

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50003743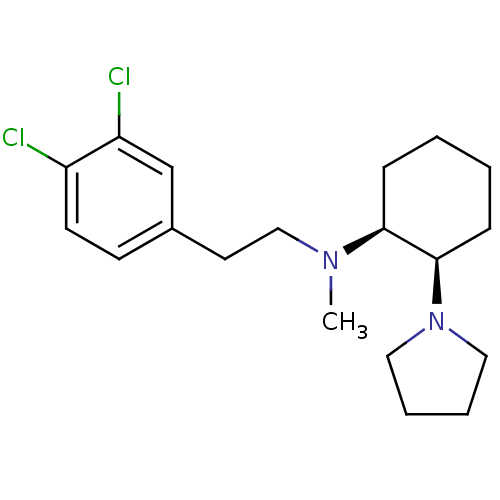 ((-)-[2-(3,4-Dichloro-phenyl)-ethyl]-methyl-(2-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Affinity of compound to Sigma opioid receptor labelled with [3H](+)-3-PPP | J Med Chem 33: 3100-10 (1990) BindingDB Entry DOI: 10.7270/Q2348KZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50003747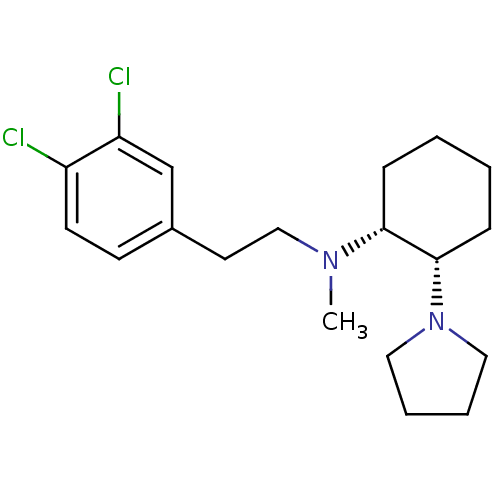 ((+)-[2-(3,4-Dichloro-phenyl)-ethyl]-methyl-(2-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Affinity of compound to Sigma opioid receptor labelled with [3H](+)-3-PPP | J Med Chem 33: 3100-10 (1990) BindingDB Entry DOI: 10.7270/Q2348KZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50013955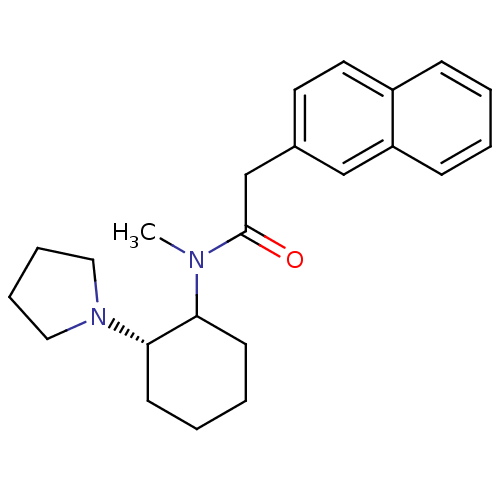 (N-Methyl-2-naphthalen-2-yl-N-(2-pyrrolidin-1-yl-cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Affinity of compound to Sigma opioid receptor labelled with [3H](+)-3-PPP | J Med Chem 33: 3100-10 (1990) BindingDB Entry DOI: 10.7270/Q2348KZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50013968 (2-(2-Amino-4,5-dichloro-phenyl)-N-methyl-N-(2-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Affinity of compound towards Opioid receptor kappa 1 was determined in presence of [3H]bremazocine radioligand | J Med Chem 33: 3100-10 (1990) BindingDB Entry DOI: 10.7270/Q2348KZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50013955 (N-Methyl-2-naphthalen-2-yl-N-(2-pyrrolidin-1-yl-cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity for sigma opioid receptor using [3H](+)-3-PPP as radioligand | J Med Chem 33: 3100-10 (1990) BindingDB Entry DOI: 10.7270/Q2348KZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50013966 ((2-Benzo[1,3]dioxol-5-yl-ethyl)-methyl-(2-pyrrolid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Affinity of compound to Sigma opioid receptor labelled with [3H](+)-3-PPP | J Med Chem 33: 3100-10 (1990) BindingDB Entry DOI: 10.7270/Q2348KZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50013966 ((2-Benzo[1,3]dioxol-5-yl-ethyl)-methyl-(2-pyrrolid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Affinity of