 Found 7774 hits with Last Name = 'ito' and Initial = 'm'
Found 7774 hits with Last Name = 'ito' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50143784
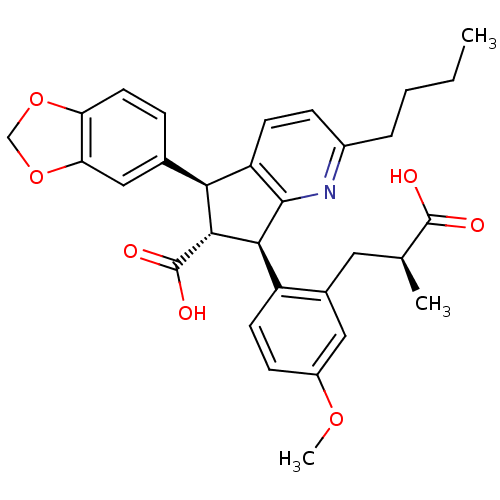
((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-2-butyl-7-[2-((...)Show SMILES CCCCc1ccc2[C@@H]([C@H]([C@@H](c2n1)c1ccc(OC)cc1C[C@H](C)C(O)=O)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C31H33NO7/c1-4-5-6-20-8-10-23-26(18-7-12-24-25(15-18)39-16-38-24)28(31(35)36)27(29(23)32-20)22-11-9-21(37-3)14-19(22)13-17(2)30(33)34/h7-12,14-15,17,26-28H,4-6,13,16H2,1-3H3,(H,33,34)(H,35,36)/t17-,26-,27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 1262-70 (1999)
BindingDB Entry DOI: 10.7270/Q2Q52N5Q |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50295693
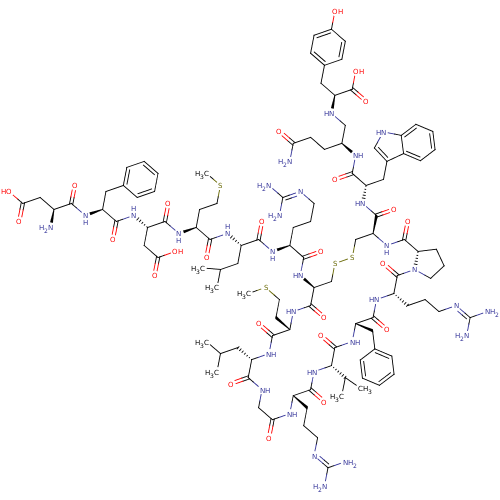
(CHEMBL557629)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCSC)NC1=O)C(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(N)=O)CN[C@@H](Cc1ccc(O)cc1)C(O)=O |r,wU:27.28,36.36,71.73,148.153,55.55,60.134,8.12,wD:112.115,4.4,97.100,93.131,82.84,16.24,134.137,44.44,64.69,156.161,120.123,(5.27,-20.83,;6.6,-20.06,;6.6,-18.52,;5.27,-17.75,;5.27,-16.21,;3.93,-15.44,;2.6,-16.21,;2.6,-17.75,;1.27,-15.44,;1.27,-13.9,;2.6,-13.13,;3.93,-13.9,;2.6,-11.59,;-.07,-16.21,;-1.4,-15.44,;-1.4,-13.9,;-2.73,-16.21,;-2.73,-17.75,;-1.4,-18.52,;-.07,-17.74,;1.27,-18.51,;1.27,-20.05,;-.08,-20.82,;-1.4,-20.05,;-4.07,-15.44,;-5.4,-16.21,;-5.4,-17.75,;-6.74,-15.44,;-8.07,-16.21,;-6.74,-13.9,;-8.07,-13.13,;-8.07,-11.59,;-9.4,-13.9,;6.6,-15.44,;6.6,-13.9,;7.93,-16.21,;9.27,-15.44,;9.27,-13.9,;10.6,-13.13,;10.6,-11.59,;11.94,-13.9,;10.6,-16.21,;10.6,-17.75,;11.94,-15.44,;13.27,-16.21,;13.27,-17.75,;11.94,-18.52,;11.94,-20.06,;10.6,-20.83,;10.6,-22.37,;9.27,-23.14,;11.94,-23.14,;14.6,-15.44,;14.6,-13.9,;15.94,-16.21,;17.27,-15.44,;17.27,-13.9,;15.94,-13.13,;15.71,-11.29,;14.63,-10.2,;15.04,-8.71,;16.59,-8.74,;17.38,-7.41,;18.92,-7.43,;16.62,-6.07,;15.22,-6.04,;14.79,-4.38,;16.14,-3.62,;17.27,-4.66,;18.6,-3.89,;18.6,-2.35,;19.94,-4.66,;19.94,-6.2,;21.27,-6.97,;21.27,-8.51,;19.94,-9.28,;19.94,-10.82,;18.6,-11.59,;21.27,-11.59,;21.27,-3.89,;22.61,-4.66,;22.61,-6.2,;23.94,-3.89,;23.94,-2.35,;22.61,-1.58,;22.61,-.04,;21.28,.73,;19.95,-.04,;19.95,-1.59,;21.27,-2.35,;25.27,-4.66,;26.61,-3.89,;26.61,-2.35,;27.94,-4.66,;27.94,-6.2,;26.61,-6.97,;25.27,-6.2,;26.61,-8.51,;27.94,-9.28,;27.94,-10.82,;29.27,-11.59,;29.27,-13.13,;30.61,-13.9,;31.94,-13.13,;30.61,-15.44,;25.27,-9.28,;25.27,-10.82,;23.94,-11.59,;26.61,-11.59,;26.61,-13.13,;25.27,-13.9,;23.94,-13.13,;25.27,-15.44,;26.61,-16.21,;26.61,-17.75,;27.94,-18.52,;25.27,-18.52,;23.94,-16.21,;22.61,-15.44,;22.61,-13.9,;21.27,-16.21,;21.27,-17.75,;19.94,-18.52,;19.94,-20.06,;18.6,-20.83,;19.94,-15.44,;18.6,-16.21,;18.6,-17.75,;29.27,-3.89,;30.61,-4.66,;29.27,-2.35,;13.71,-7.94,;13.71,-6.4,;12.38,-8.71,;11.04,-7.94,;11.05,-6.4,;9.71,-5.63,;8.29,-6.29,;7.28,-5.11,;8.05,-3.78,;7.58,-2.33,;8.6,-1.2,;10.11,-1.52,;10.57,-2.98,;9.54,-4.11,;9.71,-8.71,;9.71,-10.25,;8.38,-7.93,;7.04,-8.7,;7.04,-10.24,;5.71,-11.01,;5.7,-12.55,;7.19,-12.94,;5.3,-14.03,;5.71,-7.93,;4.38,-8.7,;3.04,-7.93,;3.04,-6.39,;1.71,-5.62,;1.72,-4.07,;.39,-3.3,;-.95,-4.07,;-2.28,-3.3,;-.94,-5.62,;.39,-6.38,;1.71,-8.7,;.38,-7.92,;1.71,-10.24,)| Show InChI InChI=1S/C109H162N30O25S4/c1-58(2)45-75-91(148)123-55-86(142)125-70(27-17-39-118-107(112)113)95(152)138-89(60(5)6)104(161)135-78(48-62-23-13-10-14-24-62)98(155)129-74(29-19-41-120-109(116)117)105(162)139-42-20-30-84(139)103(160)137-83(102(159)133-79(50-64-53-121-69-26-16-15-25-67(64)69)96(153)124-65(33-36-85(111)141)54-122-81(106(163)164)49-63-31-34-66(140)35-32-63)57-168-167-56-82(101(158)128-73(38-44-166-8)93(150)131-75)136-92(149)71(28-18-40-119-108(114)115)126-97(154)76(46-59(3)4)132-94(151)72(37-43-165-7)127-100(157)80(52-88(145)146)134-99(156)77(47-61-21-11-9-12-22-61)130-90(147)68(110)51-87(143)144/h9-16,21-26,31-32,34-35,53,58-60,65,68,70-84,89,121-122,140H,17-20,27-30,33,36-52,54-57,110H2,1-8H3,(H2,111,141)(H,123,148)(H,124,153)(H,125,142)(H,126,154)(H,127,157)(H,128,158)(H,129,155)(H,130,147)(H,131,150)(H,132,151)(H,133,159)(H,134,156)(H,135,161)(H,136,149)(H,137,160)(H,138,152)(H,143,144)(H,145,146)(H,163,164)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120)/t65-,68-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,89-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S] 4-(1-(3,4-difluorophenyl)-2-(ethyl(3-(6-fluoro-3H-spiro[isobenzofuran-1,4'-piperidine]-1'-yl)propyl)amino)-2-oxoethyl)-3-oxopip... |
Bioorg Med Chem Lett 19: 2835-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.102
BindingDB Entry DOI: 10.7270/Q2R49QS7 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Mus musculus) | BDBM50295693

