 Found 6013 hits with Last Name = 'lamb' and Initial = 'm'
Found 6013 hits with Last Name = 'lamb' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50246899

((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)c1ccc(I)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H24IN3O8/c20-12-6-4-11(5-7-12)16(26)21-10-2-1-3-13(17(27)28)22-19(31)23-14(18(29)30)8-9-15(24)25/h4-7,13-14H,1-3,8-10H2,(H,21,26)(H,24,25)(H,27,28)(H,29,30)(H2,22,23,31)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutions
Curated by ChEMBL
| Assay Description
Inhibition of PSMA in human LNCaP cell extract using [3H]NAAG by fluorescence-based assay |
J Med Chem 51: 7933-43 (2008)
Article DOI: 10.1021/jm801055h
BindingDB Entry DOI: 10.7270/Q2RV0NKJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50246899

((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)c1ccc(I)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H24IN3O8/c20-12-6-4-11(5-7-12)16(26)21-10-2-1-3-13(17(27)28)22-19(31)23-14(18(29)30)8-9-15(24)25/h4-7,13-14H,1-3,8-10H2,(H,21,26)(H,24,25)(H,27,28)(H,29,30)(H2,22,23,31)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutions
Curated by ChEMBL
| Assay Description
Inhibition of PSMA in human LNCaP cell extract using [3H]NAAG by radiometric assay |
J Med Chem 51: 7933-43 (2008)
Article DOI: 10.1021/jm801055h
BindingDB Entry DOI: 10.7270/Q2RV0NKJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50146972
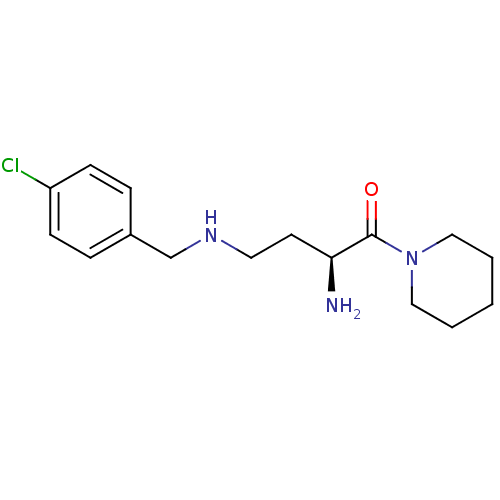
((S)-4-(4-chlorobenzylamino)-2-amino-1-(piperidin-1...)Show InChI InChI=1S/C16H24ClN3O/c17-14-6-4-13(5-7-14)12-19-9-8-15(18)16(21)20-10-2-1-3-11-20/h4-7,15,19H,1-3,8-12,18H2/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of DPP2 (unknown origin) |
Bioorg Med Chem Lett 18: 4154-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.080
BindingDB Entry DOI: 10.7270/Q2G160N3 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50146972

((S)-4-(4-chlorobenzylamino)-2-amino-1-(piperidin-1...)Show InChI InChI=1S/C16H24ClN3O/c17-14-6-4-13(5-7-14)12-19-9-8-15(18)16(21)20-10-2-1-3-11-20/h4-7,15,19H,1-3,8-12,18H2/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 16: 4777-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.082
BindingDB Entry DOI: 10.7270/Q2GM86XS |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50161518

(1-(oxazolo[4,5-b]pyridin-2-yl)-6-phenylhexan-1-one...)Show InChI InChI=1S/C18H18N2O2/c21-15(18-20-17-16(22-18)12-7-13-19-17)11-6-2-5-10-14-8-3-1-4-9-14/h1,3-4,7-9,12-13H,2,5-6,10-11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain
Curated by ChEMBL
| Assay Description
Binding affinity for human fatty acid amide hydrolase |
J Med Chem 48: 5059-87 (2005)
Article DOI: 10.1021/jm058183t
BindingDB Entry DOI: 10.7270/Q2J96753 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50246934
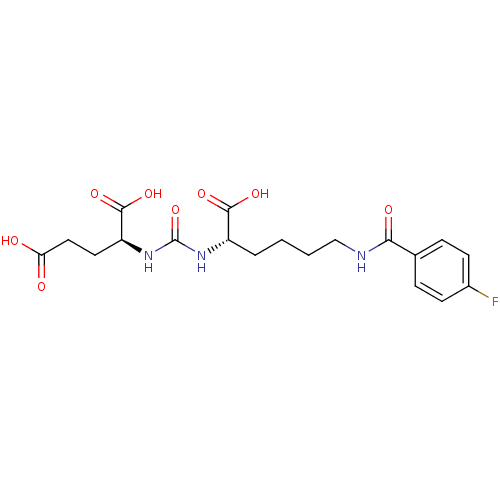
((S)-2-(3-((S)-1-carboxy-5-(4-fluorobenzamido)penty...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)c1ccc(F)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H24FN3O8/c20-12-6-4-11(5-7-12)16(26)21-10-2-1-3-13(17(27)28)22-19(31)23-14(18(29)30)8-9-15(24)25/h4-7,13-14H,1-3,8-10H2,(H,21,26)(H,24,25)(H,27,28)(H,29,30)(H2,22,23,31)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.194 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutions
Curated by ChEMBL
| Assay Description
Inhibition of PSMA in human LNCaP cell extract using [3H]NAAG by fluorescence-based assay |
J Med Chem 51: 7933-43 (2008)
Article DOI: 10.1021/jm801055h
BindingDB Entry DOI: 10.7270/Q2RV0NKJ |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50161518

(1-(oxazolo[4,5-b]pyridin-2-yl)-6-phenylhexan-1-one...)Show InChI InChI=1S/C18H18N2O2/c21-15(18-20-17-16(22-18)12-7-13-19-17)11-6-2-5-10-14-8-3-1-4-9-14/h1,3-4,7-9,12-13H,2,5-6,10-11H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain
Curated by ChEMBL
| Assay Description
Binding affinity for rat fatty acid amide hydrolase |
J Med Chem 48: 5059-87 (2005)
Article DOI: 10.1021/jm058183t
BindingDB Entry DOI: 10.7270/Q2J96753 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50246934

