 Found 641 hits with Last Name = 'follmann' and Initial = 'm'
Found 641 hits with Last Name = 'follmann' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM7840
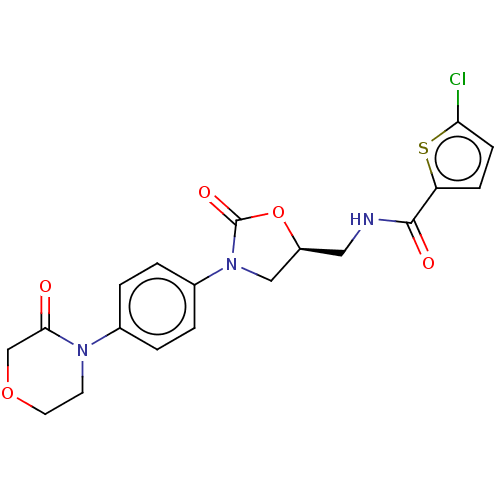
(RIVAROXABAN | US8822458, 44 | US8822458, 97)Show SMILES Clc1ccc(s1)C(=O)NC[C@H]1CN(C(=O)O1)c1ccc(cc1)N1CCOCC1=O |r| Show InChI InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of factor-10a (unknown origin) |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443853
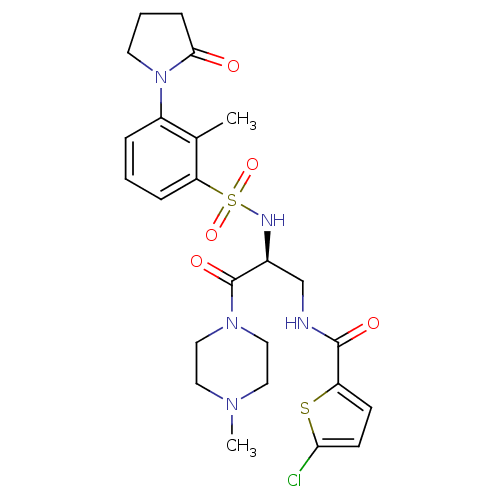
(CHEMBL3091519 | US20230391761, Reference 1)Show SMILES CN1CCN(CC1)C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCCC2=O)c1C |r| Show InChI InChI=1S/C24H30ClN5O5S2/c1-16-18(30-10-4-7-22(30)31)5-3-6-20(16)37(34,35)27-17(24(33)29-13-11-28(2)12-14-29)15-26-23(32)19-8-9-21(25)36-19/h3,5-6,8-9,17,27H,4,7,10-15H2,1-2H3,(H,26,32)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112086

(3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...)Show SMILES Cn1c(CNc2ccc(cc2)C(N)=N)nc2cc(ccc12)C(=O)N(CCC(O)=O)c1ccccn1 Show InChI InChI=1S/C25H25N7O3/c1-31-20-10-7-17(25(35)32(13-11-23(33)34)21-4-2-3-12-28-21)14-19(20)30-22(31)15-29-18-8-5-16(6-9-18)24(26)27/h2-10,12,14,29H,11,13,15H2,1H3,(H3,26,27)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of factor-10a (unknown origin) |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50443853

(CHEMBL3091519 | US20230391761, Reference 1)Show SMILES CN1CCN(CC1)C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCCC2=O)c1C |r| Show InChI InChI=1S/C24H30ClN5O5S2/c1-16-18(30-10-4-7-22(30)31)5-3-6-20(16)37(34,35)27-17(24(33)29-13-11-28(2)12-14-29)15-26-23(32)19-8-9-21(25)36-19/h3,5-6,8-9,17,27H,4,7,10-15H2,1-2H3,(H,26,32)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of t-PA (unknown origin) |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443855

(CHEMBL3091517)Show SMILES [#6]-[#6](=O)-[#7]-c1c(cccc1S(=O)(=O)[#7]-[#6@@H](-[#6]-[#6]-[#6]-c1ccc(-[#7])cn1)-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](/F)F)-c1ccccc1 |r| Show InChI InChI=1S/C30H33F2N5O4S/c1-20(38)35-28-25(21-7-3-2-4-8-21)10-6-12-27(28)42(40,41)36-26(11-5-9-24-14-13-23(33)19-34-24)30(39)37-17-15-22(16-18-37)29(31)32/h2-4,6-8,10,12-14,19,26,36H,5,9,11,15-18,33H2,1H3,(H,35,38)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of factor-10a (unknown origin) |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50443853