(CHEMBL557629)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCSC)NC1=O)C(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(N)=O)CN[C@@H](Cc1ccc(O)cc1)C(O)=O |r,wU:27.28,36.36,71.73,148.153,55.55,60.134,8.12,wD:112.115,4.4,97.100,93.131,82.84,16.24,134.137,44.44,64.69,156.161,120.123,(5.27,-20.83,;6.6,-20.06,;6.6,-18.52,;5.27,-17.75,;5.27,-16.21,;3.93,-15.44,;2.6,-16.21,;2.6,-17.75,;1.27,-15.44,;1.27,-13.9,;2.6,-13.13,;3.93,-13.9,;2.6,-11.59,;-.07,-16.21,;-1.4,-15.44,;-1.4,-13.9,;-2.73,-16.21,;-2.73,-17.75,;-1.4,-18.52,;-.07,-17.74,;1.27,-18.51,;1.27,-20.05,;-.08,-20.82,;-1.4,-20.05,;-4.07,-15.44,;-5.4,-16.21,;-5.4,-17.75,;-6.74,-15.44,;-8.07,-16.21,;-6.74,-13.9,;-8.07,-13.13,;-8.07,-11.59,;-9.4,-13.9,;6.6,-15.44,;6.6,-13.9,;7.93,-16.21,;9.27,-15.44,;9.27,-13.9,;10.6,-13.13,;10.6,-11.59,;11.94,-13.9,;10.6,-16.21,;10.6,-17.75,;11.94,-15.44,;13.27,-16.21,;13.27,-17.75,;11.94,-18.52,;11.94,-20.06,;10.6,-20.83,;10.6,-22.37,;9.27,-23.14,;11.94,-23.14,;14.6,-15.44,;14.6,-13.9,;15.94,-16.21,;17.27,-15.44,;17.27,-13.9,;15.94,-13.13,;15.71,-11.29,;14.63,-10.2,;15.04,-8.71,;16.59,-8.74,;17.38,-7.41,;18.92,-7.43,;16.62,-6.07,;15.22,-6.04,;14.79,-4.38,;16.14,-3.62,;17.27,-4.66,;18.6,-3.89,;18.6,-2.35,;19.94,-4.66,;19.94,-6.2,;21.27,-6.97,;21.27,-8.51,;19.94,-9.28,;19.94,-10.82,;18.6,-11.59,;21.27,-11.59,;21.27,-3.89,;22.61,-4.66,;22.61,-6.2,;23.94,-3.89,;23.94,-2.35,;22.61,-1.58,;22.61,-.04,;21.28,.73,;19.95,-.04,;19.95,-1.59,;21.27,-2.35,;25.27,-4.66,;26.61,-3.89,;26.61,-2.35,;27.94,-4.66,;27.94,-6.2,;26.61,-6.97,;25.27,-6.2,;26.61,-8.51,;27.94,-9.28,;27.94,-10.82,;29.27,-11.59,;29.27,-13.13,;30.61,-13.9,;31.94,-13.13,;30.61,-15.44,;25.27,-9.28,;25.27,-10.82,;23.94,-11.59,;26.61,-11.59,;26.61,-13.13,;25.27,-13.9,;23.94,-13.13,;25.27,-15.44,;26.61,-16.21,;26.61,-17.75,;27.94,-18.52,;25.27,-18.52,;23.94,-16.21,;22.61,-15.44,;22.61,-13.9,;21.27,-16.21,;21.27,-17.75,;19.94,-18.52,;19.94,-20.06,;18.6,-20.83,;19.94,-15.44,;18.6,-16.21,;18.6,-17.75,;29.27,-3.89,;30.61,-4.66,;29.27,-2.35,;13.71,-7.94,;13.71,-6.4,;12.38,-8.71,;11.04,-7.94,;11.05,-6.4,;9.71,-5.63,;8.29,-6.29,;7.28,-5.11,;8.05,-3.78,;7.58,-2.33,;8.6,-1.2,;10.11,-1.52,;10.57,-2.98,;9.54,-4.11,;9.71,-8.71,;9.71,-10.25,;8.38,-7.93,;7.04,-8.7,;7.04,-10.24,;5.71,-11.01,;5.7,-12.55,;7.19,-12.94,;5.3,-14.03,;5.71,-7.93,;4.38,-8.7,;3.04,-7.93,;3.04,-6.39,;1.71,-5.62,;1.72,-4.07,;.39,-3.3,;-.95,-4.07,;-2.28,-3.3,;-.94,-5.62,;.39,-6.38,;1.71,-8.7,;.38,-7.92,;1.71,-10.24,)| Show InChI InChI=1S/C109H162N30O25S4/c1-58(2)45-75-91(148)123-55-86(142)125-70(27-17-39-118-107(112)113)95(152)138-89(60(5)6)104(161)135-78(48-62-23-13-10-14-24-62)98(155)129-74(29-19-41-120-109(116)117)105(162)139-42-20-30-84(139)103(160)137-83(102(159)133-79(50-64-53-121-69-26-16-15-25-67(64)69)96(153)124-65(33-36-85(111)141)54-122-81(106(163)164)49-63-31-34-66(140)35-32-63)57-168-167-56-82(101(158)128-73(38-44-166-8)93(150)131-75)136-92(149)71(28-18-40-119-108(114)115)126-97(154)76(46-59(3)4)132-94(151)72(37-43-165-7)127-100(157)80(52-88(145)146)134-99(156)77(47-61-21-11-9-12-22-61)130-90(147)68(110)51-87(143)144/h9-16,21-26,31-32,34-35,53,58-60,65,68,70-84,89,121-122,140H,17-20,27-30,33,36-52,54-57,110H2,1-8H3,(H2,111,141)(H,123,148)(H,124,153)(H,125,142)(H,126,154)(H,127,157)(H,128,158)(H,129,155)(H,130,147)(H,131,150)(H,132,151)(H,133,159)(H,134,156)(H,135,161)(H,136,149)(H,137,160)(H,138,152)(H,143,144)(H,145,146)(H,163,164)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120)/t65-,68-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,89-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S] 4-(1-(3,4-difluorophenyl)-2-(ethyl(3-(6-fluoro-3H-spiro[isobenzofuran-1,4'-piperidine]-1'-yl)propyl)amino)-2-oxoethyl)-3-oxopip... |
Bioorg Med Chem Lett 19: 2835-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.102
BindingDB Entry DOI: 10.7270/Q2R49QS7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391602

(CHEMBL2147915)Show SMILES CN1CC[C@]23Cc4nc5c(O)cccc5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:18:28.22.21| Show InChI InChI=1S/C24H24N2O3/c1-26-8-7-23-13-19-16(9-15-3-2-4-20(28)22(15)25-19)12-24(23,29)21(26)10-14-5-6-17(27)11-18(14)23/h2-6,9,11,21,27-29H,7-8,10,12-13H2,1H3/t21-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50143784