((S)-2-(3-((S)-1-carboxy-5-(4-fluorobenzamido)penty...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)c1ccc(F)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H24FN3O8/c20-12-6-4-11(5-7-12)16(26)21-10-2-1-3-13(17(27)28)22-19(31)23-14(18(29)30)8-9-15(24)25/h4-7,13-14H,1-3,8-10H2,(H,21,26)(H,24,25)(H,27,28)(H,29,30)(H2,22,23,31)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.256 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutions
Curated by ChEMBL
| Assay Description
Inhibition of PSMA in human LNCaP cell extract using [3H]NAAG by radiometric assay |
J Med Chem 51: 7933-43 (2008)
Article DOI: 10.1021/jm801055h
BindingDB Entry DOI: 10.7270/Q2RV0NKJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50397390
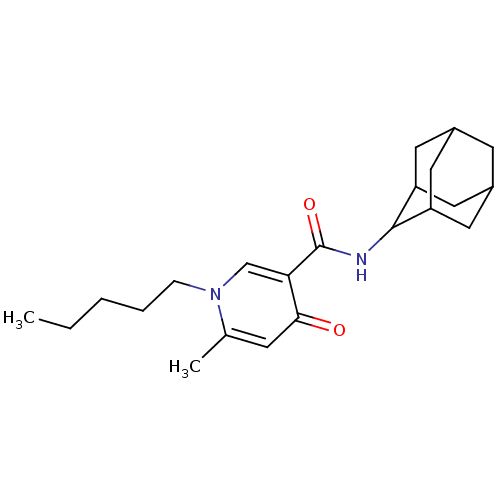
(CHEMBL2170545)Show SMILES CCCCCn1cc(C(=O)NC2C3CC4CC(C3)CC2C4)c(=O)cc1C |TLB:20:19:17:13.14.15,THB:20:14:11.19.18:17,15:14:11:18.16.17,15:16:11:13.20.14,10:11:17:13.14.15,(25.24,-38.56,;26.58,-39.34,;27.92,-38.57,;29.25,-39.35,;30.6,-38.58,;31.93,-39.36,;33.27,-38.58,;34.61,-39.36,;35.95,-38.59,;35.95,-37.05,;37.29,-39.37,;38.73,-38.65,;39.88,-37.52,;41.08,-38.03,;42.39,-37.76,;42.46,-36.35,;41.19,-35.76,;39.93,-36.15,;40.18,-36.86,;40.13,-38.32,;41.4,-38.9,;34.61,-40.91,;35.94,-41.68,;33.26,-41.67,;31.93,-40.9,;30.59,-41.67,)| Show InChI InChI=1S/C22H32N2O2/c1-3-4-5-6-24-13-19(20(25)7-14(24)2)22(26)23-21-17-9-15-8-16(11-17)12-18(21)10-15/h7,13,15-18,21H,3-6,8-12H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille Nord de France
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB2 receptor expressed in CHO cells membrane by scintillation counting |
J Med Chem 55: 8948-52 (2012)
Article DOI: 10.1021/jm3008568
BindingDB Entry DOI: 10.7270/Q27H1KQS |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50135635
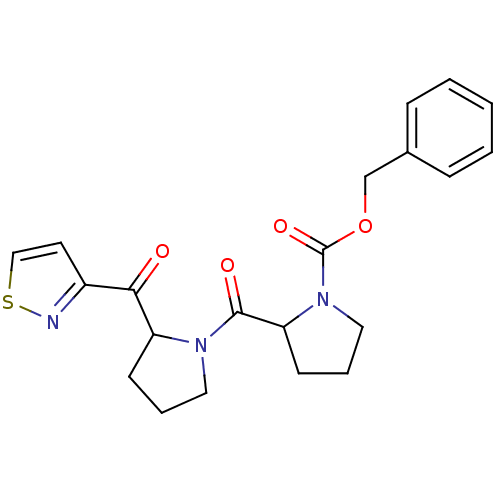
(2-[2-(Isothiazole-3-carbonyl)-pyrrolidine-1-carbon...)Show SMILES O=C(OCc1ccccc1)N1CCCC1C(=O)N1CCCC1C(=O)c1ccsn1 Show InChI InChI=1S/C21H23N3O4S/c25-19(16-10-13-29-22-16)17-8-4-11-23(17)20(26)18-9-5-12-24(18)21(27)28-14-15-6-2-1-3-7-15/h1-3,6-7,10,13,17-18H,4-5,8-9,11-12,14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi |
Bioorg Med Chem Lett 13: 2875-8 (2003)
BindingDB Entry DOI: 10.7270/Q2JW8D9K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50397387
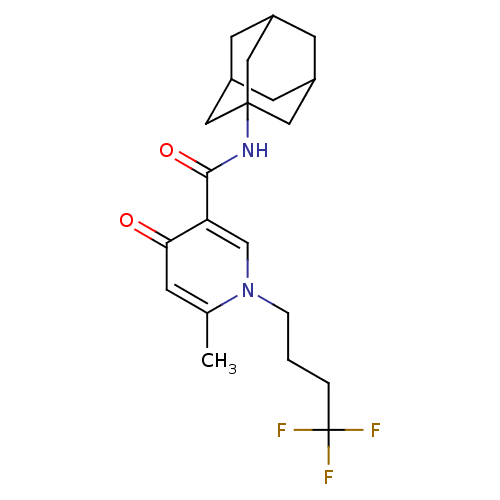
(CHEMBL2170532)Show SMILES Cc1cc(=O)c(cn1CCCC(F)(F)F)C(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:17:18:21:25.24.23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18| Show InChI InChI=1S/C21H27F3N2O2/c1-13-5-18(27)17(12-26(13)4-2-3-21(22,23)24)19(28)25-20-9-14-6-15(10-20)8-16(7-14)11-20/h5,12,14-16H,2-4,6-11H2,1H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille Nord de France
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB2 receptor expressed in CHO cells membrane by scintillation counting |
J Med Chem 55: 8948-52 (2012)
Article DOI: 10.1021/jm3008568
BindingDB Entry DOI: 10.7270/Q27H1KQS |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50135633

((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...)Show SMILES O=C(OCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1cc(COCc2ccccc2)on1 Show InChI InChI=1S/C29H31N3O6/c33-27(24-17-23(38-30-24)20-36-18-21-9-3-1-4-10-21)25-13-7-15-31(25)28(34)26-14-8-16-32(26)29(35)37-19-22-11-5-2-6-12-22/h1-6,9-12,17,25-26H,7-8,13-16,18-20H2/t25-,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi |
Bioorg Med Chem Lett 13: 2875-8 (2003)
BindingDB Entry DOI: 10.7270/Q2JW8D9K |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50135637

(1-{(S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-car...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1cc(COCc2ccccc2)on1 Show InChI InChI=1S/C31H35N3O5/c35-29(17-7-14-23-10-3-1-4-11-23)33-18-9-16-28(33)31(37)34-19-8-15-27(34)30(36)26-20-25(39-32-26)22-38-21-24-12-5-2-6-13-24/h1-6,10-13,20,27-28H,7-9,14-19,21-22H2/t27-,28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human |
Bioorg Med Chem Lett 13: 2875-8 (2003)
BindingDB Entry DOI: 10.7270/Q2JW8D9K |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50246935