(CHEMBL3091519 | US20230391761, Reference 1)Show SMILES CN1CCN(CC1)C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCCC2=O)c1C |r| Show InChI InChI=1S/C24H30ClN5O5S2/c1-16-18(30-10-4-7-22(30)31)5-3-6-20(16)37(34,35)27-17(24(33)29-13-11-28(2)12-14-29)15-26-23(32)19-8-9-21(25)36-19/h3,5-6,8-9,17,27H,4,7,10-15H2,1-2H3,(H,26,32)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50443853

(CHEMBL3091519 | US20230391761, Reference 1)Show SMILES CN1CCN(CC1)C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCCC2=O)c1C |r| Show InChI InChI=1S/C24H30ClN5O5S2/c1-16-18(30-10-4-7-22(30)31)5-3-6-20(16)37(34,35)27-17(24(33)29-13-11-28(2)12-14-29)15-26-23(32)19-8-9-21(25)36-19/h3,5-6,8-9,17,27H,4,7,10-15H2,1-2H3,(H,26,32)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443857
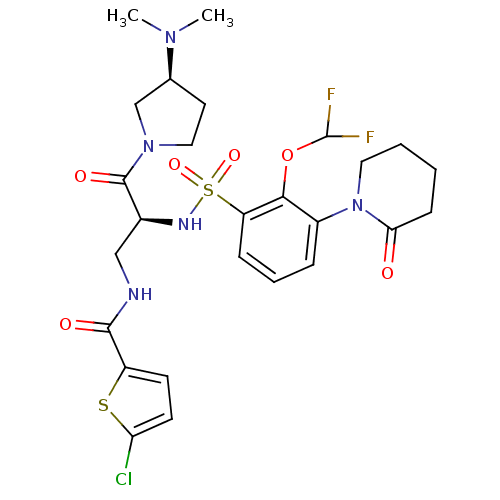
(CHEMBL3091501)Show SMILES CN(C)[C@H]1CCN(C1)C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCCCC2=O)c1OC(F)F |r| Show InChI InChI=1S/C26H32ClF2N5O6S2/c1-32(2)16-11-13-33(15-16)25(37)17(14-30-24(36)19-9-10-21(27)41-19)31-42(38,39)20-7-5-6-18(23(20)40-26(28)29)34-12-4-3-8-22(34)35/h5-7,9-10,16-17,26,31H,3-4,8,11-15H2,1-2H3,(H,30,36)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium/hydrogen exchanger 3
(Homo sapiens (Human)) | BDBM50196843
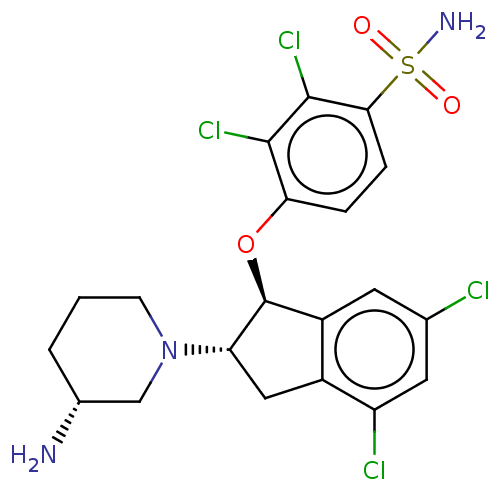
(CHEMBL3904640)Show SMILES N[C@@H]1CCCN(C1)[C@H]1Cc2c(cc(Cl)cc2Cl)[C@@H]1Oc1ccc(c(Cl)c1Cl)S(N)(=O)=O |r| Show InChI InChI=1S/C20H21Cl4N3O3S/c21-10-6-13-12(14(22)7-10)8-15(27-5-1-2-11(25)9-27)20(13)30-16-3-4-17(31(26,28)29)19(24)18(16)23/h3-4,6-7,11,15,20H,1-2,5,8-9,25H2,(H2,26,28,29)/t11-,15+,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human NHE3 expressed in LAP1 cell assessed as intracellular pH recovery measured for 2 mins by CECF-AM dye based FLIPR assay |
J Med Chem 59: 8812-8829 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00624
BindingDB Entry DOI: 10.7270/Q2FX7CDJ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM312960
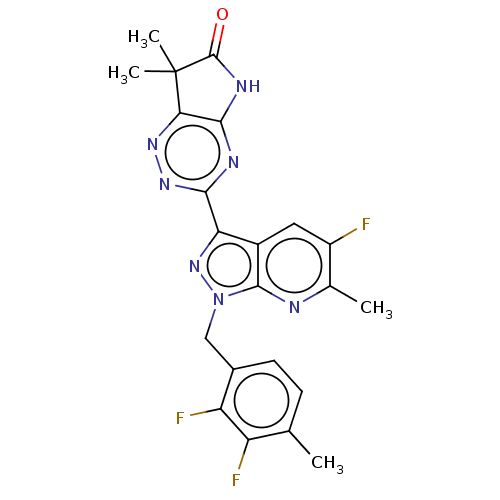
(3-[1-(2,3-Difluoro-4-methylbenzyl)-5-fluoro-6-meth...)Show SMILES Cc1ccc(Cn2nc(-c3nnc4c(NC(=O)C4(C)C)n3)c3cc(F)c(C)nc23)c(F)c1F Show InChI InChI=1S/C22H18F3N7O/c1-9-5-6-11(15(25)14(9)24)8-32-20-12(7-13(23)10(2)26-20)16(31-32)18-27-19-17(29-30-18)22(3,4)21(33)28-19/h5-7H,8H2,1-4H3,(H,27,28,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT
US Patent
| Assay Description
To determine their in vitro action on human PDE 5, the test substances are dissolved in 100% DMSO and serially diluted. Typically, dilution series (1... |
US Patent US9605008 (2017)
BindingDB Entry DOI: 10.7270/Q2DZ0BC2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443853