((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-2-butyl-7-[2-((...)Show SMILES CCCCc1ccc2[C@@H]([C@H]([C@@H](c2n1)c1ccc(OC)cc1C[C@H](C)C(O)=O)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C31H33NO7/c1-4-5-6-20-8-10-23-26(18-7-12-24-25(15-18)39-16-38-24)28(31(35)36)27(29(23)32-20)22-11-9-21(37-3)14-19(22)13-17(2)30(33)34/h7-12,14-15,17,26-28H,4-6,13,16H2,1-3H3,(H,33,34)(H,35,36)/t17-,26-,27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 1262-70 (1999)
BindingDB Entry DOI: 10.7270/Q2Q52N5Q |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50505569
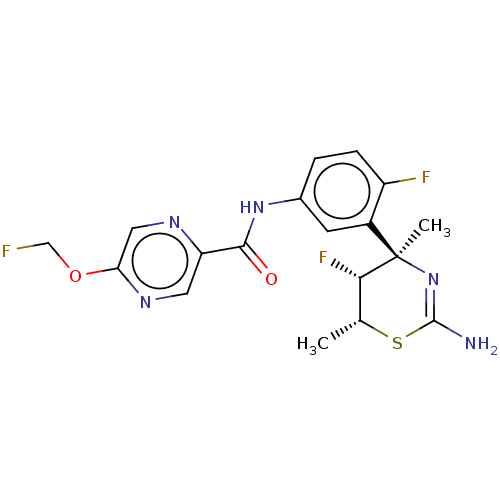
(CHEMBL4557670)Show SMILES C[C@H]1SC(N)=N[C@@](C)([C@H]1F)c1cc(NC(=O)c2cnc(OCF)cn2)ccc1F |r,c:4| Show InChI InChI=1S/C18H18F3N5O2S/c1-9-15(21)18(2,26-17(22)29-9)11-5-10(3-4-12(11)20)25-16(27)13-6-24-14(7-23-13)28-8-19/h3-7,9,15H,8H2,1-2H3,(H2,22,26)(H,25,27)/t9-,15+,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H] JNJ-962 from BACE2 (unknown origin) expressed in HEK293 cell membranes assessed as inhibition constant at pH 6.2 by scintillatio... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00359
BindingDB Entry DOI: 10.7270/Q2WW7NJB |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM210070
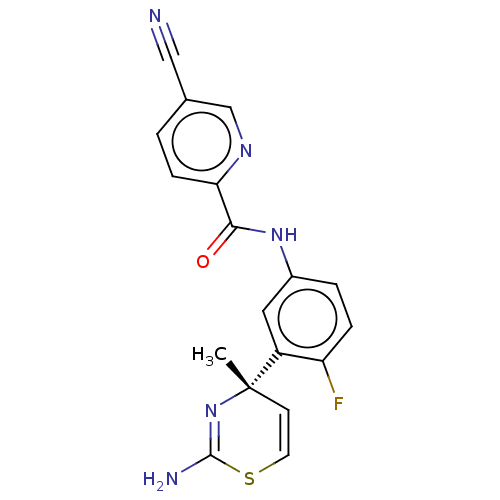
(US9270353, 17)Show SMILES C[C@]1(C=CSC(N)=N1)c1cc(NC(=O)c2ccc(cn2)C#N)ccc1F |r,c:2,6| Show InChI InChI=1S/C18H14FN5OS/c1-18(6-7-26-17(21)24-18)13-8-12(3-4-14(13)19)23-16(25)15-5-2-11(9-20)10-22-15/h2-8,10H,1H3,(H2,21,24)(H,23,25)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H] JNJ-962 from BACE1 (unknown origin) expressed in HEK293 cell membranes assessed as inhibition constant at pH 6.2 by scintillatio... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00359
BindingDB Entry DOI: 10.7270/Q2WW7NJB |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50329100
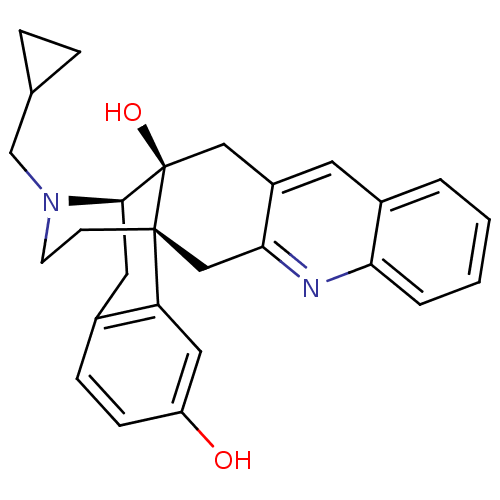
(CHEMBL1270475)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]4(Cc5nc6ccccc6cc5C[C@@]34O)c2c1 |r,THB:8:7:27:4.29.5| Show InChI InChI=1S/C27H28N2O2/c30-21-8-7-18-12-25-27(31)14-20-11-19-3-1-2-4-23(19)28-24(20)15-26(27,22(18)13-21)9-10-29(25)16-17-5-6-17/h1-4,7-8,11,13,17,25,30-31H,5-6,9-10,12,14-16H2/t25-,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain membranes |
Bioorg Med Chem Lett 20: 6302-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.083
BindingDB Entry DOI: 10.7270/Q2G73DZ1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50294428
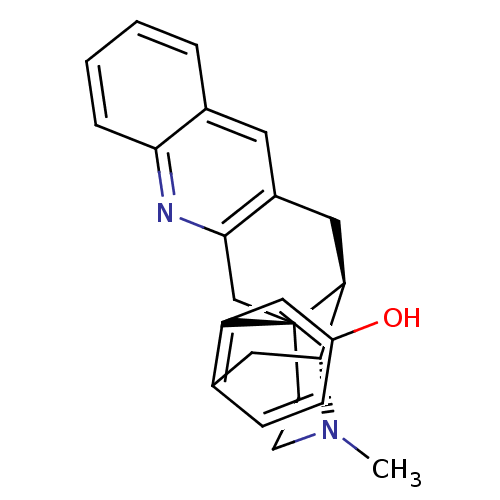
(CHEMBL552308 | SN-28)Show SMILES CN1CC[C@]23Cc4nc5ccccc5cc4C[C@H]2[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:17:26.20.19| Show InChI InChI=1S/C24H24N2O/c1-26-9-8-24-14-22-17(10-16-4-2-3-5-21(16)25-22)11-20(24)23(26)12-15-6-7-18(27)13-19(15)24/h2-7,10,13,20,23,27H,8-9,11-12,14H2,1H3/t20-,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain membranes |
Bioorg Med Chem Lett 20: 6302-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.083
BindingDB Entry DOI: 10.7270/Q2G73DZ1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391591
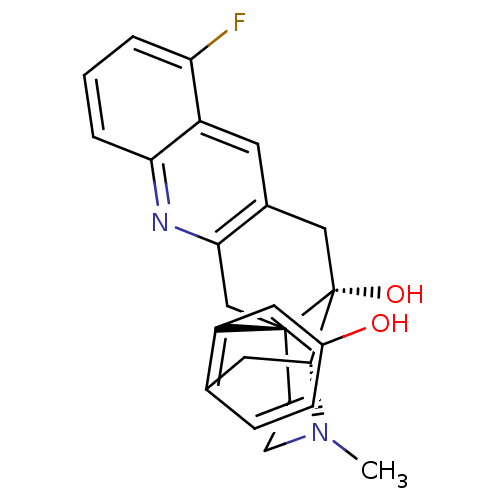
(CHEMBL2147904)Show SMILES CN1CC[C@]23Cc4nc5cccc(F)c5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:18:28.22.21| Show InChI InChI=1S/C24H23FN2O2/c1-27-8-7-23-13-21-15(9-17-19(25)3-2-4-20(17)26-21)12-24(23,29)22(27)10-14-5-6-16(28)11-18(14)23/h2-6,9,11,22,28-29H,7-8,10,12-13H2,1H3/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391594
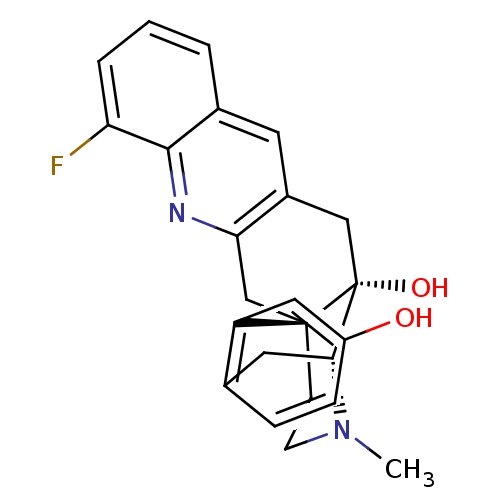
(CHEMBL2147907)Show SMILES CN1CC[C@]23Cc4nc5c(F)cccc5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:18:28.22.21| Show InChI InChI=1S/C24H23FN2O2/c1-27-8-7-23-13-20-16(9-15-3-2-4-19(25)22(15)26-20)12-24(23,29)21(27)10-14-5-6-17(28)11-18(14)23/h2-6,9,11,21,28-29H,7-8,10,12-13H2,1H3/t21-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391595
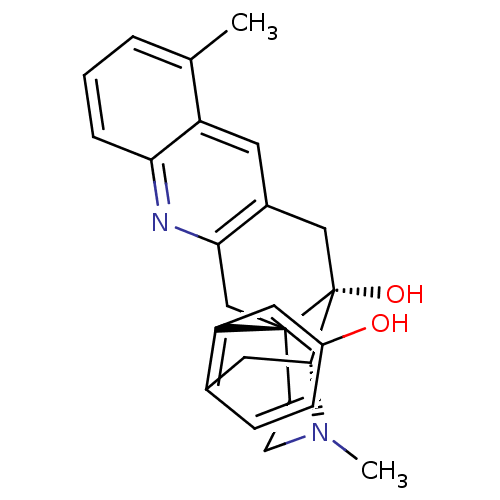
(CHEMBL2147908)Show SMILES CN1CC[C@]23Cc4nc5cccc(C)c5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:18:28.22.21| Show InChI InChI=1S/C25H26N2O2/c1-15-4-3-5-21-19(15)10-17-13-25(29)23-11-16-6-7-18(28)12-20(16)24(25,8-9-27(23)2)14-22(17)26-21/h3-7,10,12,23,28-29H,8-9,11,13-14H2,1-2H3/t23-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391603

