 Found 361 hits with Last Name = 'kohchi' and Initial = 'm'
Found 361 hits with Last Name = 'kohchi' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Chitin synthase 1
(Candida albicans) | BDBM50089536
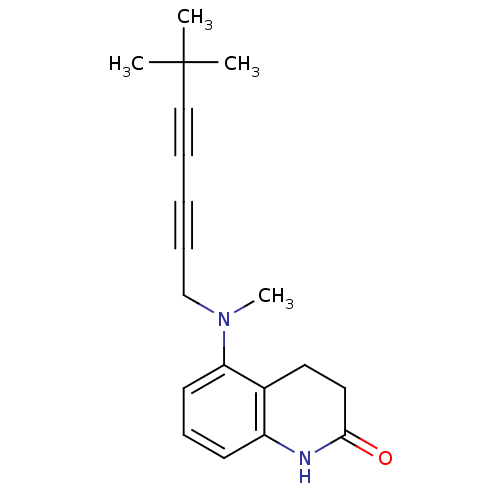
(5-[(6,6-Dimethyl-hepta-2,4-diynyl)-methyl-amino]-3...)Show InChI InChI=1S/C19H22N2O/c1-19(2,3)13-6-5-7-14-21(4)17-10-8-9-16-15(17)11-12-18(22)20-16/h8-10H,11-12,14H2,1-4H3,(H,20,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory of Candida albicans chitin synthase 1 |
Bioorg Med Chem Lett 10: 1459-62 (2000)
BindingDB Entry DOI: 10.7270/Q2R210MK |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50197690
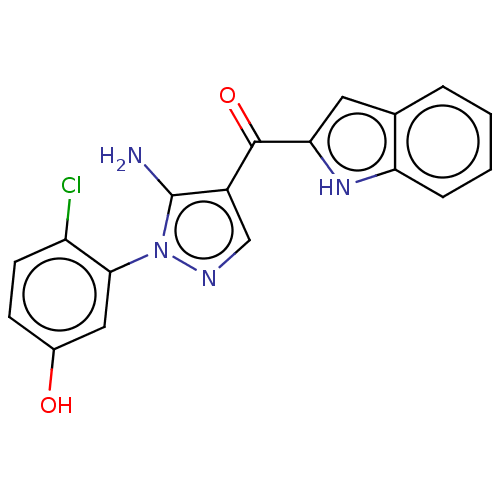
(CHEMBL3976548)Show SMILES Nc1c(cnn1-c1cc(O)ccc1Cl)C(=O)c1cc2ccccc2[nH]1 Show InChI InChI=1S/C18H13ClN4O2/c19-13-6-5-11(24)8-16(13)23-18(20)12(9-21-23)17(25)15-7-10-3-1-2-4-14(10)22-15/h1-9,22,24H,20H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human SRC by time-resolved fluorescence or time-resolved fluorescence assay |
J Med Chem 59: 10586-10600 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01156
BindingDB Entry DOI: 10.7270/Q2ZS2ZGV |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50100335
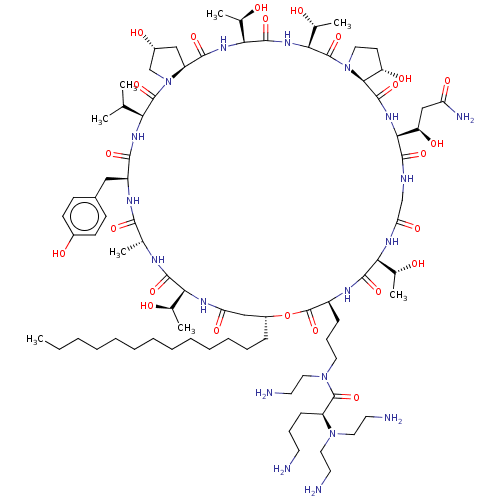
(CHEMBL2371681 | RO-09-3655 derivative)Show SMILES [H][C@@]12C[C@@H](O)CN1C(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](C)NC(=O)[C@@]([H])(NC(=O)C[C@@H](CCCCCCCCCCCCC)OC(=O)[C@H](CCCN(CCN)C(=O)[C@H](CCCN)N(CCN)CCN)NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@]1([H])[C@@H](O)CCN1C(=O)[C@@]([H])(NC(=O)[C@@]([H])(NC2=O)[C@@H](C)O)[C@@H](C)O)[C@H](O)CC(N)=O)[C@@H](C)O)[C@@H](C)O)C(C)C Show InChI InChI=1S/C82H141N19O24/c1-9-10-11-12-13-14-15-16-17-18-19-22-54-41-62(111)92-65(47(5)102)75(117)89-46(4)71(113)91-56(39-51-25-27-52(106)28-26-51)72(114)94-64(45(2)3)80(122)101-44-53(107)40-58(101)73(115)95-67(49(7)104)77(119)96-68(50(8)105)81(123)100-35-29-59(108)70(100)78(120)97-69(60(109)42-61(87)110)74(116)88-43-63(112)93-66(48(6)103)76(118)90-55(82(124)125-54)23-21-34-99(38-33-86)79(121)57(24-20-30-83)98(36-31-84)37-32-85/h25-28,45-50,53-60,64-70,102-109H,9-24,29-44,83-86H2,1-8H3,(H2,87,110)(H,88,116)(H,89,117)(H,90,118)(H,91,113)(H,92,111)(H,93,112)(H,94,114)(H,95,115)(H,96,119)(H,97,120)/t46-,47-,48-,49-,50-,53-,54-,55+,56+,57+,58+,59+,60-,64+,65+,66+,67+,68+,69+,70+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Beta glucan synthase |
Bioorg Med Chem Lett 11: 1273-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CN74FQ |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50100338

(CHEMBL267407 | RO-09-3655 derivative)Show SMILES CCCCCCCCCCCCC[C@@H]1CC(=O)N[C@H]([C@@H](C)O)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](C(C)C)C(=O)N2C[C@H](O)C[C@H]2C(=O)N[C@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N2CC[C@H](O)[C@H]2C(=O)N[C@@H]([C@H](O)CC(N)=O)C(=O)NCC(=O)N[C@H]([C@@H](C)O)C(=O)N[C@@H](CCCN(CCCN)C(=O)[C@@H](CCCN)N(CCN)CCN)C(=O)O1 Show InChI InChI=1S/C83H143N19O24/c1-9-10-11-12-13-14-15-16-17-18-19-23-55-42-63(112)93-66(48(5)103)76(118)90-47(4)72(114)92-57(40-52-26-28-53(107)29-27-52)73(115)95-65(46(2)3)81(123)102-45-54(108)41-59(102)74(116)96-68(50(7)105)78(120)97-69(51(8)106)82(124)101-37-30-60(109)71(101)79(121)98-70(61(110)43-62(88)111)75(117)89-44-64(113)94-67(49(6)104)77(119)91-56(83(125)126-55)24-21-35-100(36-22-32-85)80(122)58(25-20-31-84)99(38-33-86)39-34-87/h26-29,46-51,54-61,65-71,103-110H,9-25,30-45,84-87H2,1-8H3,(H2,88,111)(H,89,117)(H,90,118)(H,91,119)(H,92,114)(H,93,112)(H,94,113)(H,95,115)(H,96,116)(H,97,120)(H,98,121)/t47-,48-,49-,50-,51-,54-,55-,56+,57+,58-,59+,60+,61-,65+,66-,67-,68-,69+,70+,71+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Beta glucan synthase |