(CHEMBL3091519 | US20230391761, Reference 1)Show SMILES CN1CCN(CC1)C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCCC2=O)c1C |r| Show InChI InChI=1S/C24H30ClN5O5S2/c1-16-18(30-10-4-7-22(30)31)5-3-6-20(16)37(34,35)27-17(24(33)29-13-11-28(2)12-14-29)15-26-23(32)19-8-9-21(25)36-19/h3,5-6,8-9,17,27H,4,7,10-15H2,1-2H3,(H,26,32)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639340
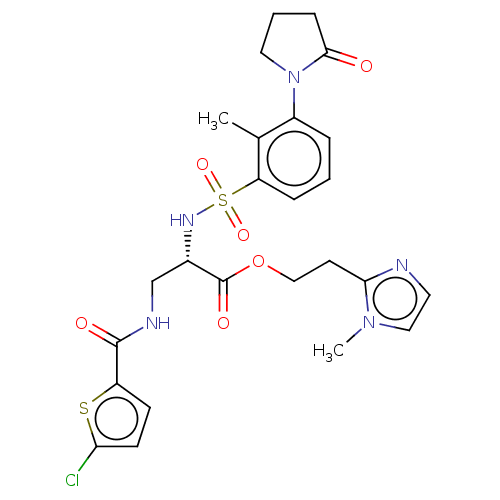
(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-{[(5-chloro-2...)Show SMILES Cc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CCCC1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639343

(3-(1-Methyl-1H-imidazol-2-yl)propyl 3-[(5-chloroth...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCCc1nccn1C)N1CCCC1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639321

(Methyl 3-{[(5-chloro-2-thienyl)carbonyl]amino}-N-{...)Show SMILES COC(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCCC2=O)c1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639349