(CHEMBL2147916)Show SMILES COc1cccc2nc3C[C@]45CCN(C)[C@H](Cc6ccc(O)cc46)[C@]5(O)Cc3cc12 |r,TLB:14:13:24:23.17.16| Show InChI InChI=1S/C25H26N2O3/c1-27-9-8-24-14-21-16(10-18-20(26-21)4-3-5-22(18)30-2)13-25(24,29)23(27)11-15-6-7-17(28)12-19(15)24/h3-7,10,12,23,28-29H,8-9,11,13-14H2,1-2H3/t23-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50329095
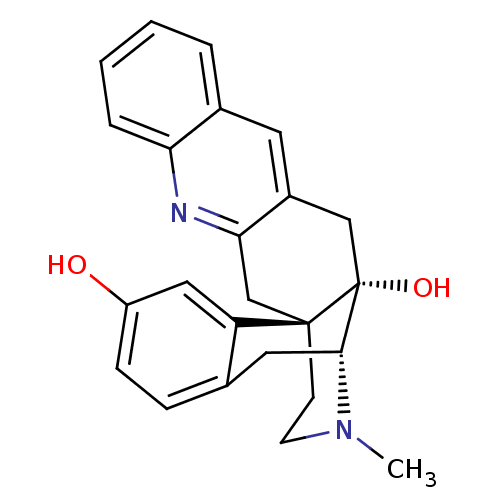
(CHEMBL1270282)Show SMILES CN1CC[C@]23Cc4nc5ccccc5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:17:21.27.20| Show InChI InChI=1S/C24H24N2O2/c1-26-9-8-23-14-21-17(10-16-4-2-3-5-20(16)25-21)13-24(23,28)22(26)11-15-6-7-18(27)12-19(15)23/h2-7,10,12,22,27-28H,8-9,11,13-14H2,1H3/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50329095

(CHEMBL1270282)Show SMILES CN1CC[C@]23Cc4nc5ccccc5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:17:21.27.20| Show InChI InChI=1S/C24H24N2O2/c1-26-9-8-23-14-21-17(10-16-4-2-3-5-20(16)25-21)13-24(23,28)22(26)11-15-6-7-18(27)12-19(15)23/h2-7,10,12,22,27-28H,8-9,11,13-14H2,1H3/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain membranes |
Bioorg Med Chem Lett 20: 6302-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.083
BindingDB Entry DOI: 10.7270/Q2G73DZ1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM21864

((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...)Show SMILES [H][C@@]12Cc3ccc(O)c4OC5c6[nH]c7ccccc7c6CC1(O)C5(CCN2CC1CC1)c34 |THB:27:26:21:31.2.3| Show InChI InChI=1S/C26H26N2O3/c29-19-8-7-15-11-20-26(30)12-17-16-3-1-2-4-18(16)27-22(17)24-25(26,21(15)23(19)31-24)9-10-28(20)13-14-5-6-14/h1-4,7-8,14,20,24,27,29-30H,5-6,9-13H2/t20-,24?,25?,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50295690
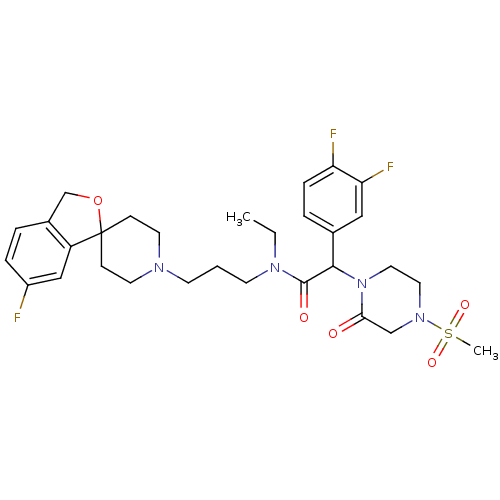
((+/-)-2-(3,4-difluorophenyl)-N-ethyl-N-(3-(6-fluor...)Show SMILES CCN(CCCN1CCC2(CC1)OCc1ccc(F)cc21)C(=O)C(N1CCN(CC1=O)S(C)(=O)=O)c1ccc(F)c(F)c1 Show InChI InChI=1S/C30H37F3N4O5S/c1-3-35(12-4-11-34-13-9-30(10-14-34)24-18-23(31)7-5-22(24)20-42-30)29(39)28(21-6-8-25(32)26(33)17-21)37-16-15-36(19-27(37)38)43(2,40)41/h5-8,17-18,28H,3-4,9-16,19-20H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human MCH1R expressed in CHO cells by scintillation counting per mg of protein |
Bioorg Med Chem Lett 19: 2835-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.102
BindingDB Entry DOI: 10.7270/Q2R49QS7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116748
BindingDB Entry DOI: 10.7270/Q2MG7TGW |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM85790
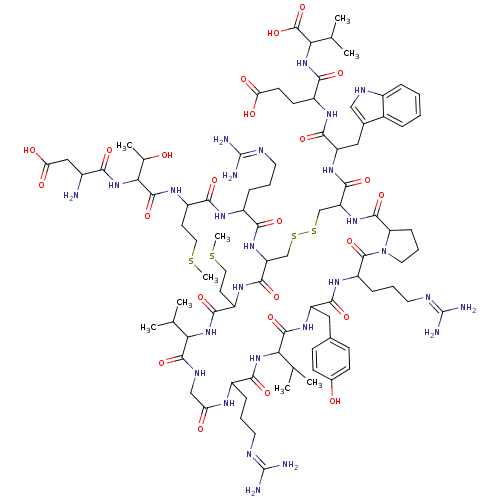
(Salmon MCH)Show SMILES CSCCC(NC(=O)C(NC(=O)C(N)CC(O)=O)C(C)O)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(NC(=O)C(CCSC)NC1=O)C(C)C)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(O)=O)C(=O)NC(C(C)C)C(O)=O |(16.32,3.32,;17.66,2.55,;18.99,3.32,;18.99,4.86,;20.32,5.63,;20.32,7.17,;21.66,7.94,;22.99,7.17,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;25.66,11.79,;24.33,14.1,;25.66,14.87,;25.66,16.41,;26.99,14.1,;20.32,10.25,;18.99,9.48,;20.32,11.79,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;26.99,-15.16,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;30.99,2.55,;32.33,3.32,;29.66,3.32,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;7.91,-1.3,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C89H139N27O24S4/c1-43(2)67-82(135)101-40-64(119)102-53(18-12-30-97-87(91)92)74(127)113-68(44(3)4)83(136)109-59(36-47-22-24-49(118)25-23-47)77(130)107-58(20-14-32-99-89(95)96)85(138)116-33-15-21-63(116)81(134)111-62(80(133)108-60(37-48-39-100-52-17-11-10-16-50(48)52)78(131)104-55(26-27-65(120)121)75(128)114-69(45(5)6)86(139)140)42-144-143-41-61(79(132)105-57(29-35-142-9)76(129)112-67)110-72(125)54(19-13-31-98-88(93)94)103-73(126)56(28-34-141-8)106-84(137)70(46(7)117)115-71(124)51(90)38-66(122)123/h10-11,16-17,22-25,39,43-46,51,53-63,67-70,100,117-118H,12-15,18-21,26-38,40-42,90H2,1-9H3,(H,101,135)(H,102,119)(H,103,126)(H,104,131)(H,105,132)(H,106,137)(H,107,130)(H,108,133)(H,109,136)(H,110,125)(H,111,134)(H,112,129)(H,113,127)(H,114,128)(H,115,124)(H,120,121)(H,122,123)(H,139,140)(H4,91,92,97)(H4,93,94,98)(H4,95,96,99) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM85790