Bioorg Med Chem Lett 11: 1273-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CN74FQ |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50100332
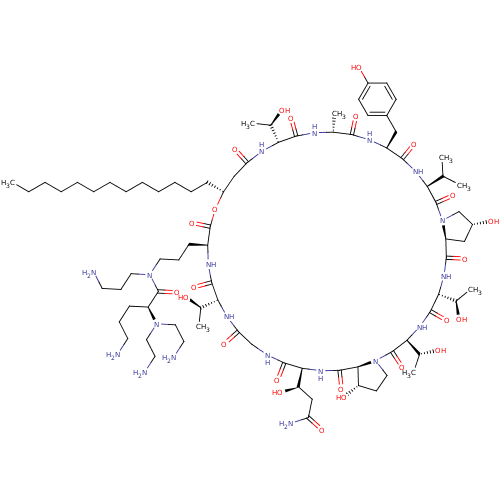
(CHEMBL415843 | RO-09-3655 derivative)Show SMILES CCCCCCCCCCCCC[C@@H]1CC(=O)N[C@H]([C@@H](C)O)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](C(C)C)C(=O)N2C[C@H](O)C[C@H]2C(=O)N[C@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N2CC[C@H](O)[C@H]2C(=O)N[C@@H]([C@H](O)CC(N)=O)C(=O)NCC(=O)N[C@H]([C@@H](C)O)C(=O)N[C@@H](CCCN(CCCN)C(=O)[C@H](CCCN)N(CCN)CCN)C(=O)O1 Show InChI InChI=1S/C83H143N19O24/c1-9-10-11-12-13-14-15-16-17-18-19-23-55-42-63(112)93-66(48(5)103)76(118)90-47(4)72(114)92-57(40-52-26-28-53(107)29-27-52)73(115)95-65(46(2)3)81(123)102-45-54(108)41-59(102)74(116)96-68(50(7)105)78(120)97-69(51(8)106)82(124)101-37-30-60(109)71(101)79(121)98-70(61(110)43-62(88)111)75(117)89-44-64(113)94-67(49(6)104)77(119)91-56(83(125)126-55)24-21-35-100(36-22-32-85)80(122)58(25-20-31-84)99(38-33-86)39-34-87/h26-29,46-51,54-61,65-71,103-110H,9-25,30-45,84-87H2,1-8H3,(H2,88,111)(H,89,117)(H,90,118)(H,91,119)(H,92,114)(H,93,112)(H,94,113)(H,95,115)(H,96,116)(H,97,120)(H,98,121)/t47-,48-,49-,50-,51-,54-,55-,56+,57+,58+,59+,60+,61-,65+,66-,67-,68-,69+,70+,71+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Beta glucan synthase |
Bioorg Med Chem Lett 11: 1273-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CN74FQ |
More data for this
Ligand-Target Pair | |
Chitin synthase 1
(Candida albicans) | BDBM50089557

(5-[Butyl-((E)-6,6-dimethyl-hept-2-en-4-ynyl)-amino...)Show InChI InChI=1S/C22H30N2O/c1-5-6-16-24(17-9-7-8-15-22(2,3)4)20-12-10-11-19-18(20)13-14-21(25)23-19/h7,9-12H,5-6,13-14,16-17H2,1-4H3,(H,23,25)/b9-7+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory of Candida albicans chitin synthase 1 |
Bioorg Med Chem Lett 10: 1459-62 (2000)
BindingDB Entry DOI: 10.7270/Q2R210MK |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50197709
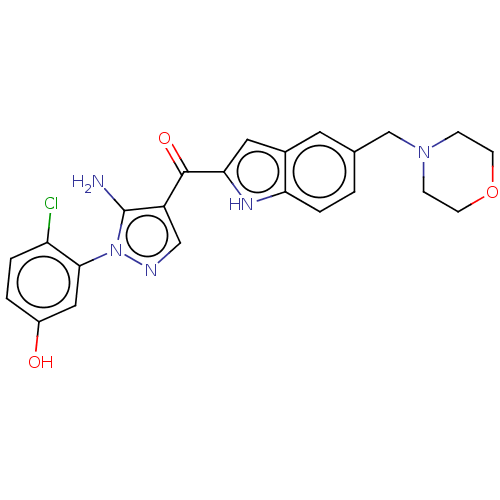
(CHEMBL3984621)Show SMILES Nc1c(cnn1-c1cc(O)ccc1Cl)C(=O)c1cc2cc(CN3CCOCC3)ccc2[nH]1 Show InChI InChI=1S/C23H22ClN5O3/c24-18-3-2-16(30)11-21(18)29-23(25)17(12-26-29)22(31)20-10-15-9-14(1-4-19(15)27-20)13-28-5-7-32-8-6-28/h1-4,9-12,27,30H,5-8,13,25H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human SRC by time-resolved fluorescence or time-resolved fluorescence assay |
J Med Chem 59: 10586-10600 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01156
BindingDB Entry DOI: 10.7270/Q2ZS2ZGV |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50100333
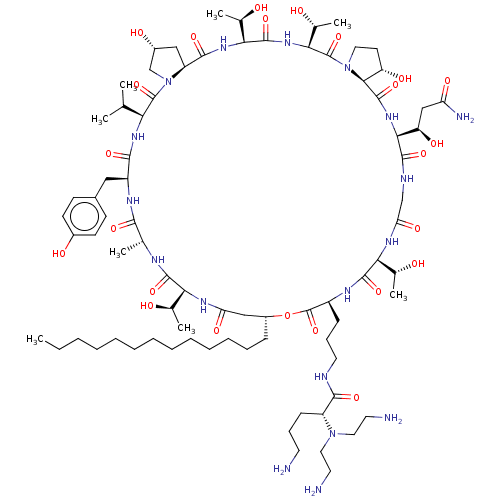
(CHEMBL2371765 | RO-09-3655 derivative)Show SMILES [H][C@@]12C[C@@H](O)CN1C(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](C)NC(=O)[C@@]([H])(NC(=O)C[C@@H](CCCCCCCCCCCCC)OC(=O)[C@H](CCCNC(=O)[C@@H](CCCN)N(CCN)CCN)NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@]1([H])[C@@H](O)CCN1C(=O)[C@@]([H])(NC(=O)[C@@]([H])(NC2=O)[C@@H](C)O)[C@@H](C)O)[C@H](O)CC(N)=O)[C@@H](C)O)[C@@H](C)O)C(C)C Show InChI