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CC[C@H](O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443846
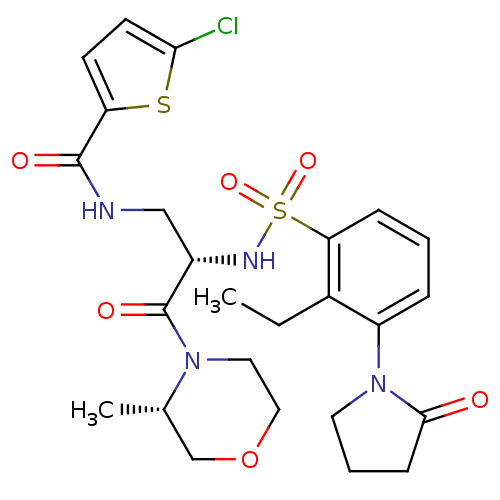
(CHEMBL3091527)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)N1CCOC[C@@H]1C)N1CCCC1=O |r| Show InChI InChI=1S/C25H31ClN4O6S2/c1-3-17-19(30-11-5-8-23(30)31)6-4-7-21(17)38(34,35)28-18(25(33)29-12-13-36-15-16(29)2)14-27-24(32)20-9-10-22(26)37-20/h4,6-7,9-10,16,18,28H,3,5,8,11-15H2,1-2H3,(H,27,32)/t16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443858
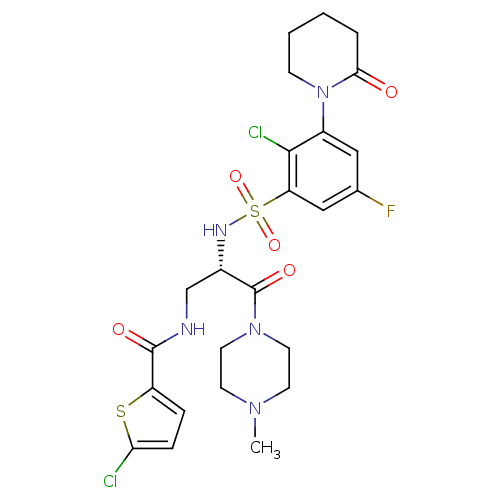
(CHEMBL3091502)Show SMILES CN1CCN(CC1)C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cc(F)cc(N2CCCCC2=O)c1Cl |r| Show InChI InChI=1S/C24H28Cl2FN5O5S2/c1-30-8-10-31(11-9-30)24(35)16(14-28-23(34)18-5-6-20(25)38-18)29-39(36,37)19-13-15(27)12-17(22(19)26)32-7-3-2-4-21(32)33/h5-6,12-13,16,29H,2-4,7-11,14H2,1H3,(H,28,34)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639349

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CC[C@H](O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639346

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CC[C@@H](O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639322

(Methyl 3-[(5-chlorothiophene-2-carbonyl)amino]-N-[...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OC)N1CCCC1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639349

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CC[C@H](O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443847
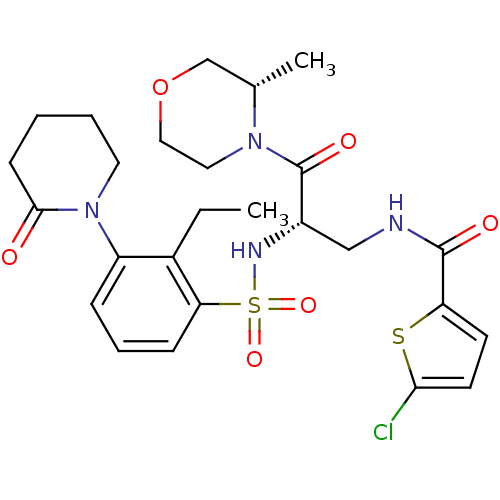
(CHEMBL3091526)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)N1CCOC[C@@H]1C)N1CCCCC1=O |r| Show InChI InChI=1S/C26H33ClN4O6S2/c1-3-18-20(31-12-5-4-9-24(31)32)7-6-8-22(18)39(35,36)29-19(26(34)30-13-14-37-16-17(30)2)15-28-25(33)21-10-11-23(27)38-21/h6-8,10-11,17,19,29H,3-5,9,12-16H2,1-2H3,(H,28,33)/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639344
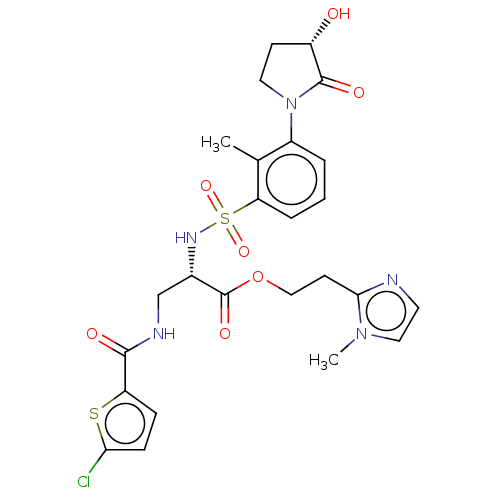
(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...)Show SMILES Cc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CC[C@H](O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639342
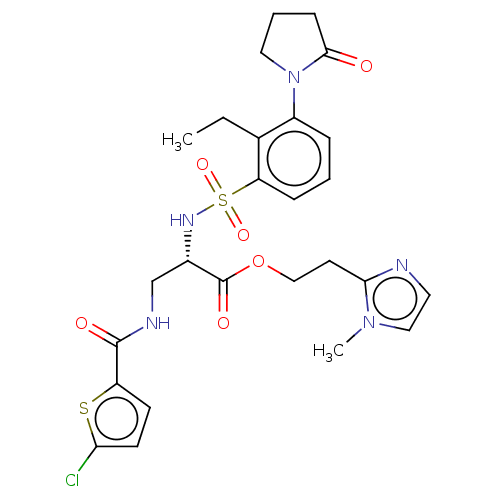
(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-{[(5-chloro-2...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CCCC1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM312961
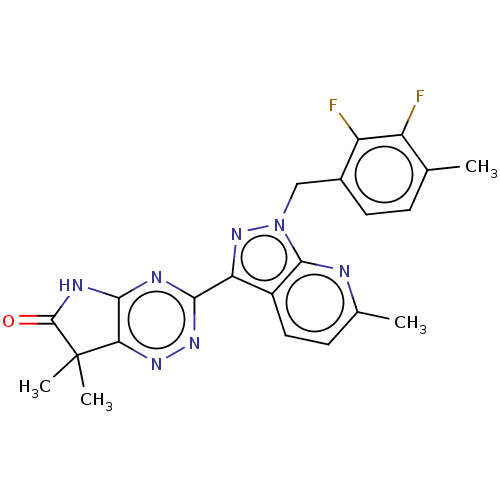
(3-[1-(2,3-Difluoro-4-methylbenzyl)-6-methyl-1H-pyr...)Show SMILES Cc1ccc2c(nn(Cc3ccc(C)c(F)c3F)c2n1)-c1nnc2c(NC(=O)C2(C)C)n1 Show InChI InChI=1S/C22H19F2N7O/c1-10-5-7-12(15(24)14(10)23)9-31-20-13(8-6-11(2)25-20)16(30-31)18-26-19-17(28-29-18)22(3,4)21(32)27-19/h5-8H,9H2,1-4H3,(H,26,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT
US Patent
| Assay Description
To determine their in vitro action on human PDE 5, the test substances are dissolved in 100% DMSO and serially diluted. Typically, dilution series (1... |
US Patent US9605008 (2017)
BindingDB Entry DOI: 10.7270/Q2DZ0BC2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639349

