10 mins f... | Bioorg Med Chem 23: 5870-80 (2015) Article DOI: 10.1016/j.bmc.2015.06.068 BindingDB Entry DOI: 10.7270/Q20P11SM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Rattus norvegicus) | BDBM50016703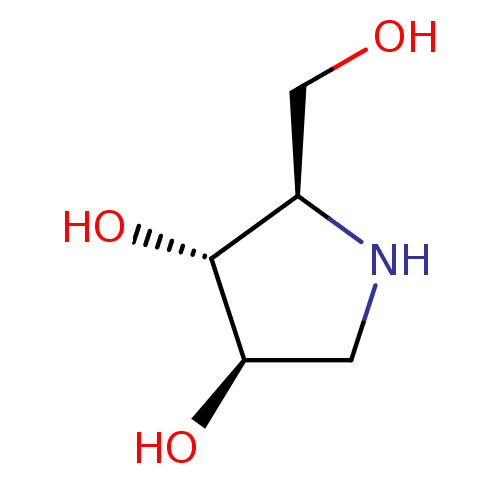 (2-Hydroxymethyl-pyrrolidine-3,4-diol | BDBM5003148...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Competitive Inhibitory activity against Golgi Alpha-mannosidase II | J Med Chem 38: 2349-56 (1995) BindingDB Entry DOI: 10.7270/Q2N878T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50108113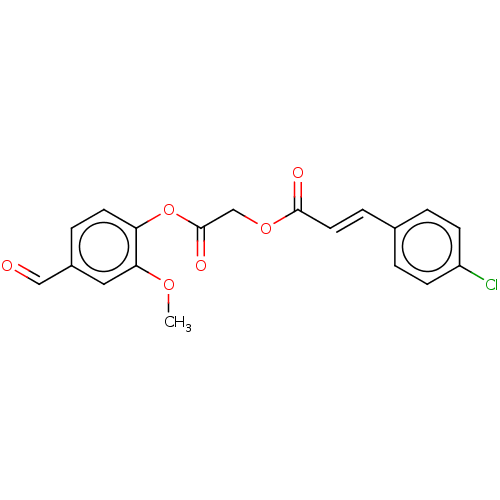 (CHEMBL3601649) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kongju National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as enzyme-inhibitor dissociation constant preincubated for 10 mins f... | Bioorg Med Chem 23: 5870-80 (2015) Article DOI: 10.1016/j.bmc.2015.06.068 BindingDB Entry DOI: 10.7270/Q20P11SM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Rattus norvegicus) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | <5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Competitive Inhibitory activity against Golgi Alpha-mannosidase II | J Med Chem 38: 2349-56 (1995) BindingDB Entry DOI: 10.7270/Q2N878T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Rattus norvegicus) | BDBM18355 ((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | <5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Competitive Inhibitory activity against Golgi Alpha-mannosidase II | J Med Chem 38: 2349-56 (1995) BindingDB Entry DOI: 10.7270/Q2N878T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Rattus norvegicus) | BDBM18353 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-methylpiperidine...