

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ataxin-2 (Homo sapiens (Human)) | BDBM258574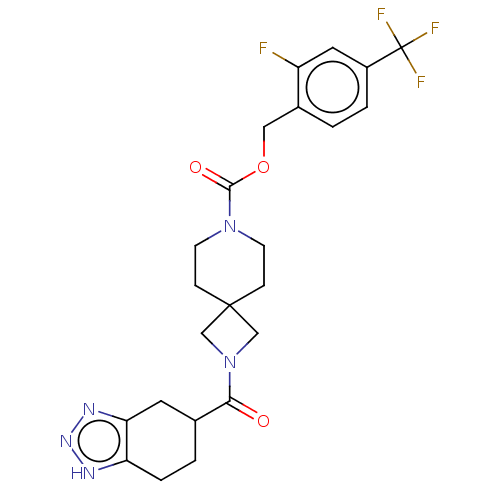 (US10633384, Example 12.36 | US9493486, 12.36) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258575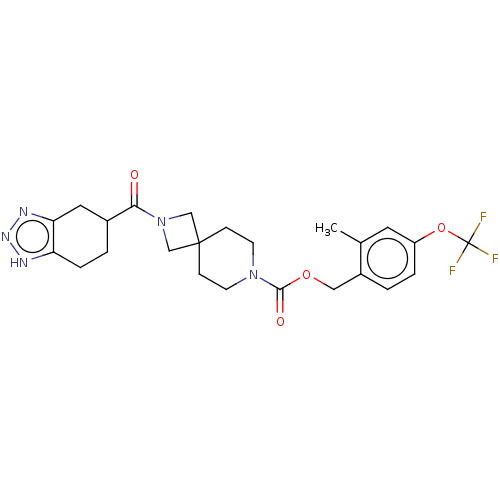 (US10633384, Example 12.37 | US9493486, 12.37) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258580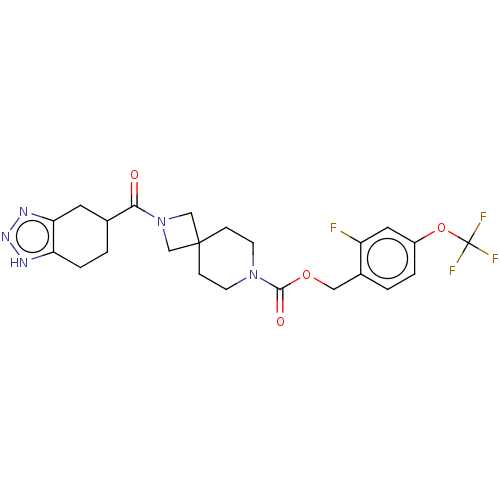 (US10633384, Example 12.42 | US9493486, 12.42) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258581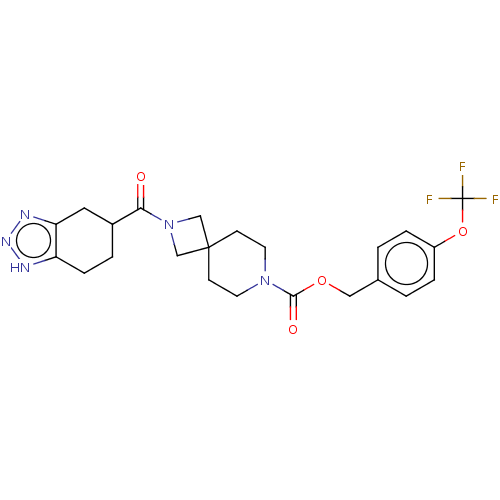 (US10633384, Example 12.43 | US9493486, 12.43) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258585 (US10633384, 13.01 | US9493486, 13.01) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM440743 ((?)-2-Fluoro-4-(trifluoromethoxy)benzyl 2-(4,5,6,7...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258501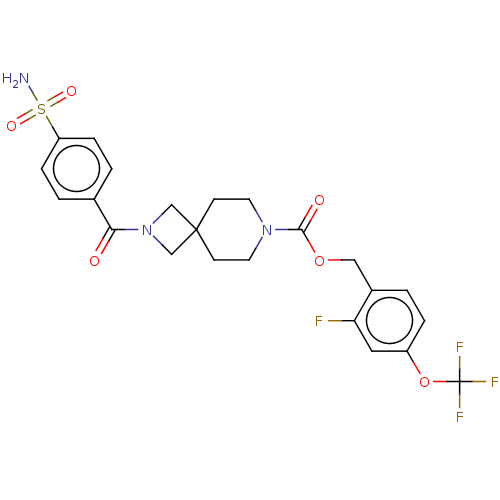 (US10633384, Example 1.23 | US9493486, 1.23) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258574 (US10633384, Example 12.36 | US9493486, 12.36) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258575 (US10633384, Example 12.37 | US9493486, 12.37) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258580 (US10633384, Example 12.42 | US9493486, 12.42) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258581 (US10633384, Example 12.43 | US9493486, 12.43) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258585 (US10633384, 13.01 | US9493486, 13.01) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258590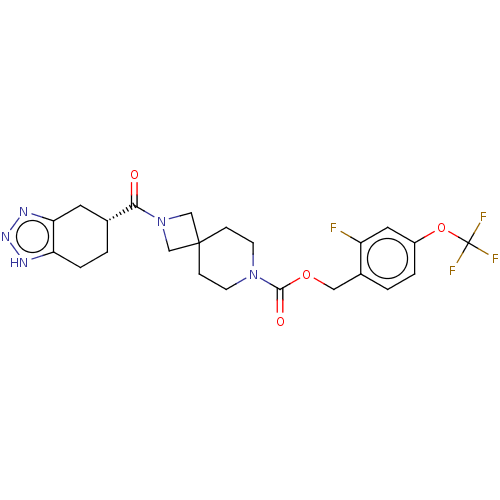 (US9493486, 15B) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258501 (US10633384, Example 1.23 | US9493486, 1.23) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258579 (US10633384, Example 12.41 | US9493486, 