((S)-2-(3-((S)-1-carboxy-5-(3-iodonicotinamido)pent...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)c1cncc(I)c1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C18H23IN4O8/c19-11-7-10(8-20-9-11)15(26)21-6-2-1-3-12(16(27)28)22-18(31)23-13(17(29)30)4-5-14(24)25/h7-9,12-13H,1-6H2,(H,21,26)(H,24,25)(H,27,28)(H,29,30)(H2,22,23,31)/t12-,13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.351 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutions
Curated by ChEMBL
| Assay Description
Inhibition of PSMA in human LNCaP cell extract using [3H]NAAG by radiometric assay |
J Med Chem 51: 7933-43 (2008)
Article DOI: 10.1021/jm801055h
BindingDB Entry DOI: 10.7270/Q2RV0NKJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50081023
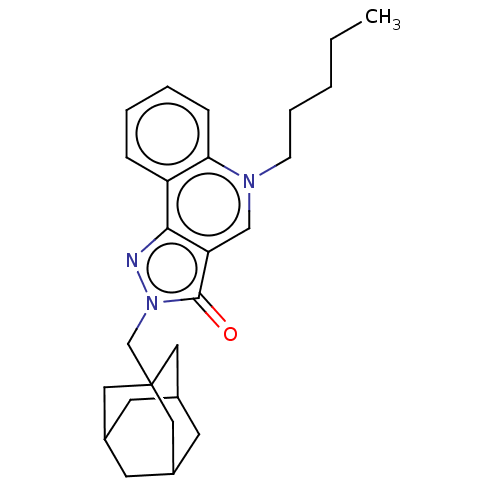
(CHEMBL3421699)Show SMILES CCCCCn1cc2c(nn(CC34CC5CC(CC(C5)C3)C4)c2=O)c2ccccc12 |TLB:19:14:21:18.20.17,19:18:21:13.14.15,THB:17:16:13:19.18.20,17:18:13:21.15.16| Show InChI InChI=1S/C26H33N3O/c1-2-3-6-9-28-16-22-24(21-7-4-5-8-23(21)28)27-29(25(22)30)17-26-13-18-10-19(14-26)12-20(11-18)15-26/h4-5,7-8,16,18-20H,2-3,6,9-15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Nord de France
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor after 1 hr by scintillation counting analysis |
ACS Med Chem Lett 6: 198-203 (2015)
Article DOI: 10.1021/ml500439x
BindingDB Entry DOI: 10.7270/Q20K2B8Z |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50135634
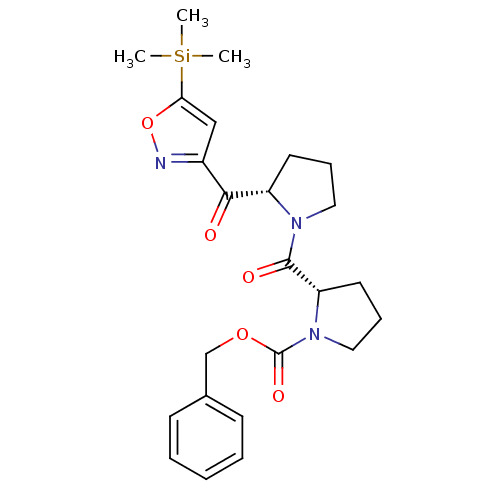
((S)-2-[(S)-2-(5-Trimethylsilanyl-isoxazole-3-carbo...)Show SMILES C[Si](C)(C)c1cc(no1)C(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)OCc1ccccc1 Show InChI InChI=1S/C24H31N3O5Si/c1-33(2,3)21-15-18(25-32-21)22(28)19-11-7-13-26(19)23(29)20-12-8-14-27(20)24(30)31-16-17-9-5-4-6-10-17/h4-6,9-10,15,19-20H,7-8,11-14,16H2,1-3H3/t19-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human |
Bioorg Med Chem Lett 13: 2875-8 (2003)
BindingDB Entry DOI: 10.7270/Q2JW8D9K |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50246935

((S)-2-(3-((S)-1-carboxy-5-(3-iodonicotinamido)pent...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)c1cncc(I)c1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C18H23IN4O8/c19-11-7-10(8-20-9-11)15(26)21-6-2-1-3-12(16(27)28)22-18(31)23-13(17(29)30)4-5-14(24)25/h7-9,12-13H,1-6H2,(H,21,26)(H,24,25)(H,27,28)(H,29,30)(H2,22,23,31)/t12-,13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.557 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutions
Curated by ChEMBL
| Assay Description
Inhibition of PSMA in human LNCaP cell extract using [3H]NAAG by fluorescence-based assay |
J Med Chem 51: 7933-43 (2008)
Article DOI: 10.1021/jm801055h
BindingDB Entry DOI: 10.7270/Q2RV0NKJ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403349
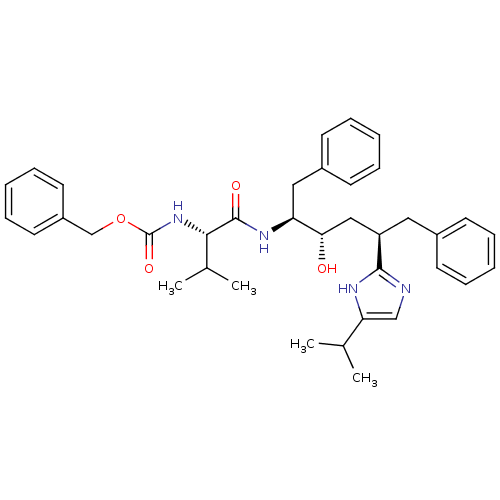
(CHEMBL407551)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C37H46N4O4/c1-25(2)32-23-38-35(39-32)30(20-27-14-8-5-9-15-27)22-33(42)31(21-28-16-10-6-11-17-28)40-36(43)34(26(3)4)41-37(44)45-24-29-18-12-7-13-19-29/h5-19,23,25-26,30-31,33-34,42H,20-22,24H2,1-4H3,(H,38,39)(H,40,43)(H,41,44)/t30-,31+,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50081021
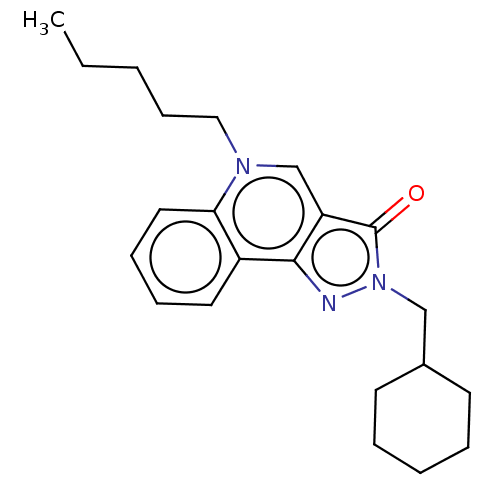
(CHEMBL3421697)Show InChI InChI=1S/C22H29N3O/c1-2-3-9-14-24-16-19-21(18-12-7-8-13-20(18)24)23-25(22(19)26)15-17-10-5-4-6-11-17/h7-8,12-13,16-17H,2-6,9-11,14-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Nord de France
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor after 1 hr by scintillation counting analysis |
ACS Med Chem Lett 6: 198-203 (2015)
Article DOI: 10.1021/ml500439x
BindingDB Entry DOI: 10.7270/Q20K2B8Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50334590
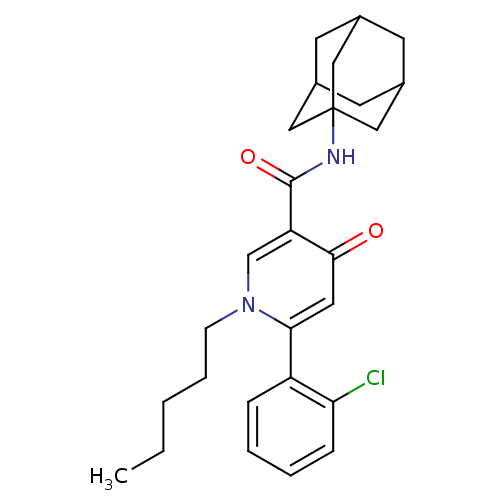
(CHEMBL1641956 | N3-(1-Adamantyl)-6-(2-chlorophenyl...)Show SMILES CCCCCn1cc(C(=O)NC23CC4CC(CC(C4)C2)C3)c(=O)cc1-c1ccccc1Cl |TLB:14:15:13.12.18:19,THB:16:15:12:18.17.19,16:17:14.15.20:12,14:13:15.20.16:19| Show InChI InChI=1S/C27H33ClN2O2/c1-2-3-6-9-30-17-22(25(31)13-24(30)21-7-4-5-8-23(21)28)26(32)29-27-14-18-10-19(15-27)12-20(11-18)16-27/h4-5,7-8,13,17-20H,2-3,6,9-12,14-16H2,1H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£? Lille-Nord de France
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells after 1 hr |
J Med Chem 53: 7918-31 (2010)
Article DOI: 10.1021/jm100286k
BindingDB Entry DOI: 10.7270/Q2BR8SFP |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508937