compound towards Opioid receptor kappa 1 was determined in presence of [3H]U-69593. radioligand | J Med Chem 33: 3100-10 (1990) BindingDB Entry DOI: 10.7270/Q2348KZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50013962 (2-(2H-1,3-benzodioxol-5-yl)-N-methyl-N-[(2R)-2-(py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 456 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Affinity of compound to Sigma opioid receptor labelled with [3H](+)-3-PPP | J Med Chem 33: 3100-10 (1990) BindingDB Entry DOI: 10.7270/Q2348KZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50013968 (2-(2-Amino-4,5-dichloro-phenyl)-N-methyl-N-(2-pyrr...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 523 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Affinity of compound towards Opioid receptor kappa 1 was determined in presence of [3H]bremazocine radioligand | J Med Chem 33: 3100-10 (1990) BindingDB Entry DOI: 10.7270/Q2348KZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50013955 (N-Methyl-2-naphthalen-2-yl-N-(2-pyrrolidin-1-yl-cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Affinity of compound to Sigma opioid receptor labelled with [3H](+)-3-PPP | J Med Chem 33: 3100-10 (1990) BindingDB Entry DOI: 10.7270/Q2348KZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50013955 (N-Methyl-2-naphthalen-2-yl-N-(2-pyrrolidin-1-yl-cy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Affinity of compound to Sigma opioid receptor labelled with [3H](+)-3-PPP | J Med Chem 33: 3100-10 (1990) BindingDB Entry DOI: 10.7270/Q2348KZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50013968 (2-(2-Amino-4,5-dichloro-phenyl)-N-methyl-N-(2-pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Affinity of compound towards Dopamine receptor D2 was determined in presence of [3H](-)-sulpiride radioligand | J Med Chem 33: 3100-10 (1990) BindingDB Entry DOI: 10.7270/Q2348KZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50013955 (N-Methyl-2-naphthalen-2-yl-N-(2-pyrrolidin-1-yl-cy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Affinity of compound towards Sigma opioid receptor was determined in presence of [3H](+)-3-PPP radioligand | J Med Chem 33: 3100-10 (1990) BindingDB Entry DOI: 10.7270/Q2348KZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50013955 (N-Methyl-2-naphthalen-2-yl-N-(2-pyrrolidin-1-yl-cy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Affinity of compound towards Opioid receptor kappa 1 was determined in presence of [3H]U-69593. radioligand | J Med Chem 33: 3100-10 (1990) BindingDB Entry DOI: 10.7270/Q2348KZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50013955 (N-Methyl-2-naphthalen-2-yl-N-(2-pyrrolidin-1-yl-cy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Affinity of compound towards Opioid receptor kappa 1 was determined in presence of [3H]U-69593 radioligand | J Med Chem 33: 3100-10 (1990) BindingDB Entry DOI: 10.7270/Q2348KZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50013962 (2-(2H-1,3-benzodioxol-5-yl)-N-methyl-N-[(2R)-2-(py...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Affinity of compound towards Opioid receptor kappa 1 was determined in presence of [3H]U-69593. radioligand | J Med Chem 33: 3100-10 (1990) BindingDB Entry DOI: 10.7270/Q2348KZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50003747 ((+)-[2-(3,4-Dichloro-phenyl)-ethyl]-methyl-(2-pyrr...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Affinity of compound towards Opioid receptor kappa 1 was determined in presence of [3H]U-69593. radioligand | J Med Chem 33: 3100-10 (1990) BindingDB Entry DOI: 10.7270/Q2348KZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50013962 (2-(2H-1,3-benzodioxol-5-yl)-N-methyl-N-[(2R)-2-(py...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 6.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Affinity of compound towards Opioid receptor kappa 1 labeled with [3H]bremazocine. | J Med Chem 33: 3100-10 (1990) BindingDB Entry DOI: 10.7270/Q2348KZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50013962 (2-(2H-1,3-benzodioxol-5-yl)-N-methyl-N-[(2R)-2-(py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 7.