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CC[C@H](O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639338
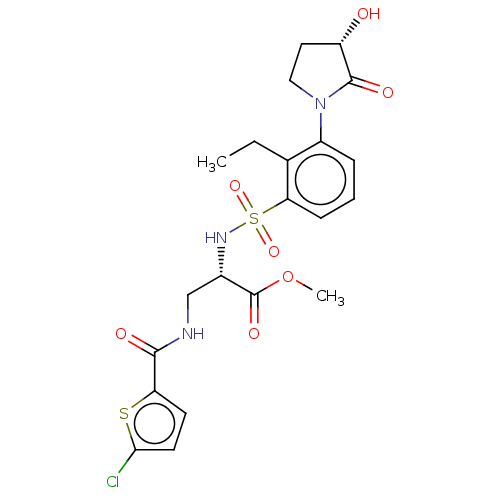
(Methyl 3-[(5-chlorothiophene-2-carbonyl)amino]-N-{...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OC)N1CC[C@H](O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639346

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CC[C@@H](O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639349

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CC[C@H](O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM639342

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-{[(5-chloro-2...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CCCC1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639339
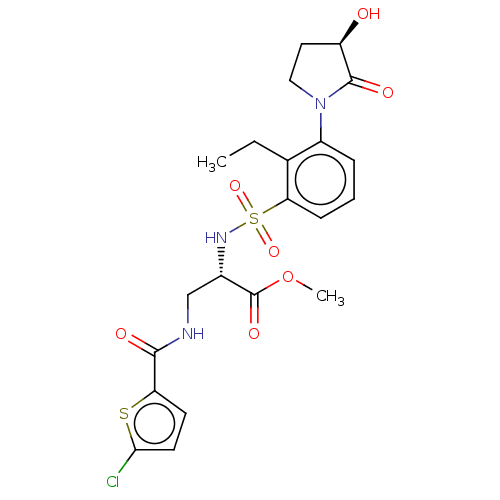
(Methyl 3-[(5-chlorothiophene-2-carbonyl)amino]-N-{...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OC)N1CC[C@@H](O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639349

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CC[C@H](O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639349