(Salmon MCH)Show SMILES CSCCC(NC(=O)C(NC(=O)C(N)CC(O)=O)C(C)O)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(NC(=O)C(CCSC)NC1=O)C(C)C)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(O)=O)C(=O)NC(C(C)C)C(O)=O |(16.32,3.32,;17.66,2.55,;18.99,3.32,;18.99,4.86,;20.32,5.63,;20.32,7.17,;21.66,7.94,;22.99,7.17,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;25.66,11.79,;24.33,14.1,;25.66,14.87,;25.66,16.41,;26.99,14.1,;20.32,10.25,;18.99,9.48,;20.32,11.79,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;26.99,-15.16,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;30.99,2.55,;32.33,3.32,;29.66,3.32,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;7.91,-1.3,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C89H139N27O24S4/c1-43(2)67-82(135)101-40-64(119)102-53(18-12-30-97-87(91)92)74(127)113-68(44(3)4)83(136)109-59(36-47-22-24-49(118)25-23-47)77(130)107-58(20-14-32-99-89(95)96)85(138)116-33-15-21-63(116)81(134)111-62(80(133)108-60(37-48-39-100-52-17-11-10-16-50(48)52)78(131)104-55(26-27-65(120)121)75(128)114-69(45(5)6)86(139)140)42-144-143-41-61(79(132)105-57(29-35-142-9)76(129)112-67)110-72(125)54(19-13-31-98-88(93)94)103-73(126)56(28-34-141-8)106-84(137)70(46(7)117)115-71(124)51(90)38-66(122)123/h10-11,16-17,22-25,39,43-46,51,53-63,67-70,100,117-118H,12-15,18-21,26-38,40-42,90H2,1-9H3,(H,101,135)(H,102,119)(H,103,126)(H,104,131)(H,105,132)(H,106,137)(H,107,130)(H,108,133)(H,109,136)(H,110,125)(H,111,134)(H,112,129)(H,113,127)(H,114,128)(H,115,124)(H,120,121)(H,122,123)(H,139,140)(H4,91,92,97)(H4,93,94,98)(H4,95,96,99) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50329098
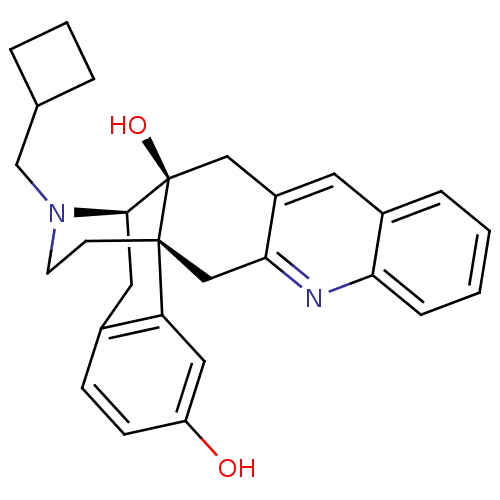
(CHEMBL1270383)Show SMILES Oc1ccc2C[C@H]3N(CC4CCC4)CC[C@@]4(Cc5nc6ccccc6cc5C[C@@]34O)c2c1 |r,THB:8:7:28:4.30.5| Show InChI InChI=1S/C28H30N2O2/c31-22-9-8-19-13-26-28(32)15-21-12-20-6-1-2-7-24(20)29-25(21)16-27(28,23(19)14-22)10-11-30(26)17-18-4-3-5-18/h1-2,6-9,12,14,18,26,31-32H,3-5,10-11,13,15-17H2/t26-,27-,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain membranes |
Bioorg Med Chem Lett 20: 6302-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.083
BindingDB Entry DOI: 10.7270/Q2G73DZ1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391582

(CHEMBL2147927)Show SMILES CN1CC[C@]23Cc4nc5c(cccc5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31)[N+]([O-])=O |r,THB:0:1:17:27.21.20| Show InChI InChI=1S/C24H23N3O4/c1-26-8-7-23-13-19-16(9-15-3-2-4-20(27(30)31)22(15)25-19)12-24(23,29)21(26)10-14-5-6-17(28)11-18(14)23/h2-6,9,11,21,28-29H,7-8,10,12-13H2,1H3/t21-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.218 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391590
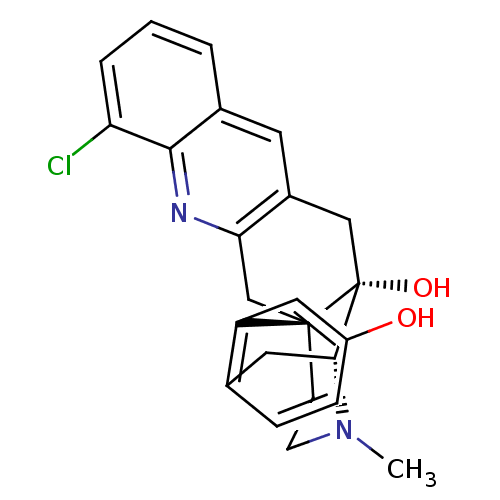
(CHEMBL2147903)Show SMILES CN1CC[C@]23Cc4nc5c(Cl)cccc5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:18:28.22.21| Show InChI InChI=1S/C24H23ClN2O2/c1-27-8-7-23-13-20-16(9-15-3-2-4-19(25)22(15)26-20)12-24(23,29)21(27)10-14-5-6-17(28)11-18(14)23/h2-6,9,11,21,28-29H,7-8,10,12-13H2,1H3/t21-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.235 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391607
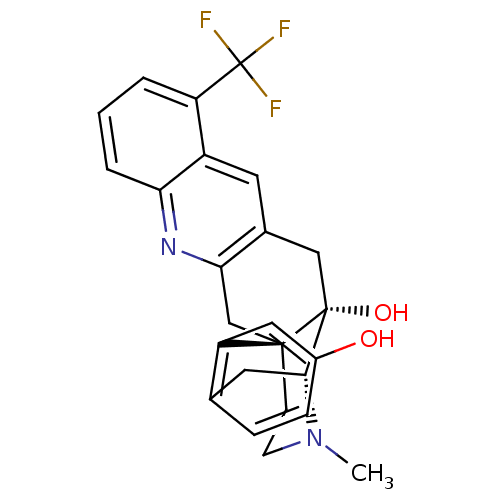
(CHEMBL2147920)Show SMILES CN1CC[C@]23Cc4nc5cccc(c5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31)C(F)(F)F |r,THB:0:1:17:27.21.20| Show InChI InChI=1S/C25H23F3N2O2/c1-30-8-7-23-13-21-15(9-17-18(25(26,27)28)3-2-4-20(17)29-21)12-24(23,32)22(30)10-14-5-6-16(31)11-19(14)23/h2-6,9,11,22,31-32H,7-8,10,12-13H2,1H3/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.244 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391593

(CHEMBL2147906)Show SMILES CN1CC[C@]23Cc4nc5cc(F)ccc5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:18:28.22.21| Show InChI InChI=1S/C24H23FN2O2/c1-27-7-6-23-13-21-16(8-15-2-4-17(25)10-20(15)26-21)12-24(23,29)22(27)9-14-3-5-18(28)11-19(14)23/h2-5,8,10-11,22,28-29H,6-7,9,12-13H2,1H3/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.255 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391598