(CHEMBL4448046)Show SMILES Cn1nc2CSCc3nn(C)c(Cl)c3-c3c(Cl)ccc4c(CCCOc5cc(SCc1c2)cc1ccccc51)c(C(O)=O)n(C)c34 Show InChI InChI=1S/C34H31Cl2N5O3S2/c1-39-31-25-10-11-26(35)29(31)30-27(38-41(3)33(30)36)18-45-16-20-14-21(40(2)37-20)17-46-22-13-19-7-4-5-8-23(19)28(15-22)44-12-6-9-24(25)32(39)34(42)43/h4-5,7-8,10-11,13-15H,6,9,12,16-18H2,1-3H3,(H,42,43) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50433602
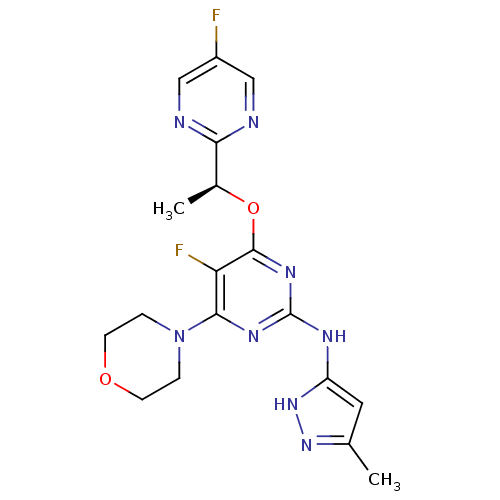
(CHEMBL2381980)Show SMILES C[C@H](Oc1nc(Nc2cc(C)n[nH]2)nc(N2CCOCC2)c1F)c1ncc(F)cn1 |r| Show InChI InChI=1S/C18H20F2N8O2/c1-10-7-13(27-26-10)23-18-24-16(28-3-5-29-6-4-28)14(20)17(25-18)30-11(2)15-21-8-12(19)9-22-15/h7-9,11H,3-6H2,1-2H3,(H2,23,24,25,26,27)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 23: 3105-10 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.111
BindingDB Entry DOI: 10.7270/Q23T9JMX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50304738
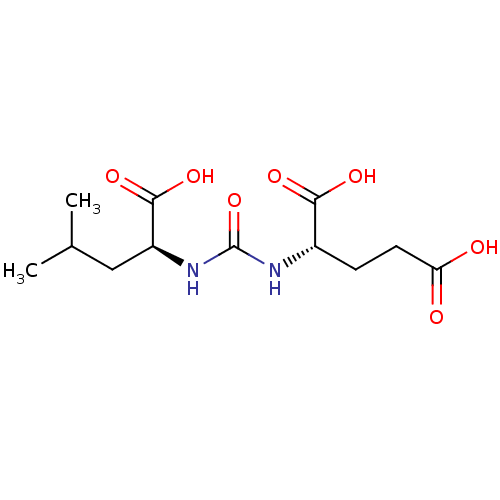
(2-(3-((S)-1-carboxy-3-methylbutyl)ureido)pentanedi...)Show SMILES CC(C)C[C@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C12H20N2O7/c1-6(2)5-8(11(19)20)14-12(21)13-7(10(17)18)3-4-9(15)16/h6-8H,3-5H2,1-2H3,(H,15,16)(H,17,18)(H,19,20)(H2,13,14,21)/t7-,8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutions
Curated by ChEMBL
| Assay Description
Inhibition of human cloned glutamate carboxypeptidase 2 |
Bioorg Med Chem Lett 20: 392-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.061
BindingDB Entry DOI: 10.7270/Q2SN092X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50080105

((3-(1,1-Dimethyl-heptyl)-9-hydroxymethyl-6,6-dimet...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(CO)CC[C@H]3C(C)(C)Oc2c1 Show InChI InChI=1S/C25H40O3/c1-6-7-8-9-12-24(2,3)18-14-21(27)23-19-13-17(16-26)10-11-20(19)25(4,5)28-22(23)15-18/h14-15,17,19-20,26-27H,6-13,16H2,1-5H3/t17?,19-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholiqu£ de Louvain
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-SR- 141716A binding to human CB1 receptor expressed in CHO cell membranes |
Bioorg Med Chem Lett 9: 2233-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TB17D5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50080105