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | <5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Competitive Inhibitory activity against Golgi Alpha-mannosidase II | J Med Chem 38: 2349-56 (1995) BindingDB Entry DOI: 10.7270/Q2N878T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Rattus norvegicus) | BDBM50031480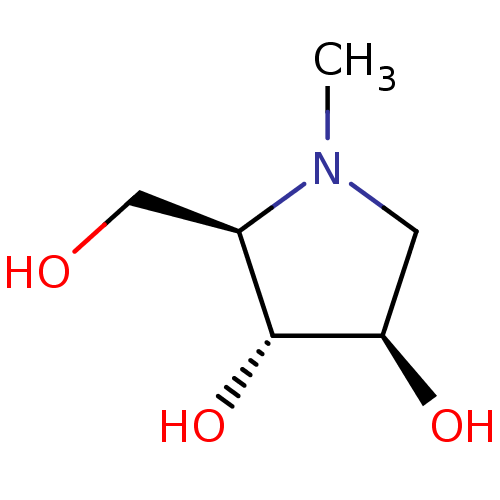 ((2R,3R,4R)-2-Hydroxymethyl-1-methyl-pyrrolidine-3,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Competitive Inhibitory activity against Golgi Alpha-mannosidase II | J Med Chem 38: 2349-56 (1995) BindingDB Entry DOI: 10.7270/Q2N878T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50108112 (CHEMBL3601648) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kongju National University Curated by ChEMBL | Assay Description Non-competitive inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as enzyme-substrate-inhibitor dissociation constant preincubated... | Bioorg Med Chem 23: 5870-80 (2015) Article DOI: 10.1016/j.bmc.2015.06.068 BindingDB Entry DOI: 10.7270/Q20P11SM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50108114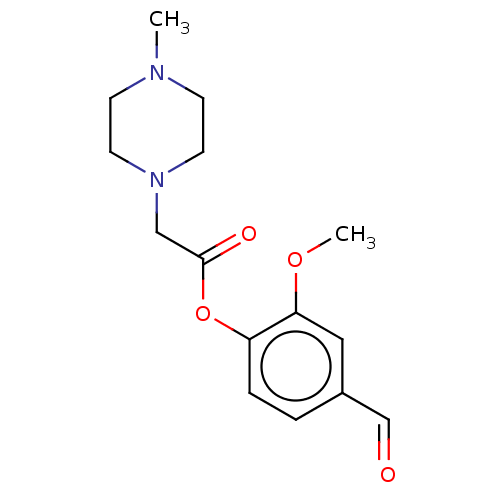 (CHEMBL3601650) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kongju National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as enzyme-inhibitor dissociation constant preincubated for 10 mins f... | Bioorg Med Chem 23: 5870-80 (2015) Article DOI: 10.1016/j.bmc.2015.06.068 BindingDB Entry DOI: 10.7270/Q20P11SM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50108109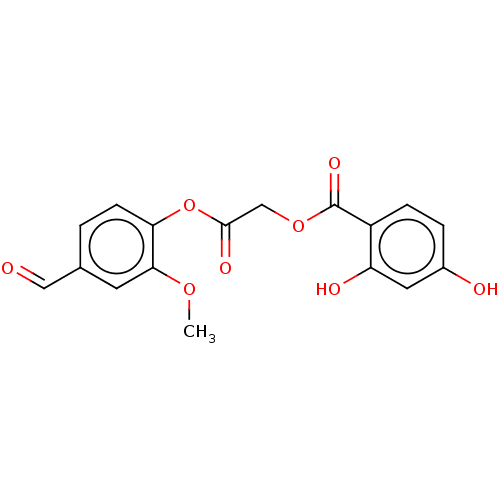 (CHEMBL3601645) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kongju National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as enzyme-inhibitor dissociation constant preincubated for 10 mins f... | Bioorg Med Chem 23: 5870-80 (2015) Article DOI: 10.1016/j.bmc.2015.06.068 BindingDB Entry DOI: 10.7270/Q20P11SM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50108113 (CHEMBL3601649) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kongju National University Curated by ChEMBL | Assay Description Non-competitive inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as enzyme-substrate-inhibitor dissociation constant preincubated... | Bioorg Med Chem 23: 5870-80 (2015) Article DOI: 10.1016/j.bmc.2015.06.068 BindingDB Entry DOI: 10.7270/Q20P11SM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Rattus norvegicus) | BDBM50031484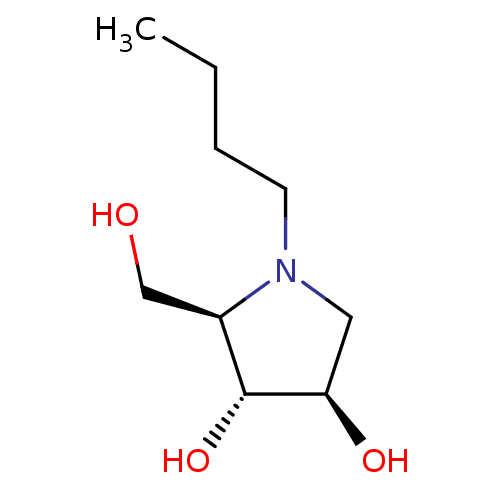 ((2R,3R,4R)-1-Butyl-2-hydroxymethyl-pyrrolidine-3,4...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Competitive Inhibitory activity against Golgi Alpha-mannosidase II | J Med Chem 38: 2349-56 (1995) BindingDB Entry DOI: 10.7270/Q2N878T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50108109 (CHEMBL3601645) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kongju National University Curated by ChEMBL | Assay Description Non-competitive inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as enzyme-substrate-inhibitor dissociation constant preincubated... | Bioorg Med Chem 23: 5870-80 (2015) Article DOI: 10.1016/j.bmc.2015.06.068 BindingDB Entry DOI: 10.7270/Q20P11SM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50422828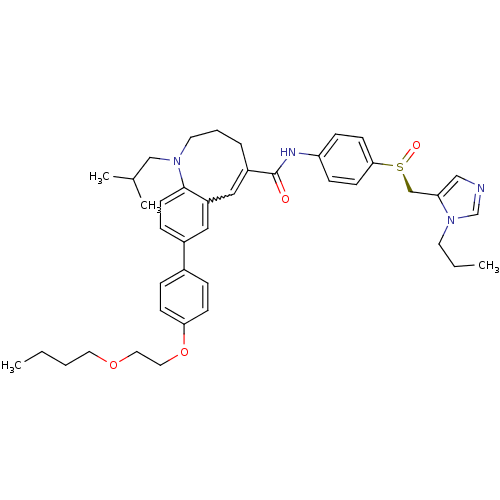 (CENICRIVIROC | CENICRIVIROC MESYLATE | TAK-652 | T...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184396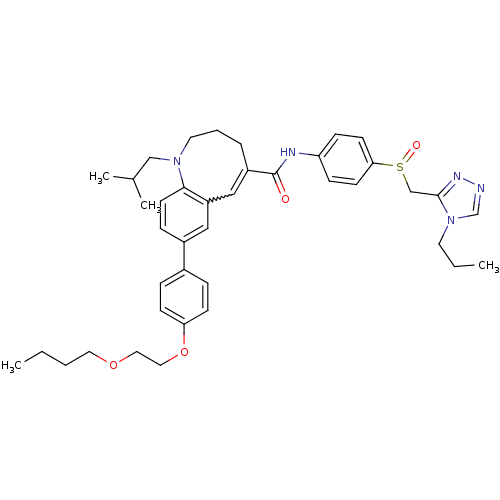 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-isobutyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50422828 (CENICRIVIROC | CENICRIVIROC MESYLATE | TAK-652 | T...