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Affinity of compound towards Opioid receptor kappa 1 labeled with [3H]bremazocine. | J Med Chem 33: 3100-10 (1990) BindingDB Entry DOI: 10.7270/Q2348KZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50013955 (N-Methyl-2-naphthalen-2-yl-N-(2-pyrrolidin-1-yl-cy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 7.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Affinity of compound towards dopamine-D2 receptor was determined in presence of [3H](-)-sulpiride radioligand | J Med Chem 33: 3100-10 (1990) BindingDB Entry DOI: 10.7270/Q2348KZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM630 ((2R,4S)-2-[(R)-({[4-(dimethylamino)phenyl]methyl}c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM626 ((2R,4S)-2-[(R)-(benzylcarbamoyl)[1-(pyridin-2-yl)a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM591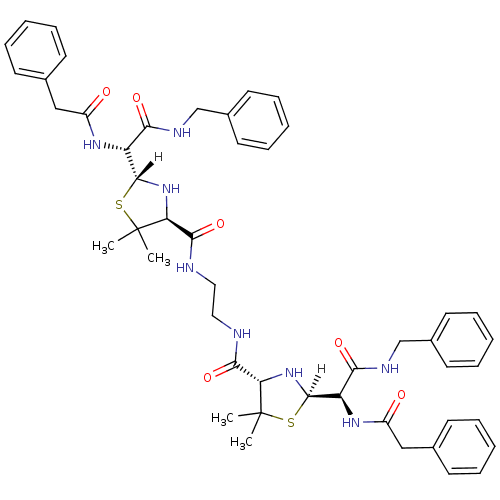 ((2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phenylacetamido...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50003743 ((-)-[2-(3,4-Dichloro-phenyl)-ethyl]-methyl-(2-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Affinity of compound to Sigma opioid receptor labelled with [3H](+)-3-PPP | J Med Chem 33: 3100-10 (1990) BindingDB Entry DOI: 10.7270/Q2348KZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM613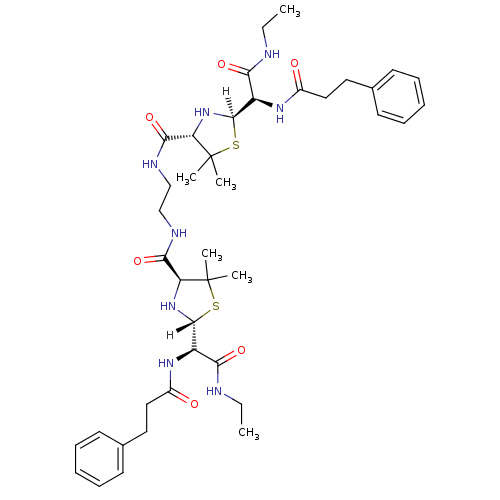 ((2R,4S)-2-[(R)-(ethylcarbamoyl)(3-phenylpropanamid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM625 ((2R,4S)-2-[(R)-(ethylcarbamoyl)[(2Z)-3-phenylprop-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM589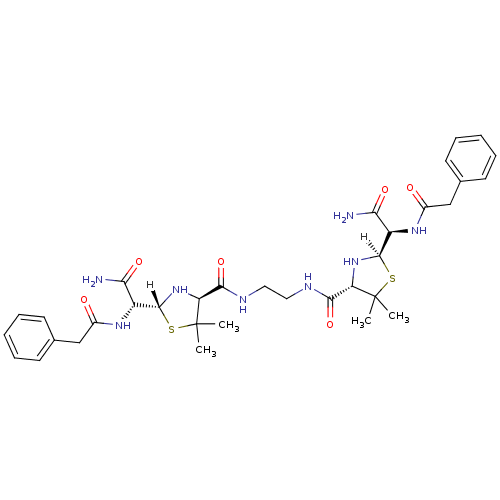 ((2R,4S)-2-[(R)-carbamoyl(1-phenylacetamido)methyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50419265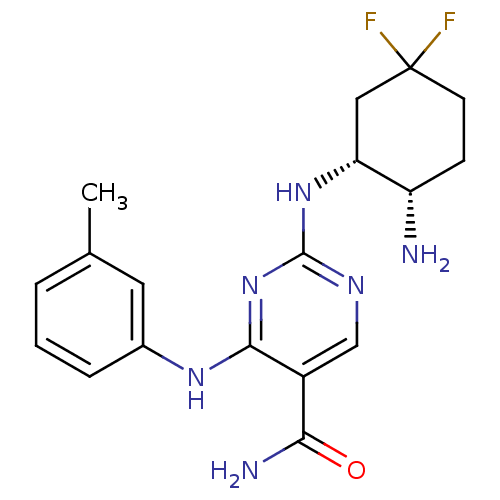 (CHEMBL1835069) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of human full-length recombinant 6His-SYK assessed as phosphorylation of Biotin-AAAEEIYGEI substrate after 60 mins by by TR-FRET assay | Bioorg Med Chem Lett 21: 6188-94 (2011) Article DOI: 10.1016/j.bmcl.2011.07.082 BindingDB Entry DOI: 10.7270/Q2XD12ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50607759 (CHEMBL5218813) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01102 BindingDB Entry DOI: 10.7270/Q2QN6BWR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1 (Homo sapiens (Human)) | BDBM50438224 (CHEMBL2407759) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of truncated TAK1-TAB1(unknown origin) using MKK7 as substrate by ALPHAScreen assay in presence of ATP | Bioorg Med Chem Lett 23: 4517-22 (2013) Article DOI: 10.1016/j.bmcl.2013.06.053 BindingDB Entry DOI: 10.7270/Q2ZK5J2K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM631 (Penicillin Et(NH)2 Sym dimer | penicillin deriv. ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM594 ((2R,4S)-N-(2-{[(2R,4S)-5,5-dimethyl-2-[(R)-(1-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM590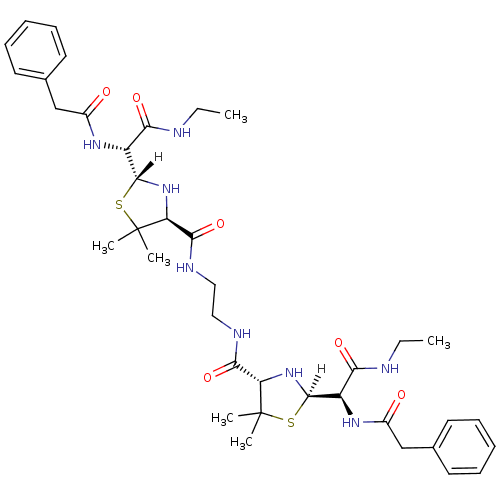 ((2R,4S)-2-[(R)-(ethylcarbamoyl)(1-phenylacetamido)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM593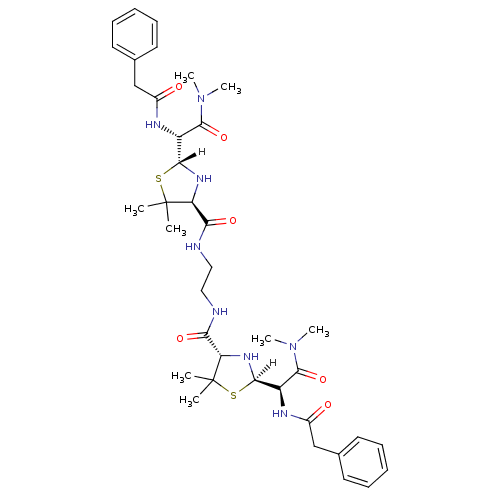 ((2R,4S)-2-[(R)-(dimethylcarbamoyl)(1-phenylacetami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal Pro-X carboxypeptidase (Homo sapiens (Human)) | BDBM50344263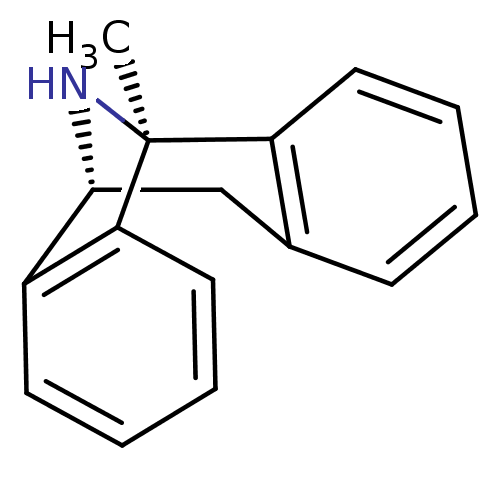 ((+)-1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle& Co. Curated by ChEMBL | Assay Description Inhibition of [3H]1-[1-(2-thienyl) piperidine ([3H]TCP) binding to phencyclidine receptor | J Med Chem 32: 1242-8 (1989) BindingDB Entry DOI: 10.7270/Q2CR5TXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM620 (Penicillin Et(NH)2 Sym dimer | [2R-[ 2a(R*),4B]]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50607759 (CHEMBL5218813) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01102 BindingDB Entry DOI: 10.7270/Q2QN6BWR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50396073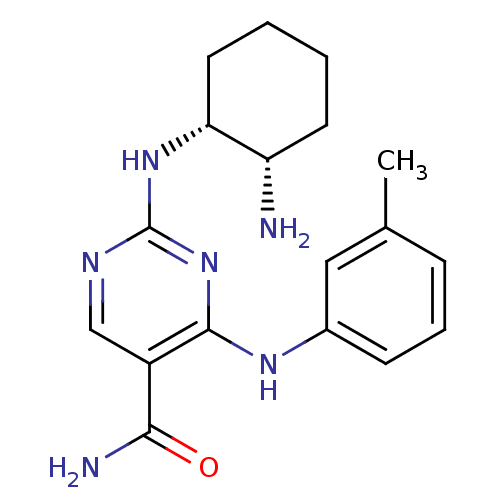 (CHEMBL1235110) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of human full-length recombinant 6His-SYK assessed as phosphorylation of Biotin-AAAEEIYGEI substrate after 60 mins by by TR-FRET assay | Bioorg Med Chem Lett 21: 6188-94 (2011) Article DOI: 10.1016/j.bmcl.2011.07.082 BindingDB Entry DOI: 10.7270/Q2XD12ZW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM595 ((2R,4S)-N-(2-{[(2R,4S)-5,5-dimethyl-2-[(1R)-2-oxo-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM627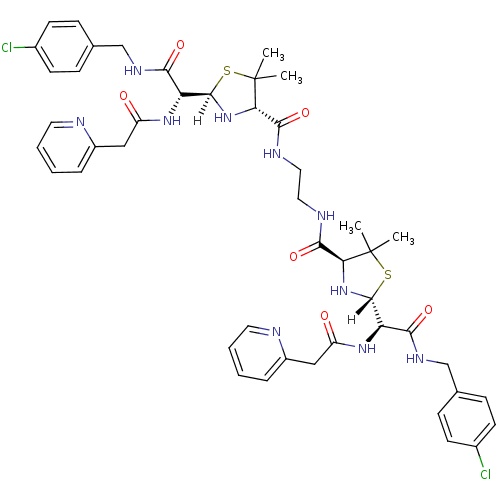 ((2R,4S)-2-[(R)-{[(4-chlorophenyl)methyl]carbamoyl}...