((3-(1,1-Dimethyl-heptyl)-9-hydroxymethyl-6,6-dimet...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(CO)CC[C@H]3C(C)(C)Oc2c1 Show InChI InChI=1S/C25H40O3/c1-6-7-8-9-12-24(2,3)18-14-21(27)23-19-13-17(16-26)10-11-20(19)25(4,5)28-22(23)15-18/h14-15,17,19-20,26-27H,6-13,16H2,1-5H3/t17?,19-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lausanne
Curated by ChEMBL
| Assay Description
Compound was evaluated for affinity towards human Cannabinoid receptor 1 using [3H]- SR-141716A as radioligand |
J Med Chem 45: 1748-56 (2002)
BindingDB Entry DOI: 10.7270/Q2VM4D02 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM203869
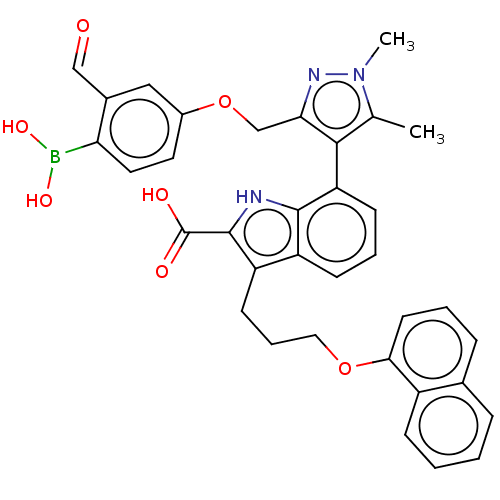
(7-(3-((4-Borono-3-formylphenoxy)methyl)-1,5-dimeth...)Show SMILES Cc1c(c(COc2ccc(B(O)O)c(C=O)c2)nn1C)-c1cccc2c(CCCOc3cccc4ccccc34)c([nH]c12)C(O)=O |(.05,-1.8,;-1.28,-2.57,;-2.53,-1.66,;-3.78,-2.57,;-5.11,-1.8,;-6.2,-2.88,;-7.53,-2.11,;-7.53,-.57,;-8.87,.2,;-10.2,-.57,;-11.29,.51,;-10.89,2,;-12.78,.91,;-10.2,-2.11,;-11.53,-2.88,;-10.76,-4.22,;-8.87,-2.88,;-3.3,-4.03,;-1.76,-4.03,;-.67,-5.12,;-2.48,-.6,;-3.81,.17,;-3.81,1.71,;-2.48,2.48,;-1.14,1.71,;.32,2.18,;-.08,3.67,;1.01,4.76,;.62,6.24,;1.7,7.33,;1.31,8.82,;-.03,9.59,;-.03,11.13,;1.31,11.9,;2.64,11.13,;3.97,11.9,;5.31,11.13,;5.31,9.59,;3.97,8.82,;2.64,9.59,;1.23,.94,;.32,-.31,;-1.14,.17,;2.77,.94,;3.86,-.15,;3.86,2.02,)| Show InChI InChI=1S/C35H32BN3O7/c1-21-32(30(38-39(21)2)20-46-24-15-16-29(36(43)44)23(18-24)19-40)28-12-6-11-26-27(34(35(41)42)37-33(26)28)13-7-17-45-31-14-5-9-22-8-3-4-10-25(22)31/h3-6,8-12,14-16,18-19,37,43-44H,7,13,17,20H2,1-2H3,(H,41,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | -51.5 | 3.40 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit
| Assay Description
TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... |
Nat Chem Biol 12: 931-936 (2016)
Article DOI: 10.1038/nchembio.2174
BindingDB Entry DOI: 10.7270/Q2FN1513 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403357

(CHEMBL419286)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C31H42N4O3/c1-20(2)27-19-32-30(34-27)25(16-23-12-8-6-9-13-23)18-28(37)26(17-24-14-10-7-11-15-24)35-31(38)29(21(3)4)33-22(5)36/h6-15,19-21,25-26,28-29,37H,16-18H2,1-5H3,(H,32,34)(H,33,36)(H,35,38)/t25-,26+,28+,29+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50171297
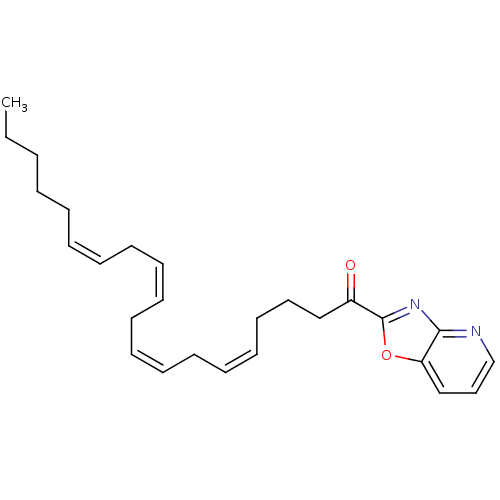
((5Z,8Z,11Z,14Z)-1-(oxazolo[4,5-b]pyridin-2-yl)icos...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)c1nc2ncccc2o1 Show InChI InChI=1S/C26H34N2O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-20-23(29)26-28-25-24(30-26)21-19-22-27-25/h6-7,9-10,12-13,15-16,19,21-22H,2-5,8,11,14,17-18,20H2,1H3/b7-6-,10-9-,13-12-,16-15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain
Curated by ChEMBL
| Assay Description
Binding affinity for fatty acid amide hydrolase |
J Med Chem 48: 5059-87 (2005)
Article DOI: 10.1021/jm058183t
BindingDB Entry DOI: 10.7270/Q2J96753 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085044