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184399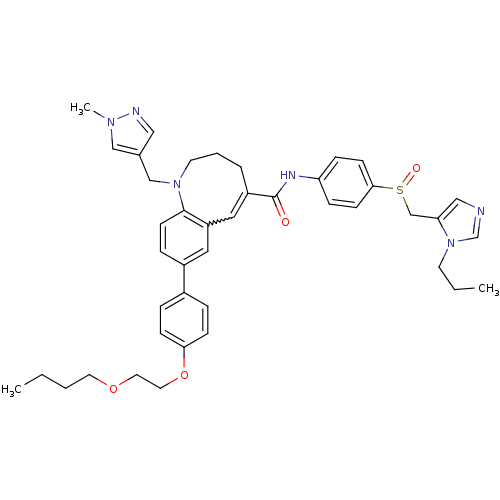 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-[(1-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184398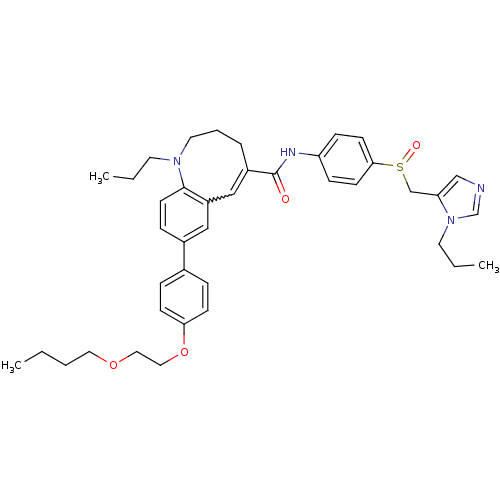 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50185979 (1-acetyl-N-{3-[4-(4-carbamoylbenzyl)piperidin-1-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2784-93 (2006) Article DOI: 10.1021/jm051034q BindingDB Entry DOI: 10.7270/Q2ZK5G8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184400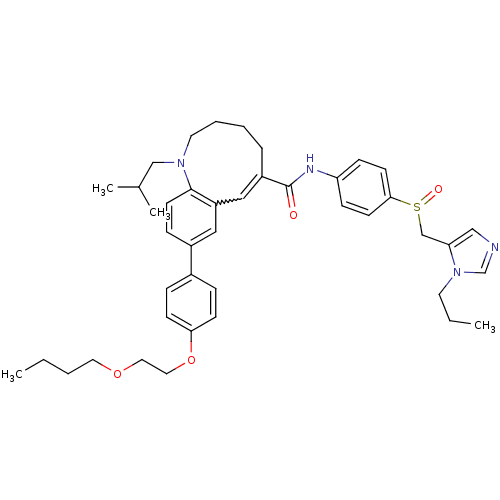 ((S)-(-)-9-{4-[2-(butoxy)ethoxy]phenyl}-1-isobutyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50185967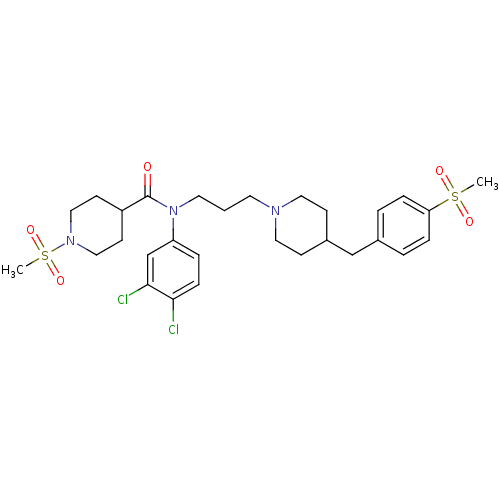 (CHEMBL207487 | N-(3-(4-(4-(methylsulfonyl)benzyl)p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2784-93 (2006) Article DOI: 10.1021/jm051034q BindingDB Entry DOI: 10.7270/Q2ZK5G8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184402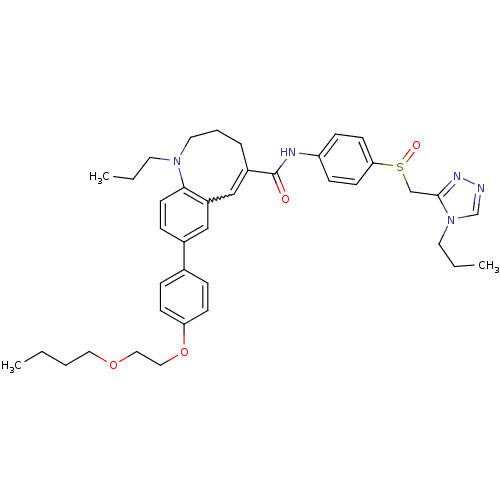 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184403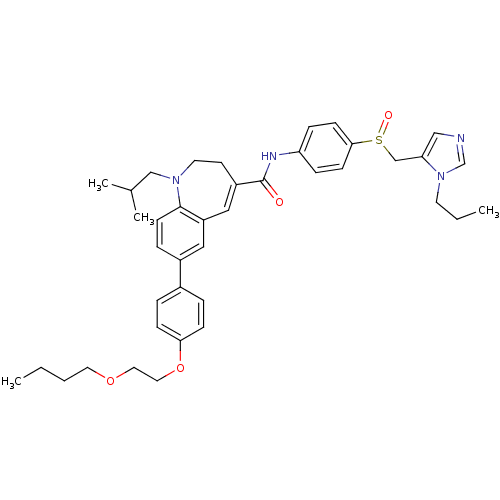 (7-(4-(2-butoxyethoxy)phenyl)-1-isobutyl-N-(4-((1-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50185972 (CHEMBL207182 | N-(3-(4-(4-fluorobenzyl)piperidin-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of [125I]RANTES binding to human CCR5 expressed in CHO cells | J Med Chem 49: 2784-93 (2006) Article DOI: 10.1021/jm051034q BindingDB Entry DOI: 10.7270/Q2ZK5G8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088321 (CHEMBL292548 | Dimethyl-(tetrahydro-pyran-4-yl)-{4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50185973 (CHEMBL207630 | N-{3-[4-(4-cyanobenzyl)piperidin-1-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2784-93 (2006) Article DOI: 10.1021/jm051034q BindingDB Entry DOI: 10.7270/Q2ZK5G8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184398 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 610 total ) | Next | Last >> |