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CC[C@H](O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639348
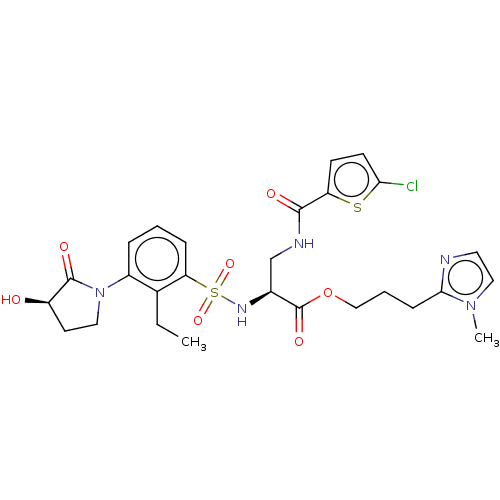
(3-(1-Methyl-1H-imidazol-2-yl)propyl 3-[(5-chloroth...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCCc1nccn1C)N1CC[C@@H](O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM639341
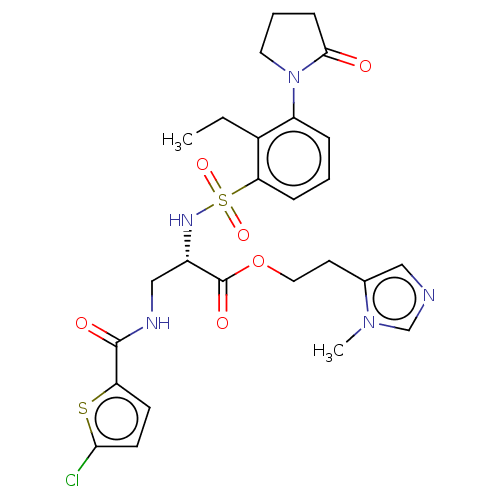
(2-(1-Methyl-1H-imidazol-5-yl)ethyl 3-[(5-chlorothi...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1cncn1C)N1CCCC1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639349

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CC[C@H](O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639341

(2-(1-Methyl-1H-imidazol-5-yl)ethyl 3-[(5-chlorothi...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1cncn1C)N1CCCC1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639347
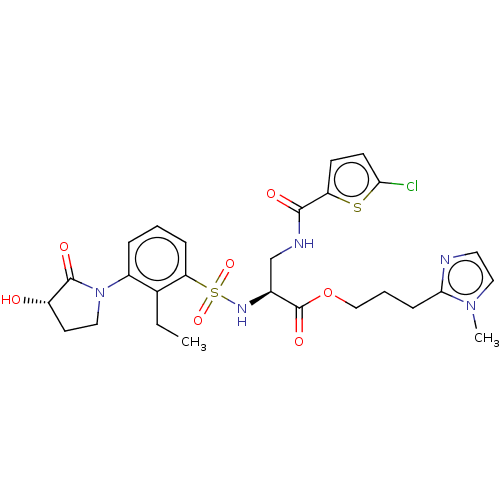
(3-(1-Methyl-1H-imidazol-2-yl)propyl 3-[(5-chloroth...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCCc1nccn1C)N1CC[C@H](O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM124886

(US8759341, 2)Show SMILES Cc1[nH]nc2ccc(cc12)C1C([N+]#[C-])C(C)=NC2=C1C(=O)OC2 |c:17,19| Show InChI InChI=1S/C17H14N4O2/c1-8-11-6-10(4-5-12(11)21-20-8)14-15-13(7-23-17(15)22)19-9(2)16(14)18-3/h4-6,14,16H,7H2,1-2H3,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
For the assay, 50 mL of a 100-fold concentrated solution of the test compound in DMSO is pipetted into a black low-volume 384-well microtiter plate (... |
US Patent US8759341 (2014)
BindingDB Entry DOI: 10.7270/Q2HQ3XMW |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM103461