InChI=1S/C80H136N18O24/c1-9-10-11-12-13-14-15-16-17-18-19-22-52-39-60(108)90-63(45(5)99)74(115)87-44(4)69(110)89-54(37-49-25-27-50(103)28-26-49)70(111)92-62(43(2)3)78(119)98-42-51(104)38-56(98)72(113)93-65(47(7)101)76(117)94-66(48(8)102)79(120)97-34-29-57(105)68(97)77(118)95-67(58(106)40-59(84)107)73(114)86-41-61(109)91-64(46(6)100)75(116)88-53(80(121)122-52)23-21-33-85-71(112)55(24-20-30-81)96(35-31-82)36-32-83/h25-28,43-48,51-58,62-68,99-106H,9-24,29-42,81-83H2,1-8H3,(H2,84,107)(H,85,112)(H,86,114)(H,87,115)(H,88,116)(H,89,110)(H,90,108)(H,91,109)(H,92,111)(H,93,113)(H,94,117)(H,95,118)/t44-,45-,46-,47-,48-,51-,52-,53+,54+,55-,56+,57+,58-,62+,63+,64+,65+,66+,67+,68+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Beta glucan synthase |
Bioorg Med Chem Lett 11: 1273-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CN74FQ |
More data for this
Ligand-Target Pair | |
Chitin synthase 1
(Candida albicans) | BDBM50089551

(5-[((E)-6,6-Dimethyl-hept-2-en-4-ynyl)-propyl-amin...)Show InChI InChI=1S/C21H28N2O/c1-5-15-23(16-8-6-7-14-21(2,3)4)19-11-9-10-18-17(19)12-13-20(24)22-18/h6,8-11H,5,12-13,15-16H2,1-4H3,(H,22,24)/b8-6+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory of Candida albicans chitin synthase 1 |
Bioorg Med Chem Lett 10: 1459-62 (2000)
BindingDB Entry DOI: 10.7270/Q2R210MK |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593047
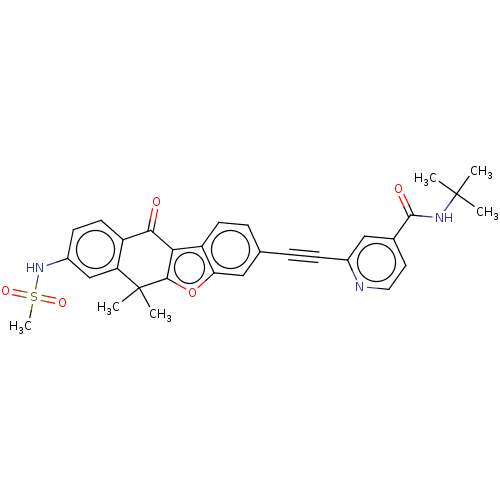
(CHEMBL5172448)Show SMILES CC(C)(C)NC(=O)c1ccnc(c1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593050
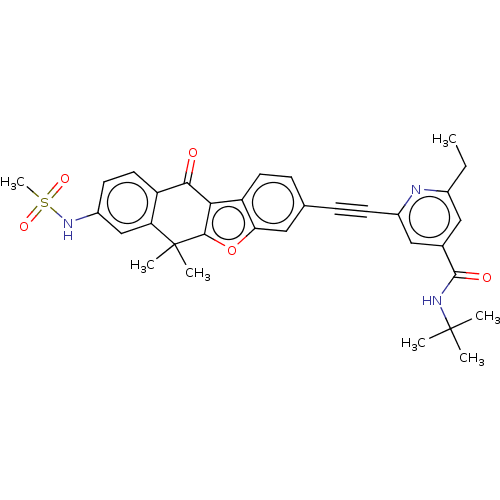
(CHEMBL5174525)Show SMILES CCc1cc(cc(n1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O)C(=O)NC(C)(C)C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479835
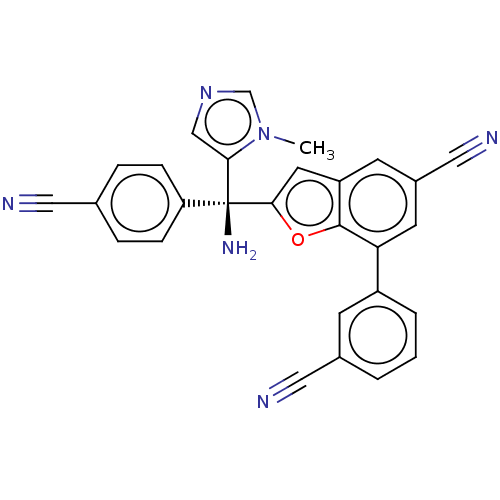
(CHEMBL510873)Show SMILES Cn1cncc1[C@](N)(c1cc2cc(cc(-c3cccc(c3)C#N)c2o1)C#N)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C28H18N6O/c1-34-17-33-16-25(34)28(32,23-7-5-18(13-29)6-8-23)26-12-22-10-20(15-31)11-24(27(22)35-26)21-4-2-3-19(9-21)14-30/h2-12,16-17H,32H2,1H3/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
Chitin synthase 1
(Candida albicans) | BDBM50089540
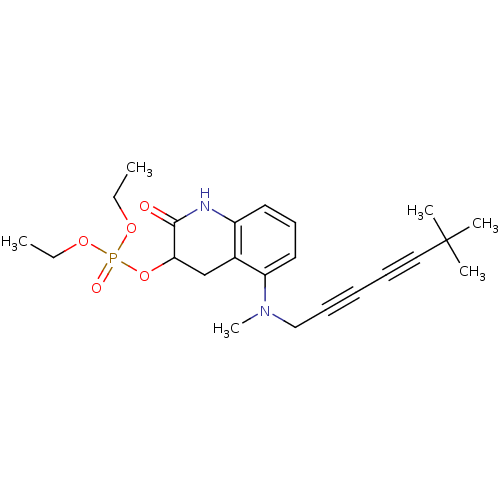
(CHEMBL32125 | Phosphoric acid 5-[(6,6-dimethyl-hep...)Show SMILES CCOP(=O)(OCC)OC1Cc2c(NC1=O)cccc2N(C)CC#CC#CC(C)(C)C Show InChI InChI=1S/C23H31N2O5P/c1-7-28-31(27,29-8-2)30-21-17-18-19(24-22(21)26)13-12-14-20(18)25(6)16-11-9-10-15-23(3,4)5/h12-14,21H,7-8,16-17H2,1-6H3,(H,24,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory of Candida albicans chitin synthase 1 |
Bioorg Med Chem Lett 10: 1459-62 (2000)
BindingDB Entry DOI: 10.7270/Q2R210MK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479863
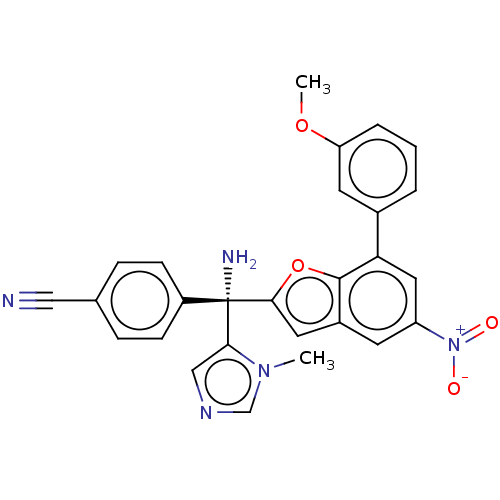
(CHEMBL521743)Show SMILES COc1cccc(c1)-c1cc(cc2cc(oc12)[C@@](N)(c1cncn1C)c1ccc(cc1)C#N)[N+]([O-])=O |r| Show InChI InChI=1S/C27H21N5O4/c1-31-16-30-15-24(31)27(29,20-8-6-17(14-28)7-9-20)25-12-19-10-21(32(33)34)13-23(26(19)36-25)18-4-3-5-22(11-18)35-2/h3-13,15-16H,29H2,1-2H3/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50100331
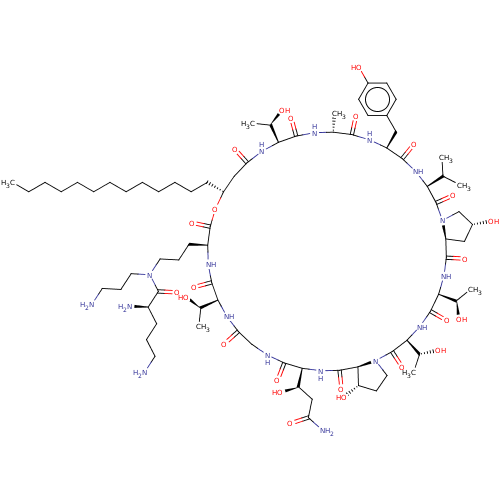