((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human Peroxisome proliferator activated receptor gamma using scintillation proximity assay |
Bioorg Med Chem Lett 11: 3111-3 (2001)
BindingDB Entry DOI: 10.7270/Q2B27TKM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM203875
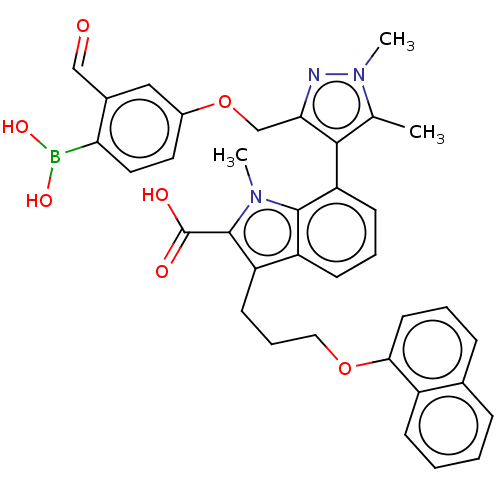
(7-(3-((4-Borono-3-formylphenoxy)methyl)-1,5-dimeth...)Show SMILES Cc1c(c(COc2ccc(B(O)O)c(C=O)c2)nn1C)-c1cccc2c(CCCOc3cccc4ccccc34)c(C(O)=O)n(C)c12 |(.34,-2.6,;-1.28,-2.57,;-2.53,-1.66,;-3.78,-2.57,;-5.11,-1.8,;-6.2,-2.88,;-7.53,-2.11,;-7.53,-.57,;-8.87,.2,;-10.2,-.57,;-11.29,.51,;-10.89,2,;-12.78,.91,;-10.2,-2.11,;-11.53,-2.88,;-10.76,-4.22,;-8.87,-2.88,;-3.3,-4.03,;-1.76,-4.03,;-.67,-5.12,;-2.48,-.6,;-3.81,.17,;-3.81,1.71,;-2.48,2.48,;-1.14,1.71,;.32,2.18,;-.08,3.67,;1.01,4.76,;.62,6.24,;1.7,7.33,;1.31,8.82,;-.03,9.59,;-.03,11.13,;1.31,11.9,;2.64,11.13,;3.97,11.9,;5.31,11.13,;5.31,9.59,;3.97,8.82,;2.64,9.59,;1.23,.94,;2.77,.94,;3.86,-.15,;3.86,2.02,;.32,-.31,;1.22,-1.52,;-1.14,.17,)| Show InChI InChI=1S/C36H34BN3O7/c1-22-33(31(38-40(22)3)21-47-25-16-17-30(37(44)45)24(19-25)20-41)29-13-7-12-27-28(35(36(42)43)39(2)34(27)29)14-8-18-46-32-15-6-10-23-9-4-5-11-26(23)32/h4-7,9-13,15-17,19-20,44-45H,8,14,18,21H2,1-3H3,(H,42,43) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | -50.7 | 4.20 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit
| Assay Description
TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... |
Nat Chem Biol 12: 931-936 (2016)
Article DOI: 10.1038/nchembio.2174
BindingDB Entry DOI: 10.7270/Q2FN1513 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM203870
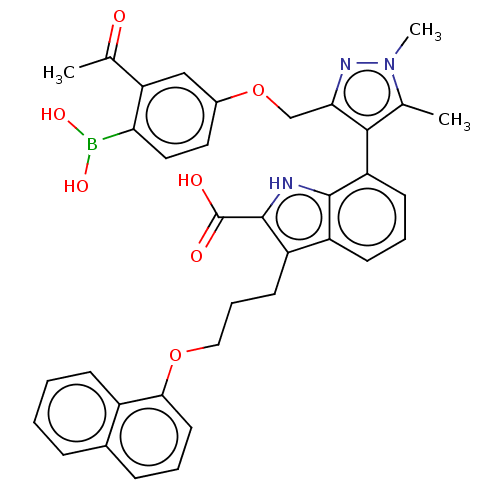
(7-(3-((3-Acetyl-4-boronophenoxy)methyl)-1,5-dimeth...)Show SMILES CC(=O)c1cc(OCc2nn(C)c(C)c2-c2cccc3c(CCCOc4cccc5ccccc45)c([nH]c23)C(O)=O)ccc1B(O)O |(-13.02,-2.49,;-11.53,-2.88,;-12.16,-4.44,;-10.2,-2.11,;-8.87,-2.88,;-7.53,-2.11,;-6.2,-2.88,;-5.11,-1.8,;-3.78,-2.57,;-3.3,-4.03,;-1.76,-4.03,;-.67,-5.12,;-1.28,-2.57,;.05,-1.8,;-2.53,-1.66,;-2.48,-.6,;-3.81,.17,;-3.81,1.71,;-2.48,2.48,;-1.14,1.71,;.32,2.18,;-.08,3.67,;1.01,4.76,;.62,6.24,;1.7,7.33,;1.31,8.82,;-.03,9.59,;-.03,11.13,;1.31,11.9,;2.64,11.13,;3.97,11.9,;5.31,11.13,;5.31,9.59,;3.97,8.82,;2.64,9.59,;1.23,.94,;.32,-.31,;-1.14,.17,;2.77,.94,;3.86,-.15,;3.86,2.02,;-7.53,-.57,;-8.87,.2,;-10.2,-.57,;-11.29,.51,;-10.89,2,;-12.78,.91,)| Show InChI InChI=1S/C36H34BN3O7/c1-21-33(31(39-40(21)3)20-47-24-16-17-30(37(44)45)29(19-24)22(2)41)28-13-7-12-26-27(35(36(42)43)38-34(26)28)14-8-18-46-32-15-6-10-23-9-4-5-11-25(23)32/h4-7,9-13,15-17,19,38,44-45H,8,14,18,20H2,1-3H3,(H,42,43) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | -50.7 | 4.70 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit
| Assay Description
TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... |
Nat Chem Biol 12: 931-936 (2016)
Article DOI: 10.1038/nchembio.2174
BindingDB Entry DOI: 10.7270/Q2FN1513 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50069273
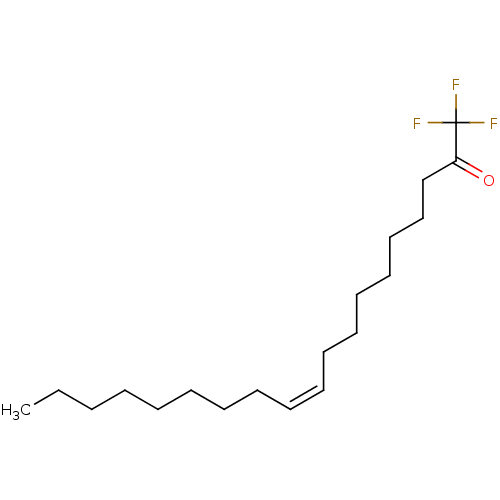
((Z)-1,1,1-Trifluoro-nonadec-10-en-2-one | 1,1,1-tr...)Show InChI InChI=1S/C19H33F3O/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(23)19(20,21)22/h9-10H,2-8,11-17H2,1H3/b10-9- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain
Curated by ChEMBL
| Assay Description
Binding affinity for fatty acid amide hydrolase |
J Med Chem 48: 5059-87 (2005)
Article DOI: 10.1021/jm058183t
BindingDB Entry DOI: 10.7270/Q2J96753 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille Nord de France