(US8551989, 18)Show SMILES CC(C)OCCOc1n[nH]c2ccc(cc12)C1C(C#N)C(C)=NC(C)=C1C#N |c:23,26| Show InChI InChI=1S/C21H23N5O2/c1-12(2)27-7-8-28-21-16-9-15(5-6-19(16)25-26-21)20-17(10-22)13(3)24-14(4)18(20)11-23/h5-6,9,12,17,20H,7-8H2,1-4H3,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
Homogeneous time-resolved fluorescence assay using c-Met receptor tyrosine kinase. |
US Patent US8551989 (2013)
BindingDB Entry DOI: 10.7270/Q24F1PCX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443861
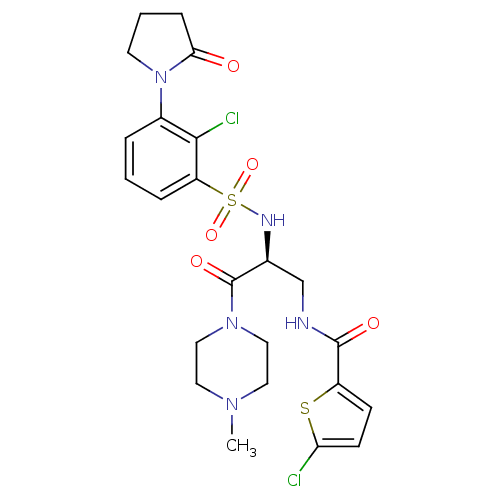
(CHEMBL3091515)Show SMILES CN1CCN(CC1)C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCCC2=O)c1Cl |r| Show InChI InChI=1S/C23H27Cl2N5O5S2/c1-28-10-12-29(13-11-28)23(33)15(14-26-22(32)17-7-8-19(24)36-17)27-37(34,35)18-5-2-4-16(21(18)25)30-9-3-6-20(30)31/h2,4-5,7-8,15,27H,3,6,9-14H2,1H3,(H,26,32)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443853

(CHEMBL3091519 | US20230391761, Reference 1)Show SMILES CN1CCN(CC1)C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCCC2=O)c1C |r| Show InChI InChI=1S/C24H30ClN5O5S2/c1-16-18(30-10-4-7-22(30)31)5-3-6-20(16)37(34,35)27-17(24(33)29-13-11-28(2)12-14-29)15-26-23(32)19-8-9-21(25)36-19/h3,5-6,8-9,17,27H,4,7,10-15H2,1-2H3,(H,26,32)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor [960-1390]
(Homo sapiens (Human)) | BDBM240734

(US9422263, 5 | US9422263, 6)Show SMILES Cc1n[nH]c2ccc(cc12)C1C(C#N)=C(C)NC(=C1C#N)C(F)(F)F |c:19,t:15| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT
US Patent
| Assay Description
The N-terminally His6-tagged recombinant kinase domain of the human c-Met (amino acids 960-1390), expressed in insect cells (SF21) and purified by Ni... |
US Patent US9422263 (2016)
BindingDB Entry DOI: 10.7270/Q2JQ0ZW2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443844