(CHEMBL2371715 | RO-09-3655 derivative)Show SMILES [H][C@@]12C[C@@H](O)CN1C(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](C)NC(=O)[C@@]([H])(NC(=O)C[C@@H](CCCCCCCCCCCCC)OC(=O)[C@H](CCCN(CCCN)C(=O)[C@H](N)CCCN)NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@]1([H])[C@@H](O)CCN1C(=O)[C@@]([H])(NC(=O)[C@@]([H])(NC2=O)[C@@H](C)O)[C@@H](C)O)[C@H](O)CC(N)=O)[C@@H](C)O)[C@@H](C)O)C(C)C Show InChI InChI=1S/C79H133N17O24/c1-9-10-11-12-13-14-15-16-17-18-19-23-51-38-59(106)88-62(44(5)97)72(112)85-43(4)68(108)87-54(36-48-26-28-49(101)29-27-48)69(109)90-61(42(2)3)77(117)96-41-50(102)37-55(96)70(110)91-64(46(7)99)74(114)92-65(47(8)100)78(118)95-35-30-56(103)67(95)75(115)93-66(57(104)39-58(83)105)71(111)84-40-60(107)89-63(45(6)98)73(113)86-53(79(119)120-51)25-21-33-94(34-22-32-81)76(116)52(82)24-20-31-80/h26-29,42-47,50-57,61-67,97-104H,9-25,30-41,80-82H2,1-8H3,(H2,83,105)(H,84,111)(H,85,112)(H,86,113)(H,87,108)(H,88,106)(H,89,107)(H,90,109)(H,91,110)(H,92,114)(H,93,115)/t43-,44-,45-,46-,47-,50-,51-,52-,53+,54+,55+,56+,57-,61+,62+,63+,64+,65+,66+,67+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Beta glucan synthase |
Bioorg Med Chem Lett 11: 1273-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CN74FQ |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50100329
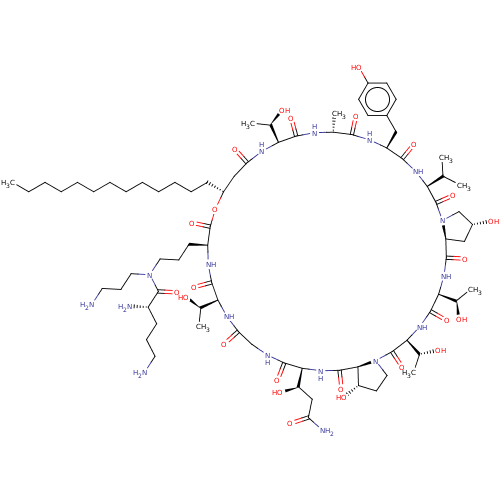
(CHEMBL2371727 | RO-09-3655 derivative)Show SMILES [H][C@@]12C[C@@H](O)CN1C(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](C)NC(=O)[C@@]([H])(NC(=O)C[C@@H](CCCCCCCCCCCCC)OC(=O)[C@H](CCCN(CCCN)C(=O)[C@@H](N)CCCN)NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@]1([H])[C@@H](O)CCN1C(=O)[C@@]([H])(NC(=O)[C@@]([H])(NC2=O)[C@@H](C)O)[C@@H](C)O)[C@H](O)CC(N)=O)[C@@H](C)O)[C@@H](C)O)C(C)C Show InChI InChI=1S/C79H133N17O24/c1-9-10-11-12-13-14-15-16-17-18-19-23-51-38-59(106)88-62(44(5)97)72(112)85-43(4)68(108)87-54(36-48-26-28-49(101)29-27-48)69(109)90-61(42(2)3)77(117)96-41-50(102)37-55(96)70(110)91-64(46(7)99)74(114)92-65(47(8)100)78(118)95-35-30-56(103)67(95)75(115)93-66(57(104)39-58(83)105)71(111)84-40-60(107)89-63(45(6)98)73(113)86-53(79(119)120-51)25-21-33-94(34-22-32-81)76(116)52(82)24-20-31-80/h26-29,42-47,50-57,61-67,97-104H,9-25,30-41,80-82H2,1-8H3,(H2,83,105)(H,84,111)(H,85,112)(H,86,113)(H,87,108)(H,88,106)(H,89,107)(H,90,109)(H,91,110)(H,92,114)(H,93,115)/t43-,44-,45-,46-,47-,50-,51-,52+,53+,54+,55+,56+,57-,61+,62+,63+,64+,65+,66+,67+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Beta glucan synthase |
Bioorg Med Chem Lett 11: 1273-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CN74FQ |
More data for this
Ligand-Target Pair | |
Chitin synthase 1
(Candida albicans) | BDBM50089563
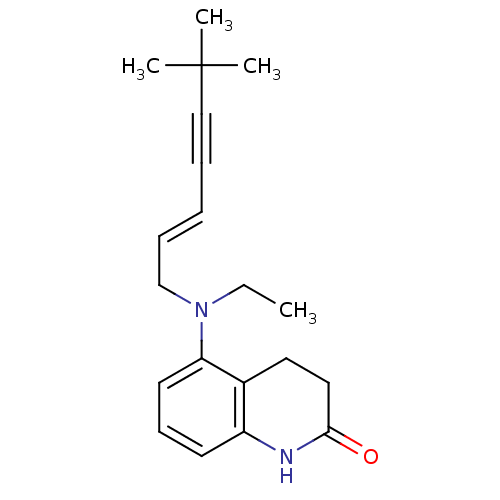
(5-[((E)-6,6-Dimethyl-hept-2-en-4-ynyl)-ethyl-amino...)Show InChI InChI=1S/C20H26N2O/c1-5-22(15-8-6-7-14-20(2,3)4)18-11-9-10-17-16(18)12-13-19(23)21-17/h6,8-11H,5,12-13,15H2,1-4H3,(H,21,23)/b8-6+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory of Candida albicans chitin synthase 1 |
Bioorg Med Chem Lett 10: 1459-62 (2000)
BindingDB Entry DOI: 10.7270/Q2R210MK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479859
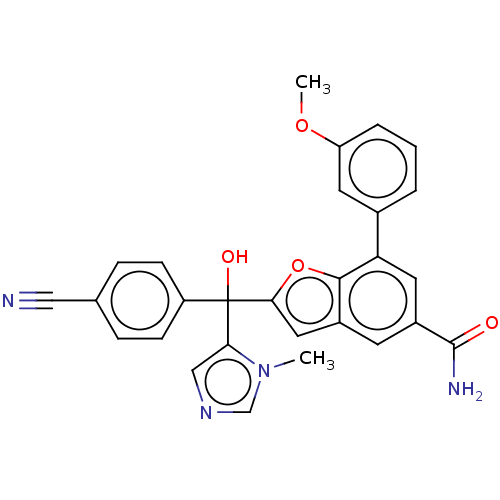
(CHEMBL508937)Show SMILES COc1cccc(c1)-c1cc(cc2cc(oc12)C(O)(c1cncn1C)c1ccc(cc1)C#N)C(N)=O Show InChI InChI=1S/C28H22N4O4/c1-32-16-31-15-24(32)28(34,21-8-6-17(14-29)7-9-21)25-13-19-10-20(27(30)33)12-23(26(19)36-25)18-4-3-5-22(11-18)35-2/h3-13,15-16,34H,1-2H3,(H2,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50100337
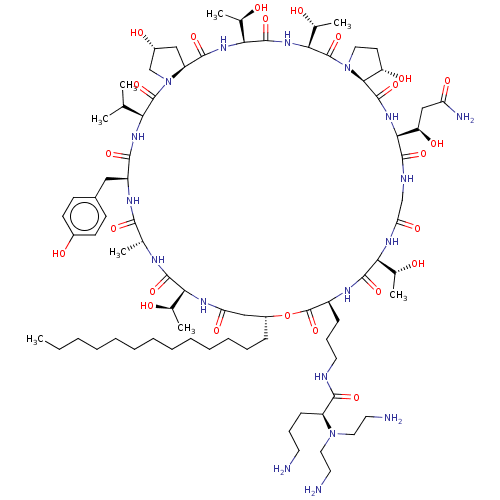