(CHEMBL2147911)Show SMILES CN1CC[C@]23Cc4nc5c(C)cccc5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:18:28.22.21| Show InChI InChI=1S/C25H26N2O2/c1-15-4-3-5-17-10-18-13-25(29)22-11-16-6-7-19(28)12-20(16)24(25,8-9-27(22)2)14-21(18)26-23(15)17/h3-7,10,12,22,28-29H,8-9,11,13-14H2,1-2H3/t22-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391601
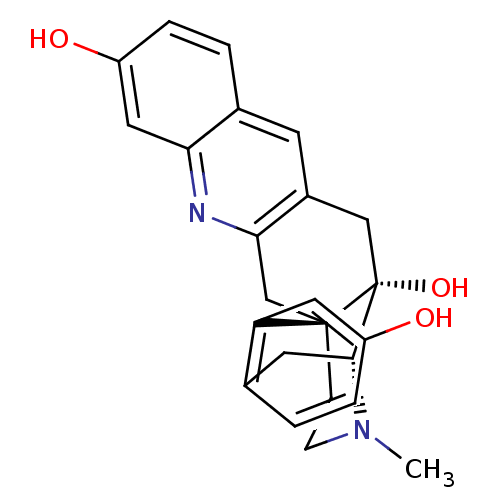
(CHEMBL2147914)Show SMILES CN1CC[C@]23Cc4nc5cc(O)ccc5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:18:28.22.21| Show InChI InChI=1S/C24H24N2O3/c1-26-7-6-23-13-21-16(8-15-3-5-18(28)11-20(15)25-21)12-24(23,29)22(26)9-14-2-4-17(27)10-19(14)23/h2-5,8,10-11,22,27-29H,6-7,9,12-13H2,1H3/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50580217
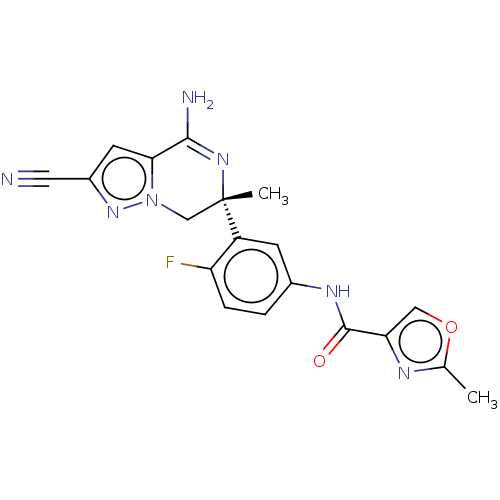
(CHEMBL5093195)Show SMILES Cc1nc(co1)C(=O)Nc1ccc(F)c(c1)[C@]1(C)Cn2nc(cc2C(N)=N1)C#N |r,c:28| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to BACE2 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00359
BindingDB Entry DOI: 10.7270/Q2WW7NJB |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM85789
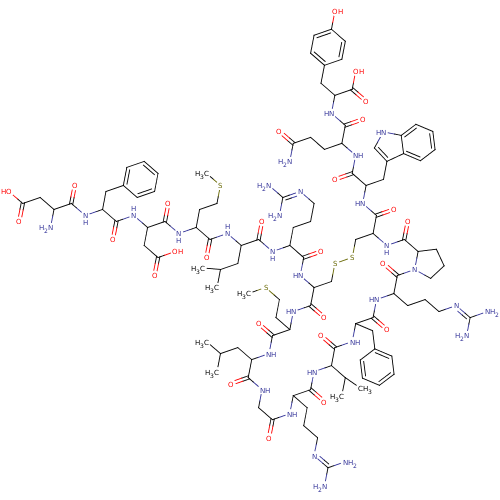
([Phe13,Tyr19]MCH)Show SMILES CSCCC(NC(=O)C(CC(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)C(N)CC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccccc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(CC(C)C)NC(=O)C(CCSC)NC1=O)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(N)=O)C(=O)NC(Cc1ccc(O)cc1)C(O)=O |(16.32,9.48,;17.66,10.25,;18.99,9.48,;20.32,10.25,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;24.33,14.1,;23.24,15.19,;23.63,16.68,;21.75,14.79,;25.66,11.79,;26.99,12.56,;26.99,14.1,;28.33,11.79,;28.33,10.25,;29.66,9.48,;29.66,7.94,;30.99,7.17,;32.33,7.94,;32.33,9.48,;30.99,10.25,;29.66,12.56,;31.15,12.16,;31.55,10.67,;32.48,12.93,;33.81,12.16,;32.48,14.47,;33.81,15.24,;33.81,16.78,;35.3,14.84,;21.66,7.94,;22.99,7.17,;20.32,7.17,;20.32,5.63,;18.99,4.86,;18.99,3.32,;17.66,2.55,;20.32,2.55,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;30.99,2.55,;32.33,3.32,;32.33,4.86,;33.66,2.55,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;5.24,.24,;3.91,1.01,;2.57,.24,;1.24,1.01,;2.57,-1.3,;3.91,-2.07,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C109H160N30O26S4/c1-57(2)45-74-90(148)122-54-85(142)123-68(27-17-39-118-107(112)113)95(153)138-88(59(5)6)104(162)134-77(48-61-23-13-10-14-24-61)97(155)128-73(29-19-41-120-109(116)117)105(163)139-42-20-30-83(139)103(161)137-82(102(160)132-78(50-63-53-121-67-26-16-15-25-65(63)67)99(157)125-70(35-36-84(111)141)92(150)135-80(106(164)165)49-62-31-33-64(140)34-32-62)56-169-168-55-81(101(159)127-72(38-44-167-8)93(151)130-74)136-91(149)69(28-18-40-119-108(114)115)124-96(154)75(46-58(3)4)131-94(152)71(37-43-166-7)126-100(158)79(52-87(145)146)133-98(156)76(47-60-21-11-9-12-22-60)129-89(147)66(110)51-86(143)144/h9-16,21-26,31-34,53,57-59,66,68-83,88,121,140H,17-20,27-30,35-52,54-56,110H2,1-8H3,(H2,111,141)(H,122,148)(H,123,142)(H,124,154)(H,125,157)(H,126,158)(H,127,159)(H,128,155)(H,129,147)(H,130,151)(H,131,152)(H,132,160)(H,133,156)(H,134,162)(H,135,150)(H,136,149)(H,137,161)(H,138,153)(H,143,144)(H,145,146)(H,164,165)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM85789

([Phe13,Tyr19]MCH)Show SMILES CSCCC(NC(=O)C(CC(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)C(N)CC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccccc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(CC(C)C)NC(=O)C(CCSC)NC1=O)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(N)=O)C(=O)NC(Cc1ccc(O)cc1)C(O)=O |(16.32,9.48,;17.66,10.25,;18.99,9.48,;20.32,10.25,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;24.33,14.1,;23.24,15.19,;23.63,16.68,;21.75,14.79,;25.66,11.79,;26.99,12.56,;26.99,14.1,;28.33,11.79,;28.33,10.25,;29.66,9.48,;29.66,7.94,;30.99,7.17,;32.33,7.94,;32.33,9.48,;30.99,10.25,;29.66,12.56,;31.15,12.16,;31.55,10.67,;32.48,12.93,;33.81,12.16,;32.48,14.47,;33.81,15.24,;33.81,16.78,;35.3,14.84,;21.66,7.94,;22.99,7.17,;20.32,7.17,;20.32,5.63,;18.99,4.86,;18.99,3.32,;17.66,2.55,;20.32,2.55,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;30.99,2.55,;32.33,3.32,;32.33,4.86,;33.66,2.55,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;5.24,.24,;3.91,1.01,;2.57,.24,;1.24,1.01,;2.57,-1.3,;3.91,-2.07,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C109H160N30O26S4/c1-57(2)45-74-90(148)122-54-85(142)123-68(27-17-39-118-107(112)113)95(153)138-88(59(5)6)104(162)134-77(48-61-23-13-10-14-24-61)97(155)128-73(29-19-41-120-109(116)117)105(163)139-42-20-30-83(139)103(161)137-82(102(160)132-78(50-63-53-121-67-26-16-15-25-65(63)67)99(157)125-70(35-36-84(111)141)92(150)135-80(106(164)165)49-62-31-33-64(140)34-32-62)56-169-168-55-81(101(159)127-72(38-44-167-8)93(151)130-74)136-91(149)69(28-18-40-119-108(114)115)124-96(154)75(46-58(3)4)131-94(152)71(37-43-166-7)126-100(158)79(52-87(145)146)133-98(156)76(47-60-21-11-9-12-22-60)129-89(147)66(110)51-86(143)144/h9-16,21-26,31-34,53,57-59,66,68-83,88,121,140H,17-20,27-30,35-52,54-56,110H2,1-8H3,(H2,111,141)(H,122,148)(H,123,142)(H,124,154)(H,125,157)(H,126,158)(H,127,159)(H,128,155)(H,129,147)(H,130,151)(H,131,152)(H,132,160)(H,133,156)(H,134,162)(H,135,150)(H,136,149)(H,137,161)(H,138,153)(H,143,144)(H,145,146)(H,164,165)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391596
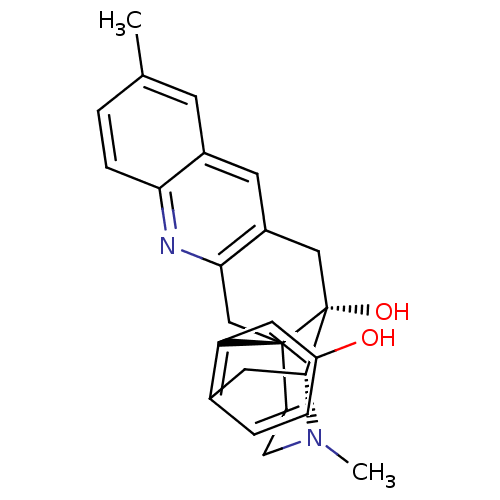
(CHEMBL2147909)Show SMILES CN1CC[C@]23Cc4nc5ccc(C)cc5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:18:28.22.21| Show InChI InChI=1S/C25H26N2O2/c1-15-3-6-21-17(9-15)10-18-13-25(29)23-11-16-4-5-19(28)12-20(16)24(25,7-8-27(23)2)14-22(18)26-21/h3-6,9-10,12,23,28-29H,7-8,11,13-14H2,1-2H3/t23-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50505569

(CHEMBL4557670)Show SMILES C[C@H]1SC(N)=N[C@@](C)([C@H]1F)c1cc(NC(=O)c2cnc(OCF)cn2)ccc1F |r,c:4| Show InChI InChI=1S/C18H18F3N5O2S/c1-9-15(21)18(2,26-17(22)29-9)11-5-10(3-4-12(11)20)25-16(27)13-6-24-14(7-23-13)28-8-19/h3-7,9,15H,8H2,1-2H3,(H2,22,26)(H,25,27)/t9-,15+,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H] JNJ-962 from BACE1 (unknown origin) expressed in HEK293 cell membranes assessed as inhibition constant at pH 6.2 by scintillatio... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00359
BindingDB Entry DOI: 10.7270/Q2WW7NJB |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391579