(CHEMBL3091506)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)N1CCOCC1)N1CCOCC1=O |r| Show InChI InChI=1S/C24H29ClN4O7S2/c1-2-16-18(29-10-13-36-15-22(29)30)4-3-5-20(16)38(33,34)27-17(24(32)28-8-11-35-12-9-28)14-26-23(31)19-6-7-21(25)37-19/h3-7,17,27H,2,8-15H2,1H3,(H,26,31)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089758
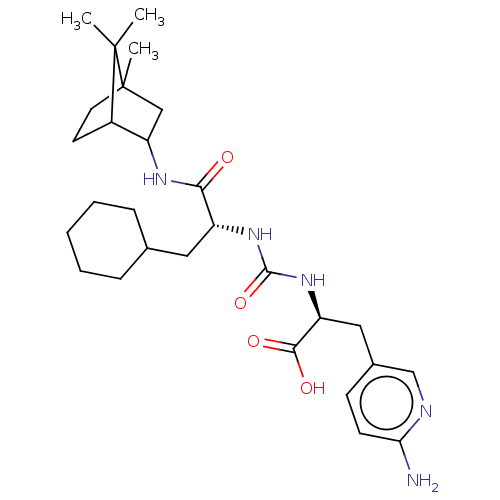
(CHEMBL3577442)Show SMILES CC12CCC(C(C1)NC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](Cc1ccc(N)nc1)C(O)=O)C2(C)C |r,THB:7:5:34:2.3| Show InChI InChI=1S/C28H43N5O4/c1-27(2)19-11-12-28(27,3)15-22(19)31-24(34)20(13-17-7-5-4-6-8-17)32-26(37)33-21(25(35)36)14-18-9-10-23(29)30-16-18/h9-10,16-17,19-22H,4-8,11-15H2,1-3H3,(H2,29,30)(H,31,34)(H,35,36)(H2,32,33,37)/t19?,20-,21+,22?,28?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50089691
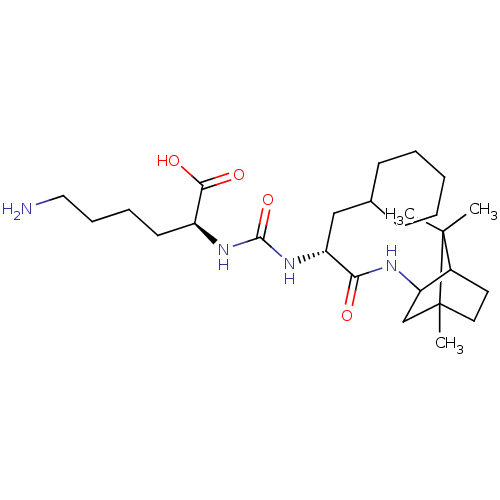
(CHEMBL3577425)Show SMILES CC1(C)C2CCC1(C)CC2NC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](CCCCN)C(O)=O |r,TLB:10:9:5.4:1| Show InChI InChI=1S/C26H46N4O4/c1-25(2)18-12-13-26(25,3)16-21(18)28-22(31)20(15-17-9-5-4-6-10-17)30-24(34)29-19(23(32)33)11-7-8-14-27/h17-21H,4-16,27H2,1-3H3,(H,28,31)(H,32,33)(H2,29,30,34)/t18?,19-,20+,21?,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of CBP (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM168604
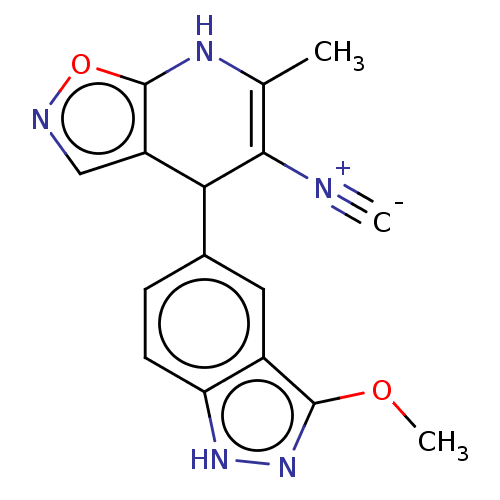
(US9073939, 3)Show SMILES COc1n[nH]c2ccc(cc12)C1c2cnoc2NC(C)=C1[N+]#[C-] |c:22| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
Recombinant human c-Met protein (Invitrogen, Carlsbad, Calif., USA) is used. As substrate for the kinase reaction the peptide KKKSPGEYVNIEFG (JPT, Ge... |
US Patent US9073939 (2015)
BindingDB Entry DOI: 10.7270/Q2TM78WW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443848

(CHEMBL3091525)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)N1CCOC[C@@H]1C)-c1ccccn1 |r| Show InChI InChI=1S/C26H29ClN4O5S2/c1-3-18-19(20-8-4-5-12-28-20)7-6-9-23(18)38(34,35)30-21(26(33)31-13-14-36-16-17(31)2)15-29-25(32)22-10-11-24(27)37-22/h4-12,17,21,30H,3,13-16H2,1-2H3,(H,29,32)/t17-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443869
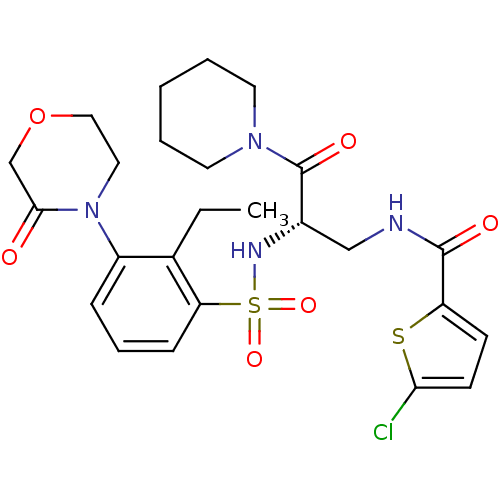
(CHEMBL3091507)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)N1CCCCC1)N1CCOCC1=O |r| Show InChI InChI=1S/C25H31ClN4O6S2/c1-2-17-19(30-13-14-36-16-23(30)31)7-6-8-21(17)38(34,35)28-18(25(33)29-11-4-3-5-12-29)15-27-24(32)20-9-10-22(26)37-20/h6-10,18,28H,2-5,11-16H2,1H3,(H,27,32)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639345

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...)Show SMILES Cc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CC[C@@H](O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data