(CHEMBL2371766 | RO-09-3655 derivative)Show SMILES [H][C@@]12C[C@@H](O)CN1C(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](C)NC(=O)[C@@]([H])(NC(=O)C[C@@H](CCCCCCCCCCCCC)OC(=O)[C@H](CCCNC(=O)[C@H](CCCN)N(CCN)CCN)NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@]1([H])[C@@H](O)CCN1C(=O)[C@@]([H])(NC(=O)[C@@]([H])(NC2=O)[C@@H](C)O)[C@@H](C)O)[C@H](O)CC(N)=O)[C@@H](C)O)[C@@H](C)O)C(C)C Show InChI InChI=1S/C80H136N18O24/c1-9-10-11-12-13-14-15-16-17-18-19-22-52-39-60(108)90-63(45(5)99)74(115)87-44(4)69(110)89-54(37-49-25-27-50(103)28-26-49)70(111)92-62(43(2)3)78(119)98-42-51(104)38-56(98)72(113)93-65(47(7)101)76(117)94-66(48(8)102)79(120)97-34-29-57(105)68(97)77(118)95-67(58(106)40-59(84)107)73(114)86-41-61(109)91-64(46(6)100)75(116)88-53(80(121)122-52)23-21-33-85-71(112)55(24-20-30-81)96(35-31-82)36-32-83/h25-28,43-48,51-58,62-68,99-106H,9-24,29-42,81-83H2,1-8H3,(H2,84,107)(H,85,112)(H,86,114)(H,87,115)(H,88,116)(H,89,110)(H,90,108)(H,91,109)(H,92,111)(H,93,113)(H,94,117)(H,95,118)/t44-,45-,46-,47-,48-,51-,52-,53+,54+,55+,56+,57+,58-,62+,63+,64+,65+,66+,67+,68+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Beta glucan synthase |
Bioorg Med Chem Lett 11: 1273-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CN74FQ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479847
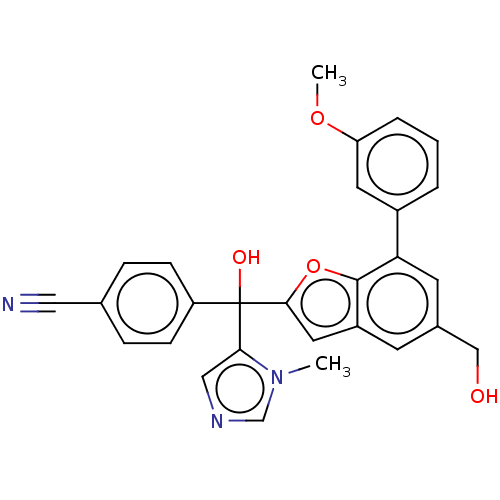
(CHEMBL514300)Show SMILES COc1cccc(c1)-c1cc(CO)cc2cc(oc12)C(O)(c1cncn1C)c1ccc(cc1)C#N Show InChI InChI=1S/C28H23N3O4/c1-31-17-30-15-25(31)28(33,22-8-6-18(14-29)7-9-22)26-13-21-10-19(16-32)11-24(27(21)35-26)20-4-3-5-23(12-20)34-2/h3-13,15,17,32-33H,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479856
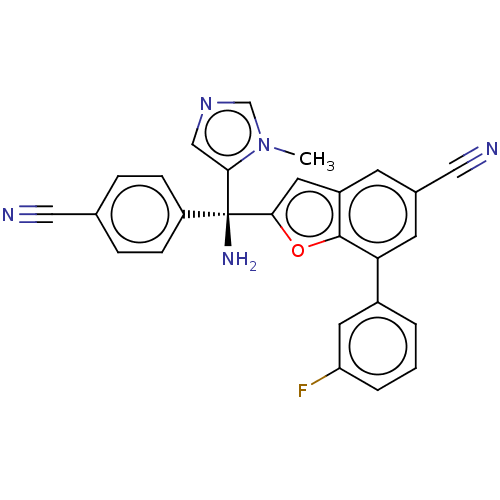
(CHEMBL454405)Show SMILES Cn1cncc1[C@](N)(c1cc2cc(cc(-c3cccc(F)c3)c2o1)C#N)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C27H18FN5O/c1-33-16-32-15-24(33)27(31,21-7-5-17(13-29)6-8-21)25-12-20-9-18(14-30)10-23(26(20)34-25)19-3-2-4-22(28)11-19/h2-12,15-16H,31H2,1H3/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
Chitin synthase 1
(Candida albicans) | BDBM50089538
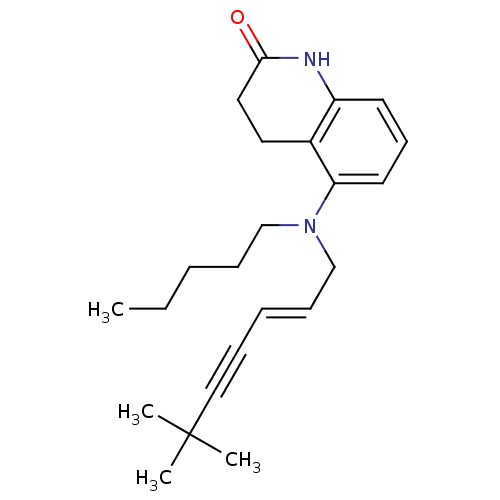
(5-[((E)-6,6-Dimethyl-hept-2-en-4-ynyl)-pentyl-amin...)Show SMILES CCCCCN(C\C=C\C#CC(C)(C)C)c1cccc2NC(=O)CCc12 Show InChI InChI=1S/C23H32N2O/c1-5-6-9-17-25(18-10-7-8-16-23(2,3)4)21-13-11-12-20-19(21)14-15-22(26)24-20/h7,10-13H,5-6,9,14-15,17-18H2,1-4H3,(H,24,26)/b10-7+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory of Candida albicans chitin synthase 1 |
Bioorg Med Chem Lett 10: 1459-62 (2000)
BindingDB Entry DOI: 10.7270/Q2R210MK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479848
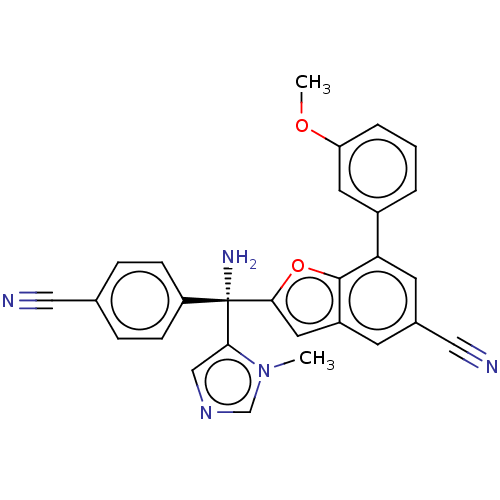
(CHEMBL489318)Show SMILES COc1cccc(c1)-c1cc(cc2cc(oc12)[C@@](N)(c1cncn1C)c1ccc(cc1)C#N)C#N |r| Show InChI InChI=1S/C28H21N5O2/c1-33-17-32-16-25(33)28(31,22-8-6-18(14-29)7-9-22)26-13-21-10-19(15-30)11-24(27(21)35-26)20-4-3-5-23(12-20)34-2/h3-13,16-17H,31H2,1-2H3/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50525707

(CHEMBL4457566)Show SMILES Cc1cc(cc(n1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O)C(=O)NC(C)(C)C Show InChI InChI=1S/C32H31N3O5S/c1-18-14-20(30(37)34-31(2,3)4)16-21(33-18)10-8-19-9-12-24-26(15-19)40-29-27(24)28(36)23-13-11-22(35-41(7,38)39)17-25(23)32(29,5)6/h9,11-17,35H,1-7H3,(H,34,37) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Chitin synthase 1
(Candida albicans) | BDBM50089559
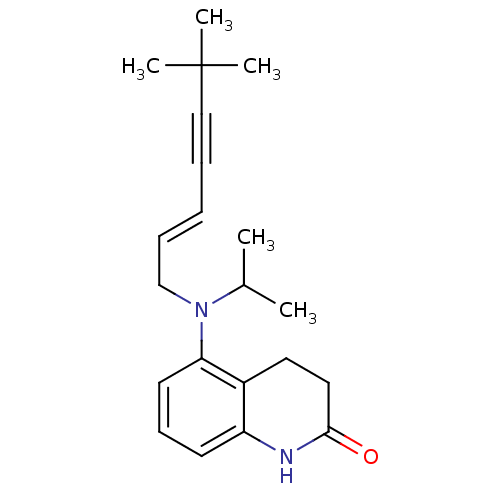
(5-[((E)-6,6-Dimethyl-hept-2-en-4-ynyl)-isopropyl-a...)Show SMILES CC(C)N(C\C=C\C#CC(C)(C)C)c1cccc2NC(=O)CCc12 Show InChI InChI=1S/C21H28N2O/c1-16(2)23(15-8-6-7-14-21(3,4)5)19-11-9-10-18-17(19)12-13-20(24)22-18/h6,8-11,16H,12-13,15H2,1-5H3,(H,22,24)/b8-6+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory of Candida albicans chitin synthase 1 |
Bioorg Med Chem Lett 10: 1459-62 (2000)