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SR141716A from human CB1 receptor expressed in CHO membranes after 1 hr by liquid scintillation counting |
Bioorg Med Chem 21: 5383-94 (2013)
Article DOI: 10.1016/j.bmc.2013.06.010
BindingDB Entry DOI: 10.7270/Q27S7RQW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain
Curated by ChEMBL
| Assay Description
Binding affinity for cannabinoid receptor 1 |
J Med Chem 48: 5059-87 (2005)
Article DOI: 10.1021/jm058183t
BindingDB Entry DOI: 10.7270/Q2J96753 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50433601
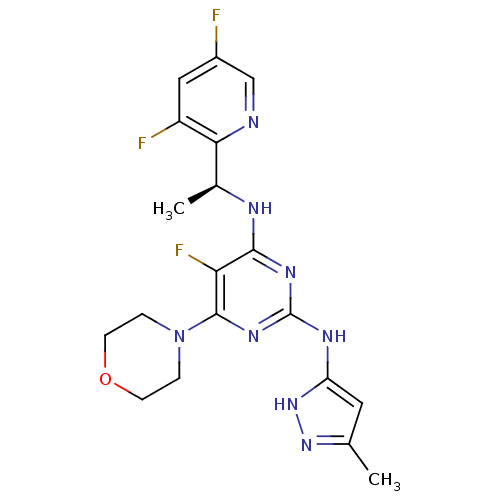
(CHEMBL2381983)Show SMILES C[C@H](Nc1nc(Nc2cc(C)n[nH]2)nc(N2CCOCC2)c1F)c1ncc(F)cc1F |r| Show InChI InChI=1S/C19H21F3N8O/c1-10-7-14(29-28-10)25-19-26-17(15(22)18(27-19)30-3-5-31-6-4-30)24-11(2)16-13(21)8-12(20)9-23-16/h7-9,11H,3-6H2,1-2H3,(H3,24,25,26,27,28,29)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 23: 3105-10 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.111
BindingDB Entry DOI: 10.7270/Q23T9JMX |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403360
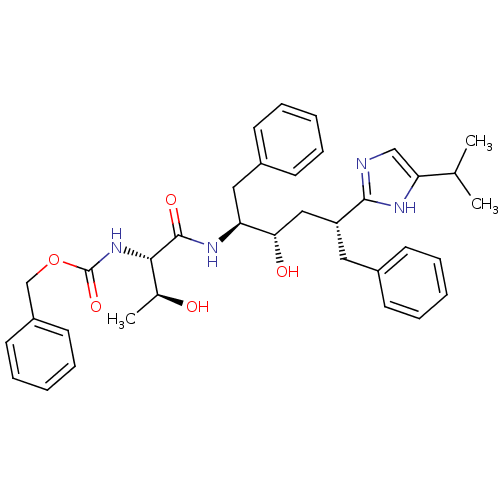
(CHEMBL1790592)Show SMILES CC(C)c1cnc([nH]1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)[C@H](C)O)Cc1ccccc1 Show InChI InChI=1S/C36H44N4O5/c1-24(2)31-22-37-34(38-31)29(19-26-13-7-4-8-14-26)21-32(42)30(20-27-15-9-5-10-16-27)39-35(43)33(25(3)41)40-36(44)45-23-28-17-11-6-12-18-28/h4-18,22,24-25,29-30,32-33,41-42H,19-21,23H2,1-3H3,(H,37,38)(H,39,43)(H,40,44)/t25-,29+,30-,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM203876
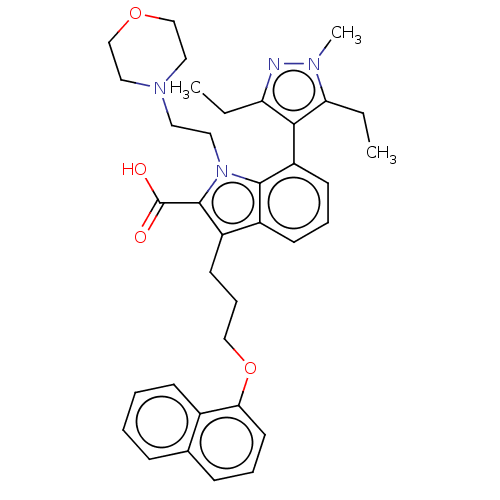
(Mcl-1 inhibitor 12)Show SMILES CCc1nn(C)c(CC)c1-c1cccc2c(CCCOc3cccc4ccccc34)c(C(O)=O)n(CCN3CCOCC3)c12 |(-6.2,-2.88,;-5.11,-1.8,;-3.78,-2.57,;-3.3,-4.03,;-1.76,-4.03,;-.67,-5.12,;-1.28,-2.57,;-.02,-2.6,;.3,-4.24,;-2.53,-1.66,;-2.48,-.6,;-3.81,.17,;-3.81,1.71,;-2.48,2.48,;-1.14,1.71,;.32,2.18,;-.08,3.67,;1.01,4.76,;.62,6.24,;1.7,7.33,;1.31,8.82,;-.03,9.59,;-.03,11.13,;1.31,11.9,;2.64,11.13,;3.97,11.9,;5.31,11.13,;5.31,9.59,;3.97,8.82,;2.64,9.59,;1.23,.94,;2.77,.94,;3.86,-.15,;3.86,2.02,;.32,-.31,;1.22,-1.52,;2.76,-1.52,;3.16,-3.01,;1.82,-3.78,;1.82,-5.32,;3.16,-6.09,;4.49,-5.32,;4.49,-3.78,;-1.14,.17,)| Show InChI InChI=1S/C36H42N4O4/c1-4-30-33(31(5-2)38(3)37-30)29-15-9-14-27-28(16-10-22-44-32-17-8-12-25-11-6-7-13-26(25)32)35(36(41)42)40(34(27)29)19-18-39-20-23-43-24-21-39/h6-9,11-15,17H,4-5,10,16,18-24H2,1-3H3,(H,41,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | -50.0 | 5.96 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit
| Assay Description
TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... |
Nat Chem Biol 12: 931-936 (2016)
Article DOI: 10.1038/nchembio.2174
BindingDB Entry DOI: 10.7270/Q2FN1513 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50334589
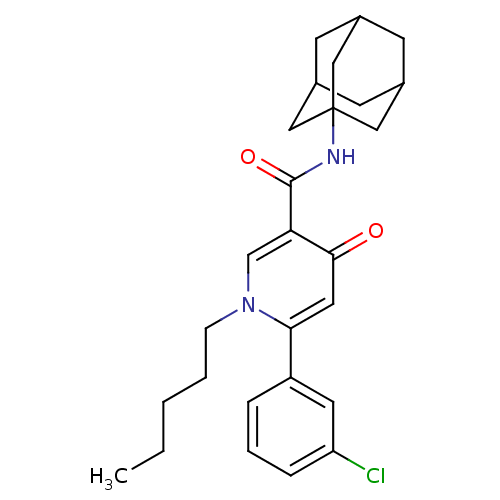
(CHEMBL1641955 | N3-(1-Adamantyl)-6-(3-chlorophenyl...)Show SMILES CCCCCn1cc(C(=O)NC23CC4CC(CC(C4)C2)C3)c(=O)cc1-c1cccc(Cl)c1 |TLB:14:15:13.12.18:19,THB:16:15:12:18.17.19,16:17:14.15.20:12,14:13:15.20.16:19| Show InChI InChI=1S/C27H33ClN2O2/c1-2-3-4-8-30-17-23(25(31)13-24(30)21-6-5-7-22(28)12-21)26(32)29-27-14-18-9-19(15-27)11-20(10-18)16-27/h5-7,12-13,17-20H,2-4,8-11,14-16H2,1H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£? Lille-Nord de France
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells after 1 hr |
J Med Chem 53: 7918-31 (2010)
Article DOI: 10.1021/jm100286k
BindingDB Entry DOI: 10.7270/Q2BR8SFP |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50135630