(CHEMBL2147924)Show SMILES CN1CC[C@]23Cc4nc5cccc([N+]([O-])=O)c5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:20:30.24.23| Show InChI InChI=1S/C24H23N3O4/c1-26-8-7-23-13-20-15(9-17-19(25-20)3-2-4-21(17)27(30)31)12-24(23,29)22(26)10-14-5-6-16(28)11-18(14)23/h2-6,9,11,22,28-29H,7-8,10,12-13H2,1H3/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.344 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391587
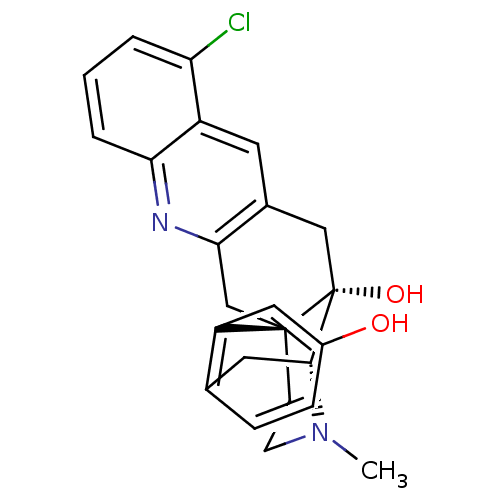
(CHEMBL2147891)Show SMILES CN1CC[C@]23Cc4nc5cccc(Cl)c5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:18:28.22.21| Show InChI InChI=1S/C24H23ClN2O2/c1-27-8-7-23-13-21-15(9-17-19(25)3-2-4-20(17)26-21)12-24(23,29)22(27)10-14-5-6-16(28)11-18(14)23/h2-6,9,11,22,28-29H,7-8,10,12-13H2,1H3/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.353 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391597

(CHEMBL2147910)Show SMILES CN1CC[C@]23Cc4nc5cc(C)ccc5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:18:28.22.21| Show InChI InChI=1S/C25H26N2O2/c1-15-3-4-17-10-18-13-25(29)23-11-16-5-6-19(28)12-20(16)24(25,7-8-27(23)2)14-22(18)26-21(17)9-15/h3-6,9-10,12,23,28-29H,7-8,11,13-14H2,1-2H3/t23-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.362 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50580216
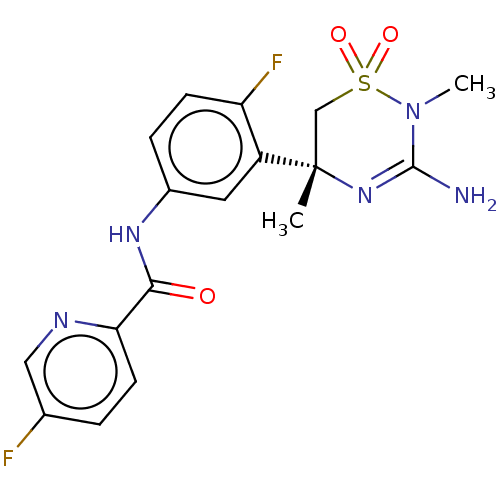
(MK-8931 | SCH 900931 | SCH-900931 | SCH900931 | VE...)Show SMILES CN1C(N)=N[C@@](C)(CS1(=O)=O)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r,c:3| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H] JNJ-962 from BACE1 (unknown origin) expressed in HEK293 cell membranes assessed as inhibition constant at pH 6.2 by scintillatio... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00359
BindingDB Entry DOI: 10.7270/Q2WW7NJB |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391589

(CHEMBL2147902)Show SMILES CN1CC[C@]23Cc4nc5cc(Cl)ccc5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:18:28.22.21| Show InChI InChI=1S/C24H23ClN2O2/c1-27-7-6-23-13-21-16(8-15-2-4-17(25)10-20(15)26-21)12-24(23,29)22(27)9-14-3-5-18(28)11-19(14)23/h2-5,8,10-11,22,28-29H,6-7,9,12-13H2,1H3/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.403 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50244722
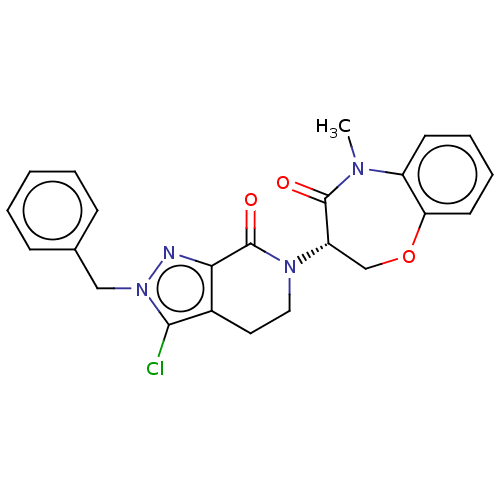
(CHEMBL4075976)Show SMILES CN1c2ccccc2OC[C@H](N2CCc3c(Cl)n(Cc4ccccc4)nc3C2=O)C1=O |r| Show InChI InChI=1S/C23H21ClN4O3/c1-26-17-9-5-6-10-19(17)31-14-18(22(26)29)27-12-11-16-20(23(27)30)25-28(21(16)24)13-15-7-3-2-4-8-15/h2-10,18H,11-14H2,1H3/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... |
J Med Chem 61: 2384-2409 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01647
BindingDB Entry DOI: 10.7270/Q2V98BGX |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50295690

((+/-)-2-(3,4-difluorophenyl)-N-ethyl-N-(3-(6-fluor...)Show SMILES CCN(CCCN1CCC2(CC1)OCc1ccc(F)cc21)C(=O)C(N1CCN(CC1=O)S(C)(=O)=O)c1ccc(F)c(F)c1 Show InChI InChI=1S/C30H37F3N4O5S/c1-3-35(12-4-11-34-13-9-30(10-14-34)24-18-23(31)7-5-22(24)20-42-30)29(39)28(21-6-8-25(32)26(33)17-21)37-16-15-36(19-27(37)38)43(2,40)41/h5-8,17-18,28H,3-4,9-16,19-20H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S] 4-(1-(3,4-difluorophenyl)-2-(ethyl(3-(6-fluoro-3H-spiro[isobenzofuran-1,4'-piperidine]-1'-yl)propyl)amino)-2-oxoethyl)-3-oxopip... |
Bioorg Med Chem Lett 19: 2835-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.102
BindingDB Entry DOI: 10.7270/Q2R49QS7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391578
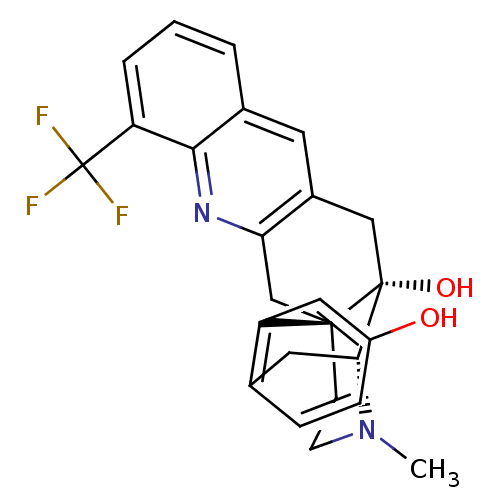
(CHEMBL2147923)Show SMILES CN1CC[C@]23Cc4nc5c(cccc5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31)C(F)(F)F |r,THB:0:1:17:27.21.20| Show InChI InChI=1S/C25H23F3N2O2/c1-30-8-7-23-13-20-16(9-15-3-2-4-18(22(15)29-20)25(26,27)28)12-24(23,32)21(30)10-14-5-6-17(31)11-19(14)23/h2-6,9,11,21,31-32H,7-8,10,12-13H2,1H3/t21-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.493 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391606

(CHEMBL2147919)Show SMILES COc1cccc2cc3C[C@@]4(O)[C@H]5Cc6ccc(O)cc6[C@@]4(CCN5C)Cc3nc12 |r,THB:25:24:10:20.14.13| Show InChI InChI=1S/C25H26N2O3/c1-27-9-8-24-14-20-17(10-16-4-3-5-21(30-2)23(16)26-20)13-25(24,29)22(27)11-15-6-7-18(28)12-19(15)24/h3-7,10,12,22,28-29H,8-9,11,13-14H2,1-2H3/t22-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.498 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Mus musculus) | BDBM50295690