BindingDB Entry DOI: 10.7270/Q2R210MK |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593051
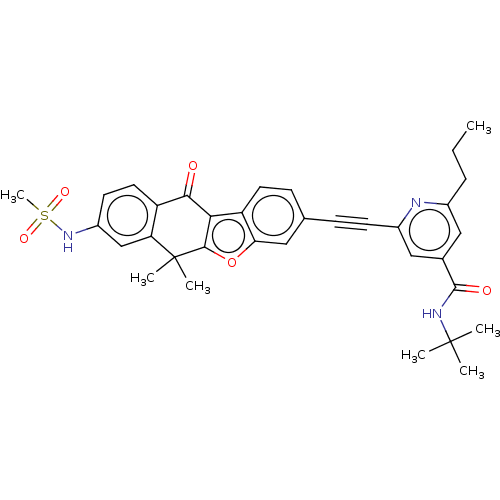
(CHEMBL5171942)Show SMILES CCCc1cc(cc(n1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O)C(=O)NC(C)(C)C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50197709

(CHEMBL3984621)Show SMILES Nc1c(cnn1-c1cc(O)ccc1Cl)C(=O)c1cc2cc(CN3CCOCC3)ccc2[nH]1 Show InChI InChI=1S/C23H22ClN5O3/c24-18-3-2-16(30)11-21(18)29-23(25)17(12-26-29)22(31)20-10-15-9-14(1-4-19(15)27-20)13-28-5-7-32-8-6-28/h1-4,9-12,27,30H,5-8,13,25H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR1 by time-resolved fluorescence or time-resolved fluorescence assay |
J Med Chem 59: 10586-10600 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01156
BindingDB Entry DOI: 10.7270/Q2ZS2ZGV |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50366679
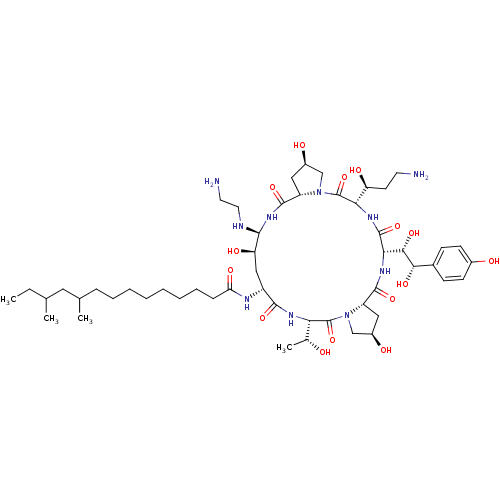
(CHEMBL1793852 | MK-991)Show SMILES CCC(C)CC(C)CCCCCCCCC(=O)N[C@@H]1C[C@@H](O)[C@@H](NCCN)NC(=O)[C@@H]2C[C@@H](O)CN2C(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H]2C[C@@H](O)CN2C(=O)[C@@H](NC1=O)[C@@H](C)O)[C@H](O)[C@@H](O)c1ccc(O)cc1)[C@@H](O)CCN Show InChI InChI=1S/C52H88N10O15/c1-5-28(2)22-29(3)12-10-8-6-7-9-11-13-40(69)56-35-25-39(68)46(55-21-20-54)60-49(74)37-24-34(66)27-62(37)52(77)42(38(67)18-19-53)58-50(75)43(45(71)44(70)31-14-16-32(64)17-15-31)59-48(73)36-23-33(65)26-61(36)51(76)41(30(4)63)57-47(35)72/h14-17,28-30,33-39,41-46,55,63-68,70-71H,5-13,18-27,53-54H2,1-4H3,(H,56,69)(H,57,72)(H,58,75)(H,59,73)(H,60,74)/t28?,29?,30-,33-,34-,35-,36+,37+,38+,39-,41+,42+,43+,44+,45+,46+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Beta glucan synthase |
Bioorg Med Chem Lett 11: 1273-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CN74FQ |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593035

(CHEMBL5183149)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCOCC3)cc12)C#Cc1cc(ccn1)C(=O)NC1CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50100336
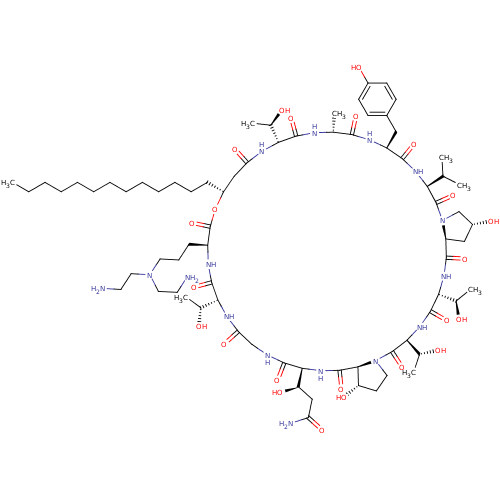
(CHEMBL405487 | RO-09-3655 derivative)Show SMILES CCCCCCCCCCCCC[C@@H]1CC(=O)N[C@H]([C@@H](C)O)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](C(C)C)C(=O)N2C[C@H](O)C[C@H]2C(=O)N[C@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N2CC[C@H](O)[C@H]2C(=O)N[C@@H]([C@H](O)CC(N)=O)C(=O)NCC(=O)N[C@H]([C@@H](C)O)C(=O)N[C@@H](CCCN(CCN)CCN)C(=O)O1 Show InChI InChI=1S/C75H126N16O23/c1-9-10-11-12-13-14-15-16-17-18-19-21-49-36-56(101)83-59(42(5)92)69(107)80-41(4)65(103)82-51(34-46-23-25-47(96)26-24-46)66(104)85-58(40(2)3)73(111)91-39-48(97)35-52(91)67(105)86-61(44(7)94)71(109)87-62(45(8)95)74(112)90-31-27-53(98)64(90)72(110)88-63(54(99)37-55(78)100)68(106)79-38-57(102)84-60(43(6)93)70(108)81-50(75(113)114-49)22-20-30-89(32-28-76)33-29-77/h23-26,40-45,48-54,58-64,92-99H,9-22,27-39,76-77H2,1-8H3,(H2,78,100)(H,79,106)(H,80,107)(H,81,108)(H,82,103)(H,83,101)(H,84,102)(H,85,104)(H,86,105)(H,87,109)(H,88,110)/t41-,42-,43-,44-,45-,48-,49-,50+,51+,52+,53+,54-,58+,59-,60-,61-,62+,63+,64+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Beta glucan synthase |
Bioorg Med Chem Lett 11: 1273-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CN74FQ |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593043
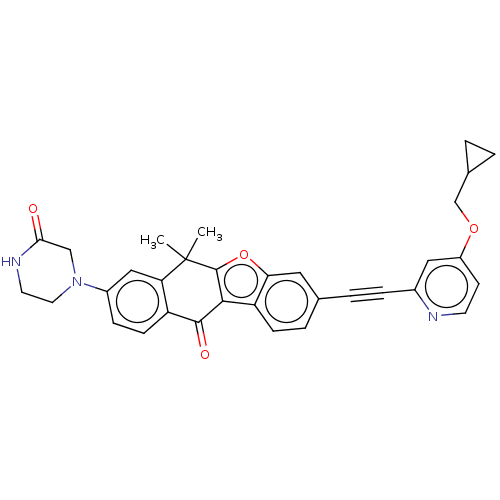
(CHEMBL5189763)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(cc12)N1CCNC(=O)C1)C#Cc1cc(OCC2CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479849