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM629 ((2R,4S)-2-[(R)-(benzylcarbamoyl)[(2-phenylphenyl)f...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50607758 (CHEMBL5219991) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01102 BindingDB Entry DOI: 10.7270/Q2QN6BWR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM619 ((2R,4S)-2-[(R)-(ethylcarbamoyl)(3-methylbutanamido...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM621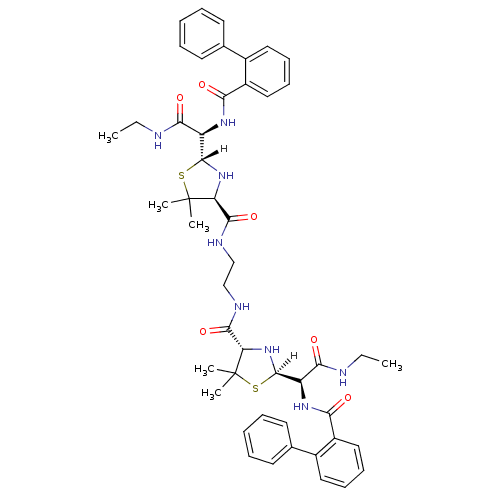 ((2R,4S)-2-[(R)-(ethylcarbamoyl)[(2-phenylphenyl)fo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50607758 (CHEMBL5219991) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01102 BindingDB Entry DOI: 10.7270/Q2QN6BWR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50607761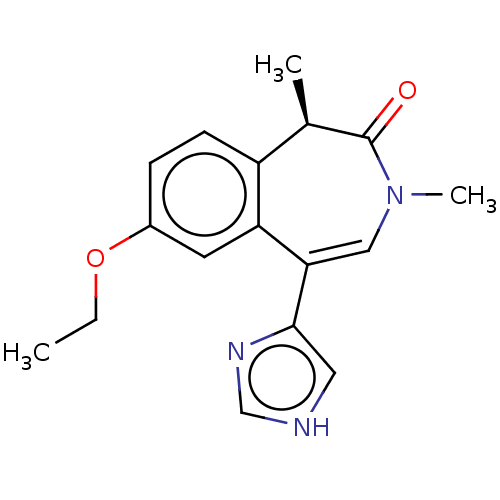 (CHEMBL5221069) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01102 BindingDB Entry DOI: 10.7270/Q2QN6BWR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 7 (Homo sapiens (Human)) | BDBM50438223 (CHEMBL2407758) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of TAK1 in human HCT116 cells assessed as inhibition of TNF-alpha-stimulated JNK phosphorylation | Bioorg Med Chem Lett 23: 4517-22 (2013) Article DOI: 10.1016/j.bmcl.2013.06.053 BindingDB Entry DOI: 10.7270/Q2ZK5J2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50419258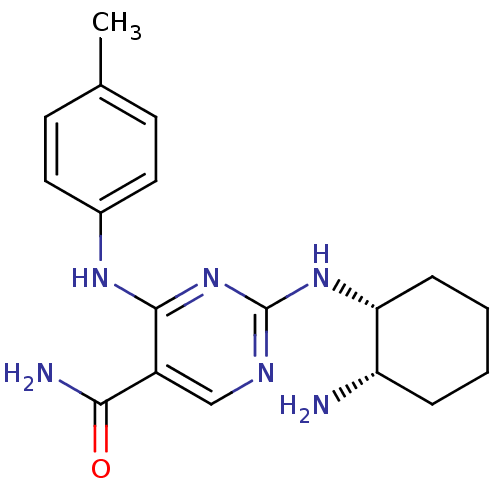 (CHEMBL1835070) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of human full-length recombinant 6His-SYK assessed as phosphorylation of Biotin-AAAEEIYGEI substrate after 60 mins by by TR-FRET assay | Bioorg Med Chem Lett 21: 6188-94 (2011) Article DOI: 10.1016/j.bmcl.2011.07.082 BindingDB Entry DOI: 10.7270/Q2XD12ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50607761 (CHEMBL5221069) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01102 BindingDB Entry DOI: 10.7270/Q2QN6BWR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM601 ((2R,4S)-N-(2-{[(2R,4S)-5,5-dimethyl-2-[(R)-(1-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 540 total ) | Next | Last >> |