((+/-)-2-(3,4-difluorophenyl)-N-ethyl-N-(3-(6-fluor...)Show SMILES CCN(CCCN1CCC2(CC1)OCc1ccc(F)cc21)C(=O)C(N1CCN(CC1=O)S(C)(=O)=O)c1ccc(F)c(F)c1 Show InChI InChI=1S/C30H37F3N4O5S/c1-3-35(12-4-11-34-13-9-30(10-14-34)24-18-23(31)7-5-22(24)20-42-30)29(39)28(21-6-8-25(32)26(33)17-21)37-16-15-36(19-27(37)38)43(2,40)41/h5-8,17-18,28H,3-4,9-16,19-20H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S] 4-(1-(3,4-difluorophenyl)-2-(ethyl(3-(6-fluoro-3H-spiro[isobenzofuran-1,4'-piperidine]-1'-yl)propyl)amino)-2-oxoethyl)-3-oxopip... |
Bioorg Med Chem Lett 19: 2835-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.102
BindingDB Entry DOI: 10.7270/Q2R49QS7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50329099
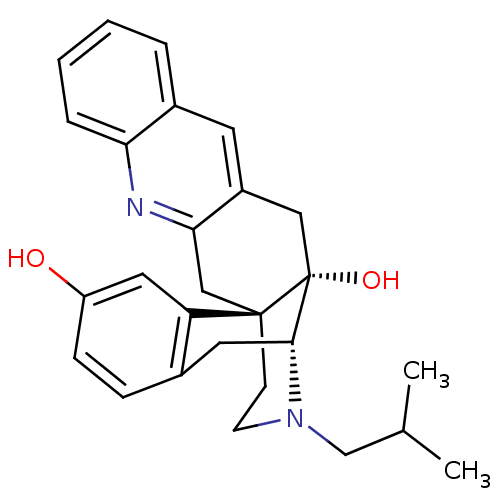
(CHEMBL1270474)Show SMILES CC(C)CN1CC[C@]23Cc4nc5ccccc5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31 |r,THB:3:4:20:24.30.23| Show InChI InChI=1S/C27H30N2O2/c1-17(2)16-29-10-9-26-15-24-20(11-19-5-3-4-6-23(19)28-24)14-27(26,31)25(29)12-18-7-8-21(30)13-22(18)26/h3-8,11,13,17,25,30-31H,9-10,12,14-16H2,1-2H3/t25-,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain membranes |
Bioorg Med Chem Lett 20: 6302-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.083
BindingDB Entry DOI: 10.7270/Q2G73DZ1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50329097
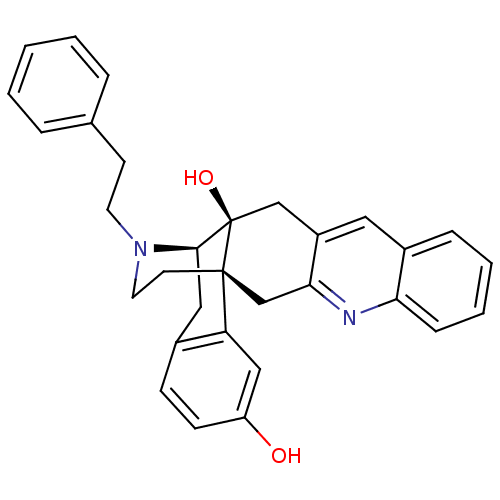
(CHEMBL1270185)Show SMILES Oc1ccc2C[C@H]3N(CCc4ccccc4)CC[C@@]4(Cc5nc6ccccc6cc5C[C@@]34O)c2c1 |r,THB:8:7:31:4.33.5| Show InChI InChI=1S/C31H30N2O2/c34-25-11-10-22-17-29-31(35)19-24-16-23-8-4-5-9-27(23)32-28(24)20-30(31,26(22)18-25)13-15-33(29)14-12-21-6-2-1-3-7-21/h1-11,16,18,29,34-35H,12-15,17,19-20H2/t29-,30-,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain membranes |
Bioorg Med Chem Lett 20: 6302-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.083
BindingDB Entry DOI: 10.7270/Q2G73DZ1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391588

(CHEMBL2147901)Show SMILES CN1CC[C@]23Cc4nc5ccc(Cl)cc5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:18:28.22.21| Show InChI InChI=1S/C24H23ClN2O2/c1-27-7-6-23-13-21-16(8-15-9-17(25)3-5-20(15)26-21)12-24(23,29)22(27)10-14-2-4-18(28)11-19(14)23/h2-5,8-9,11,22,28-29H,6-7,10,12-13H2,1H3/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.583 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391583
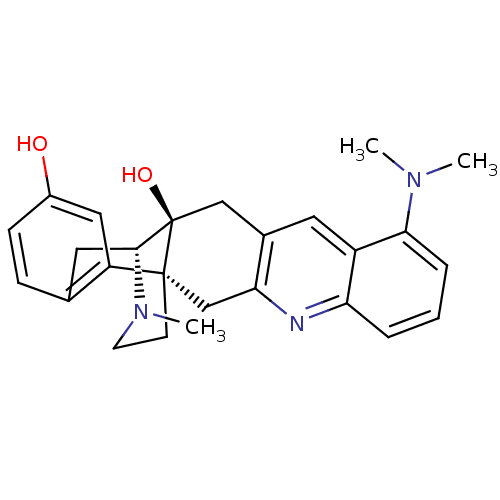
(CHEMBL2147928)Show SMILES CN(C)c1cccc2nc3C[C@]45CCN(C)[C@H](Cc6ccc(O)cc46)[C@]5(O)Cc3cc12 |r,TLB:15:14:25:24.18.17| Show InChI InChI=1S/C26H29N3O2/c1-28(2)23-6-4-5-21-19(23)11-17-14-26(31)24-12-16-7-8-18(30)13-20(16)25(26,9-10-29(24)3)15-22(17)27-21/h4-8,11,13,24,30-31H,9-10,12,14-15H2,1-3H3/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.621 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391586
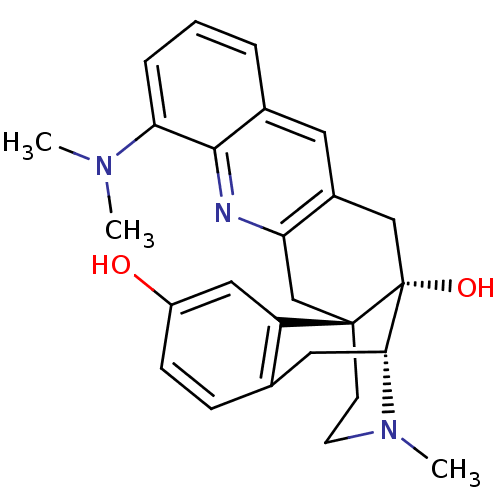
(CHEMBL2147931)Show SMILES CN(C)c1cccc2cc3C[C@@]4(O)[C@H]5Cc6ccc(O)cc6[C@@]4(CCN5C)Cc3nc12 |r,THB:26:25:11:21.15.14| Show InChI InChI=1S/C26H29N3O2/c1-28(2)22-6-4-5-17-11-18-14-26(31)23-12-16-7-8-19(30)13-20(16)25(26,9-10-29(23)3)15-21(18)27-24(17)22/h4-8,11,13,23,30-31H,9-10,12,14-15H2,1-3H3/t23-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50580217

(CHEMBL5093195)Show SMILES Cc1nc(co1)C(=O)Nc1ccc(F)c(c1)[C@]1(C)Cn2nc(cc2C(N)=N1)C#N |r,c:28| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to BACE1 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00359
BindingDB Entry DOI: 10.7270/Q2WW7NJB |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391592

(CHEMBL2147905)Show SMILES CN1CC[C@]23Cc4nc5ccc(F)cc5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:18:28.22.21| Show InChI InChI=1S/C24H23FN2O2/c1-27-7-6-23-13-21-16(8-15-9-17(25)3-5-20(15)26-21)12-24(23,29)22(27)10-14-2-4-18(28)11-19(14)23/h2-5,8-9,11,22,28-29H,6-7,10,12-13H2,1H3/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50329101
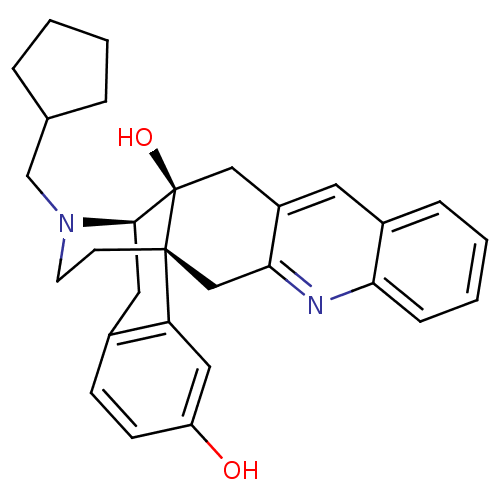
(CHEMBL1270573)Show SMILES Oc1ccc2C[C@H]3N(CC4CCCC4)CC[C@@]4(Cc5nc6ccccc6cc5C[C@@]34O)c2c1 |r,THB:8:7:29:4.31.5| Show InChI InChI=1S/C29H32N2O2/c32-23-10-9-20-14-27-29(33)16-22-13-21-7-3-4-8-25(21)30-26(22)17-28(29,24(20)15-23)11-12-31(27)18-19-5-1-2-6-19/h3-4,7-10,13,15,19,27,32-33H,1-2,5-6,11-12,14,16-18H2/t27-,28-,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain membranes |
Bioorg Med Chem Lett 20: 6302-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.083
BindingDB Entry DOI: 10.7270/Q2G73DZ1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data