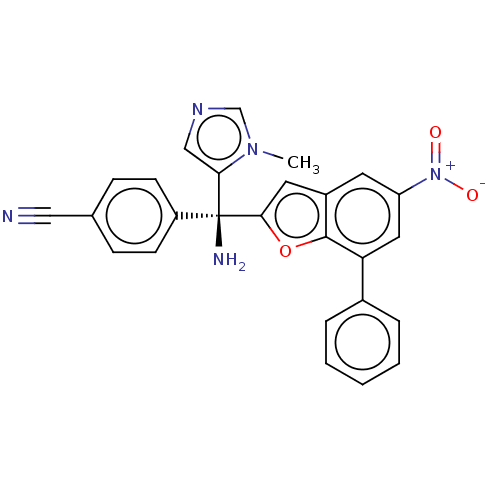
(CHEMBL489121)Show SMILES Cn1cncc1[C@](N)(c1cc2cc(cc(-c3ccccc3)c2o1)[N+]([O-])=O)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C26H19N5O3/c1-30-16-29-15-23(30)26(28,20-9-7-17(14-27)8-10-20)24-12-19-11-21(31(32)33)13-22(25(19)34-24)18-5-3-2-4-6-18/h2-13,15-16H,28H2,1H3/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50197685

(CHEMBL3681278 | US10689705, Compound 1)Show SMILES Cc1nc2cc(ccc2[nH]1)-n1ncc(C(=O)c2cc3ccc(CN4CCOCC4)cc3[nH]2)c1N Show InChI InChI=1S/C25H25N7O2/c1-15-28-20-5-4-18(12-22(20)29-15)32-25(26)19(13-27-32)24(33)23-11-17-3-2-16(10-21(17)30-23)14-31-6-8-34-9-7-31/h2-5,10-13,30H,6-9,14,26H2,1H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR1 by time-resolved fluorescence or time-resolved fluorescence assay |
J Med Chem 59: 10586-10600 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01156
BindingDB Entry DOI: 10.7270/Q2ZS2ZGV |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479853
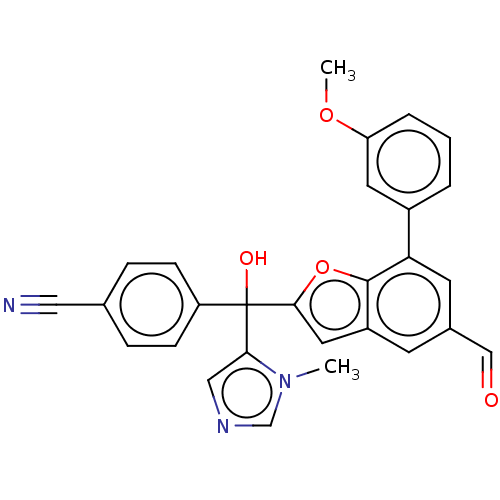
(CHEMBL511113)Show SMILES COc1cccc(c1)-c1cc(C=O)cc2cc(oc12)C(O)(c1cncn1C)c1ccc(cc1)C#N Show InChI InChI=1S/C28H21N3O4/c1-31-17-30-15-25(31)28(33,22-8-6-18(14-29)7-9-22)26-13-21-10-19(16-32)11-24(27(21)35-26)20-4-3-5-23(12-20)34-2/h3-13,15-17,33H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593029
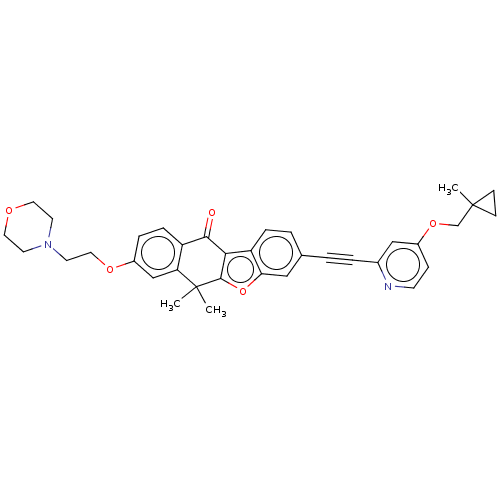
(CHEMBL5195158)Show SMILES CC1(COc2ccnc(c2)C#Cc2ccc3c4c(oc3c2)C(C)(C)c2cc(OCCN3CCOCC3)ccc2C4=O)CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593031

(CHEMBL5191460)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCOCC3)cc12)C#Cc1cc(OCCC2CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
BDNF/NT-3 growth factors receptor
(Homo sapiens (Human)) | BDBM50525707

(CHEMBL4457566)Show SMILES Cc1cc(cc(n1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O)C(=O)NC(C)(C)C Show InChI InChI=1S/C32H31N3O5S/c1-18-14-20(30(37)34-31(2,3)4)16-21(33-18)10-8-19-9-12-24-26(15-19)40-29-27(24)28(36)23-13-11-22(35-41(7,38)39)17-25(23)32(29,5)6/h9,11-17,35H,1-7H3,(H,34,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50222709
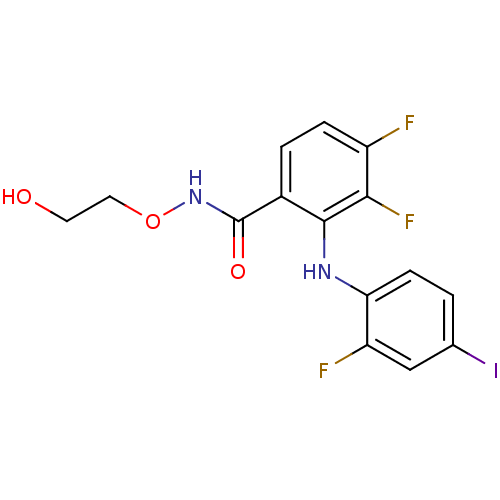
(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-N-(2-h...)Show InChI InChI=1S/C15H12F3IN2O3/c16-10-3-2-9(15(23)21-24-6-5-22)14(13(10)18)20-12-4-1-8(19)7-11(12)17/h1-4,7,20,22H,5-6H2,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50096797
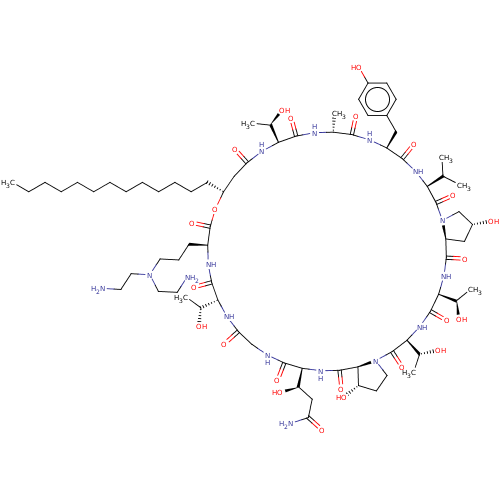
(CHEMBL2370665 | macrocyclic lipopeptidolactone der...)Show SMILES [H][C@@]12C[C@@H](O)CN1C(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](C)NC(=O)[C@@]([H])(NC(=O)C[C@@H](CCCCCCCCCCCCC)OC(=O)[C@H](CCCN(CCN)CCN)NC(=O)[C@]([H])(NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@]1([H])[C@@H](O)CCN1C(=O)[C@@]([H])(NC(=O)[C@@]([H])(NC2=O)[C@@H](C)O)[C@@H](C)O)[C@H](O)CC(N)=O)[C@@H](C)O)[C@@H](C)O)C(C)C Show InChI InChI=1S/C75H126N16O23/c1-9-10-11-12-13-14-15-16-17-18-19-21-49-36-56(101)83-59(42(5)92)69(107)80-41(4)65(103)82-51(34-46-23-25-47(96)26-24-46)66(104)85-58(40(2)3)73(111)91-39-48(97)35-52(91)67(105)86-61(44(7)94)71(109)87-62(45(8)95)74(112)90-31-27-53(98)64(90)72(110)88-63(54(99)37-55(78)100)68(106)79-38-57(102)84-60(43(6)93)70(108)81-50(75(113)114-49)22-20-30-89(32-28-76)33-29-77/h23-26,40-45,48-54,58-64,92-99H,9-22,27-39,76-77H2,1-8H3,(H2,78,100)(H,79,106)(H,80,107)(H,81,108)(H,82,103)(H,83,101)(H,84,102)(H,85,104)(H,86,105)(H,87,109)(H,88,110)/t41-,42-,43-,44-,45-,48-,49-,50+,51+,52+,53+,54-,58+,59+,60-,61+,62+,63+,64+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase (GS) from candida albicans |