((S)-2-[(S)-2-(5-Phenyl-isoxazole-3-carbonyl)-pyrro...)Show SMILES O=C(OCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1cc(on1)-c1ccccc1 Show InChI InChI=1S/C27H27N3O5/c31-25(21-17-24(35-28-21)20-11-5-2-6-12-20)22-13-7-15-29(22)26(32)23-14-8-16-30(23)27(33)34-18-19-9-3-1-4-10-19/h1-6,9-12,17,22-23H,7-8,13-16,18H2/t22-,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi |
Bioorg Med Chem Lett 13: 2875-8 (2003)
BindingDB Entry DOI: 10.7270/Q2JW8D9K |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50135630

((S)-2-[(S)-2-(5-Phenyl-isoxazole-3-carbonyl)-pyrro...)Show SMILES O=C(OCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1cc(on1)-c1ccccc1 Show InChI InChI=1S/C27H27N3O5/c31-25(21-17-24(35-28-21)20-11-5-2-6-12-20)22-13-7-15-29(22)26(32)23-14-8-16-30(23)27(33)34-18-19-9-3-1-4-10-19/h1-6,9-12,17,22-23H,7-8,13-16,18H2/t22-,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi |
Bioorg Med Chem Lett 13: 2875-8 (2003)
BindingDB Entry DOI: 10.7270/Q2JW8D9K |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063920
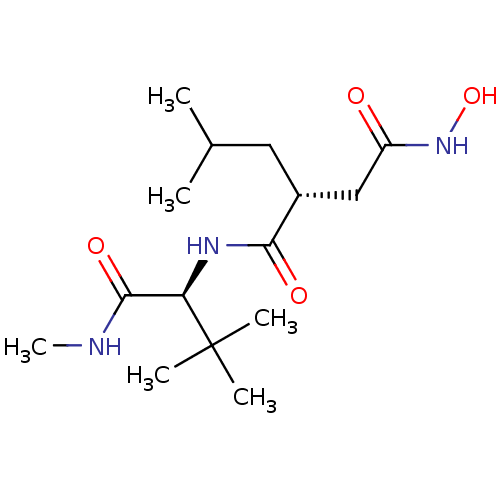
((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)CC(=O)NO)C(C)(C)C Show InChI InChI=1S/C15H29N3O4/c1-9(2)7-10(8-11(19)18-22)13(20)17-12(14(21)16-6)15(3,4)5/h9-10,12,22H,7-8H2,1-6H3,(H,16,21)(H,17,20)(H,18,19)/t10-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50063920

((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)CC(=O)NO)C(C)(C)C Show InChI InChI=1S/C15H29N3O4/c1-9(2)7-10(8-11(19)18-22)13(20)17-12(14(21)16-6)15(3,4)5/h9-10,12,22H,7-8H2,1-6H3,(H,16,21)(H,17,20)(H,18,19)/t10-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA)
Curated by ChEMBL
| Assay Description
Inhibition of Dipeptidyl Peptidase IV |
Bioorg Med Chem Lett 12: 2825-8 (2002)
BindingDB Entry DOI: 10.7270/Q279457M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403351
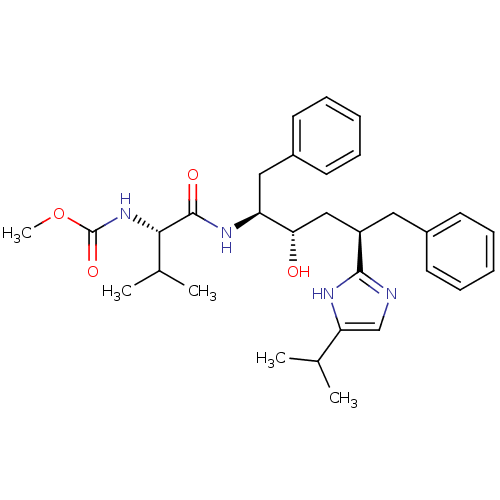
(CHEMBL79698)Show SMILES COC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C31H42N4O4/c1-20(2)26-19-32-29(33-26)24(16-22-12-8-6-9-13-22)18-27(36)25(17-23-14-10-7-11-15-23)34-30(37)28(21(3)4)35-31(38)39-5/h6-15,19-21,24-25,27-28,36H,16-18H2,1-5H3,(H,32,33)(H,34,37)(H,35,38)/t24-,25+,27+,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50100865
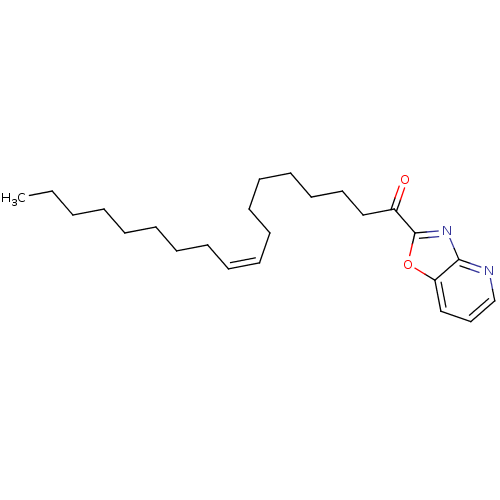
((Z)-1-(oxazolo[4,5-b]pyridin-2-yl)octadec-9-en-1-o...)Show InChI InChI=1S/C24H36N2O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-18-21(27)24-26-23-22(28-24)19-17-20-25-23/h9-10,17,19-20H,2-8,11-16,18H2,1H3/b10-9- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain
Curated by ChEMBL
| Assay Description
Binding affinity for fatty acid amide hydrolase |
J Med Chem 48: 5059-87 (2005)
Article DOI: 10.1021/jm058183t
BindingDB Entry DOI: 10.7270/Q2J96753 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50067499

((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(CO)=CC[C@H]3C(C)(C)Oc2c1 |r,c:18| Show InChI InChI=1S/C25H38O3/c1-6-7-8-9-12-24(2,3)18-14-21(27)23-19-13-17(16-26)10-11-20(19)25(4,5)28-22(23)15-18/h10,14-15,19-20,26-27H,6-9,11-13,16H2,1-5H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403358
(CHEMBL78531)Show SMILES CCOC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C32H44N4O4/c1-6-40-32(39)36-29(22(4)5)31(38)35-26(18-24-15-11-8-12-16-24)28(37)19-25(17-23-13-9-7-10-14-23)30-33-20-27(34-30)21(2)3/h7-16,20-22,25-26,28-29,37H,6,17-19H2,1-5H3,(H,33,34)(H,35,38)(H,36,39)/t25-,26+,28+,29+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase FAP
(Mus musculus (Mouse)) | BDBM50434188

(CHEMBL2385281 | US9346814, Cmpd No 2 Example 3)Show SMILES O=C(CNC(=O)c1ccnc2ccccc12)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C17H16N4O2/c18-10-12-4-3-9-21(12)16(22)11-20-17(23)14-7-8-19-15-6-2-1-5-13(14)15/h1-2,5-8,12H,3-4,9,11H2,(H,20,23)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA)
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant FAP expressed in HEK293 cells using Ala-Pro-p-nitroanilide as substrate incubated for 15 mins prior to substrate addi... |
ACS Med Chem Lett 4: 491-6 (2013)
Article DOI: 10.1021/ml300410d
BindingDB Entry DOI: 10.7270/Q2KP83J7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Nord de France
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor after 1 hr by scintillation counting analysis |
ACS Med Chem Lett 6: 198-203 (2015)
Article DOI: 10.1021/ml500439x
BindingDB Entry DOI: 10.7270/Q20K2B8Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data