Bioorg Med Chem Lett 11: 395-8 (2001)
BindingDB Entry DOI: 10.7270/Q28051W2 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593039

(CHEMBL5199099)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCNC(=O)C3)cc12)C#Cc1cc(OCC2CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593053
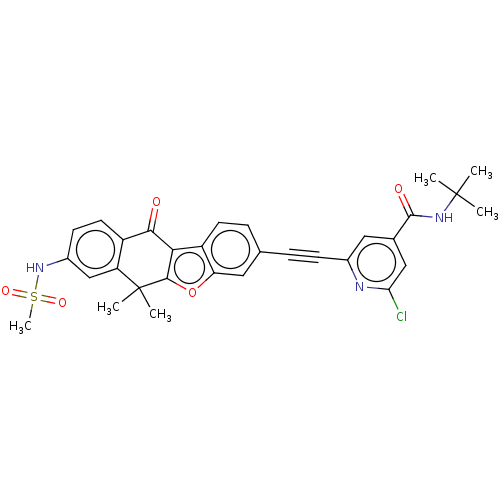
(CHEMBL5198357)Show SMILES CC(C)(C)NC(=O)c1cc(Cl)nc(c1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593045
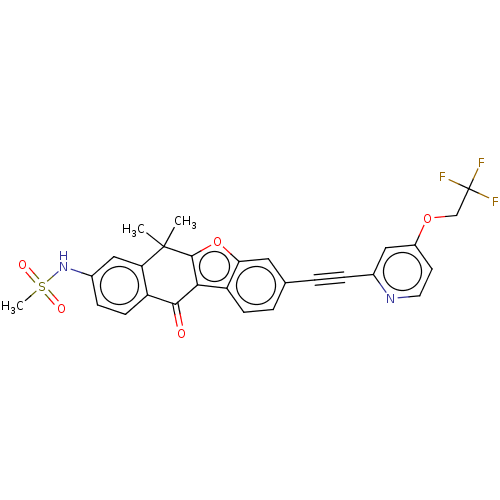
(CHEMBL5205778)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(NS(C)(=O)=O)cc12)C#Cc1cc(OCC(F)(F)F)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50197709

(CHEMBL3984621)Show SMILES Nc1c(cnn1-c1cc(O)ccc1Cl)C(=O)c1cc2cc(CN3CCOCC3)ccc2[nH]1 Show InChI InChI=1S/C23H22ClN5O3/c24-18-3-2-16(30)11-21(18)29-23(25)17(12-26-29)22(31)20-10-15-9-14(1-4-19(15)27-20)13-28-5-7-32-8-6-28/h1-4,9-12,27,30H,5-8,13,25H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human KDR by time-resolved fluorescence or time-resolved fluorescence assay |
J Med Chem 59: 10586-10600 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01156
BindingDB Entry DOI: 10.7270/Q2ZS2ZGV |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479850
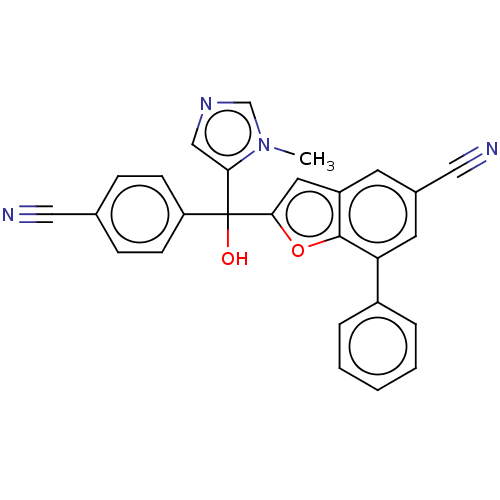
(CHEMBL475149)Show SMILES Cn1cncc1C(O)(c1cc2cc(cc(-c3ccccc3)c2o1)C#N)c1ccc(cc1)C#N Show InChI InChI=1S/C27H18N4O2/c1-31-17-30-16-24(31)27(32,22-9-7-18(14-28)8-10-22)25-13-21-11-19(15-29)12-23(26(21)33-25)20-5-3-2-4-6-20/h2-13,16-17,32H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593046

(CHEMBL5199353)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(NS(C)(=O)=O)cc12)C#Cc1cc(ccn1)C(=O)NC1CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479864

(CHEMBL510021)Show SMILES COc1cccc(c1)-c1cc(cc2cc(oc12)C(O)(c1cncn1C)c1ccc(cc1)C#N)C(=O)NN1CCOCC1 Show InChI InChI=1S/C32H29N5O5/c1-36-20-34-19-28(36)32(39,25-8-6-21(18-33)7-9-25)29-17-23-14-24(31(38)35-37-10-12-41-13-11-37)16-27(30(23)42-29)22-4-3-5-26(15-22)40-2/h3-9,14-17,19-20,39H,10-13H2,1-2H3,(H,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593052
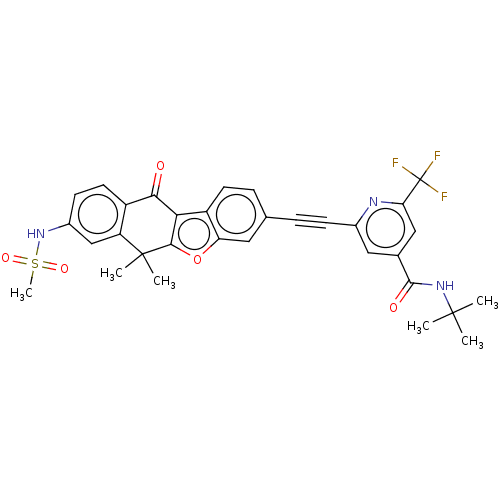
(CHEMBL5178903)Show SMILES CC(C)(C)NC(=O)c1cc(nc(c1)C(F)(F)F)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593030
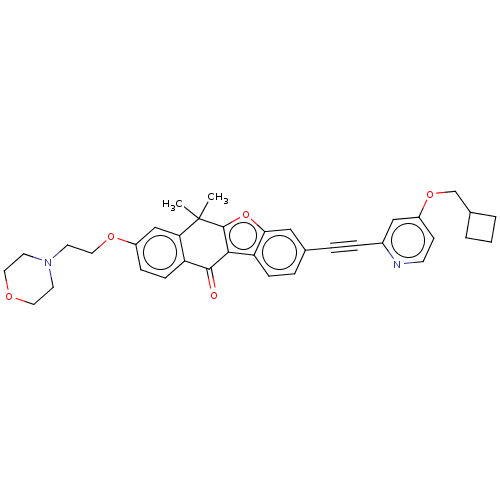
(CHEMBL5193231)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCOCC3)cc12)C#Cc1cc(OCC2CCC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Chitin synthase 1
(Candida albicans) | BDBM50089543
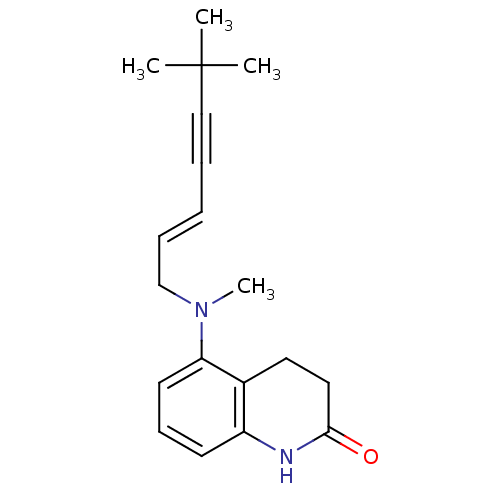
(5-[((E)-6,6-Dimethyl-hept-2-en-4-ynyl)-methyl-amin...)Show InChI InChI=1S/C19H24N2O/c1-19(2,3)13-6-5-7-14-21(4)17-10-8-9-16-15(17)11-12-18(22)20-16/h5,7-10H,11-12,14H2,1-4H3,(H,20,22)/b7-5+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory of Candida albicans chitin synthase 1 |
Bioorg Med Chem Lett 10: 1459-62 (2000)
BindingDB Entry DOI: 10.7270/Q2R210MK |
More data for this
Ligand-Target Pair | |
NT-3 growth factor receptor
(Homo sapiens (Human)) | BDBM50525707

(CHEMBL4457566)Show SMILES Cc1cc(cc(n1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O)C(=O)NC(C)(C)C Show InChI InChI=1S/C32H31N3O5S/c1-18-14-20(30(37)34-31(2,3)4)16-21(33-18)10-8-19-9-12-24-26(15-19)40-29-27(24)28(36)23-13-11-22(35-41(7,38)39)17-25(23)32(29,5)6/h9,11-17,35H,1-7H3,(H,34,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data