12.41) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258579 (US10633384, Example 12.41 | US9493486, 12.41) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258576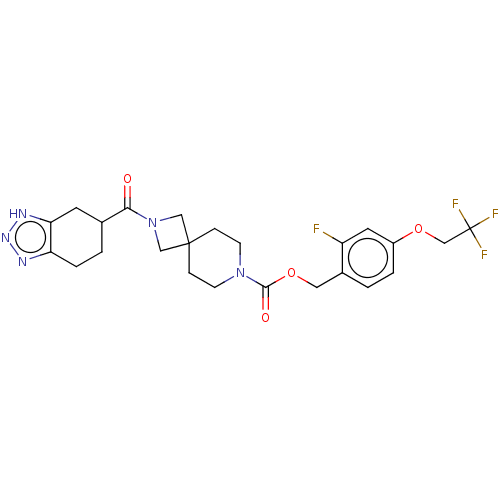 (US10633384, Example 12.38 | US9493486, 12.38) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258578 (US10633384, Example 12.40 | US9493486, 12.40) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258576 (US10633384, Example 12.38 | US9493486, 12.38) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258578 (US10633384, Example 12.40 | US9493486, 12.40) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258584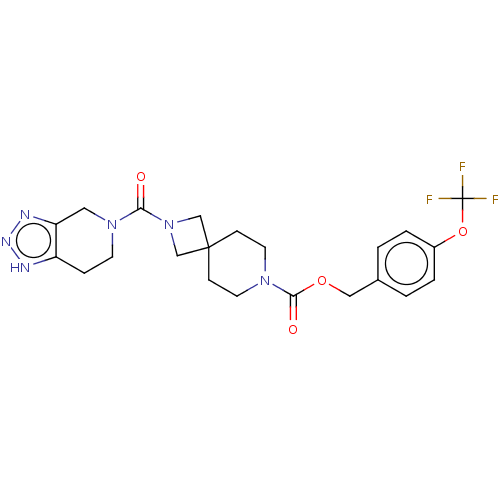 (US10633384, Example 13 | US9493486, 13) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258584 (US10633384, Example 13 | US9493486, 13) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258573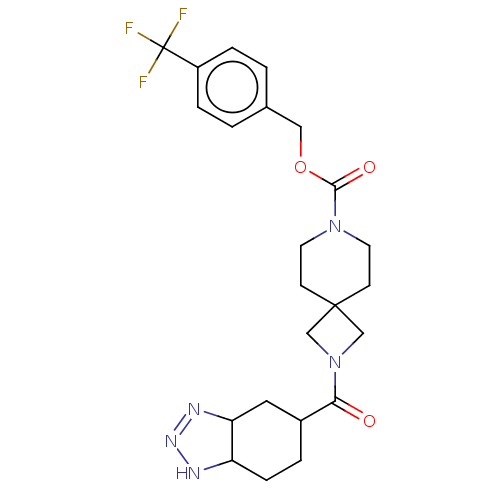 (US10633384, Example 12.35 | US9493486, 12.35) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258573 (US10633384, Example 12.35 | US9493486, 12.35) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258577 (US10633384, Example 12.39 | US9493486, 12.39) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258577 (US10633384, Example 12.39 | US9493486, 12.39) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258510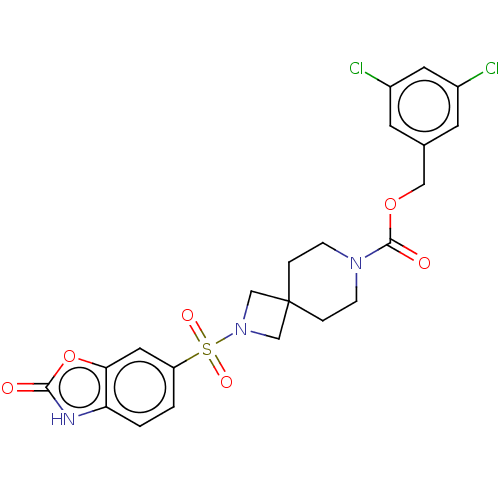 (US10633384, Example 3 | US9493486, 3) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258510 (US10633384, Example 3 | US9493486, 3) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258508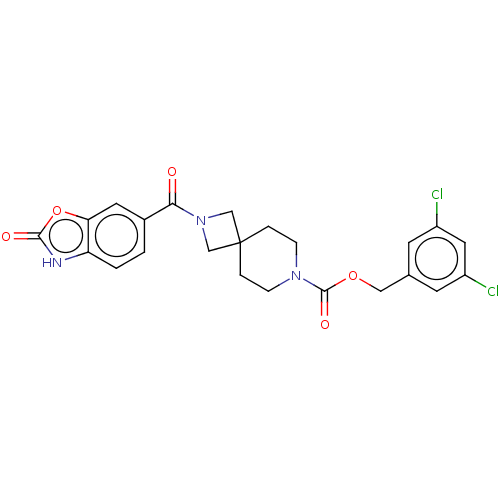 (BDBM258518 | US10633384, Example 5 | US9493486, 2....) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258508 (BDBM258518 | US10633384, Example 5 | US9493486, 2....) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258582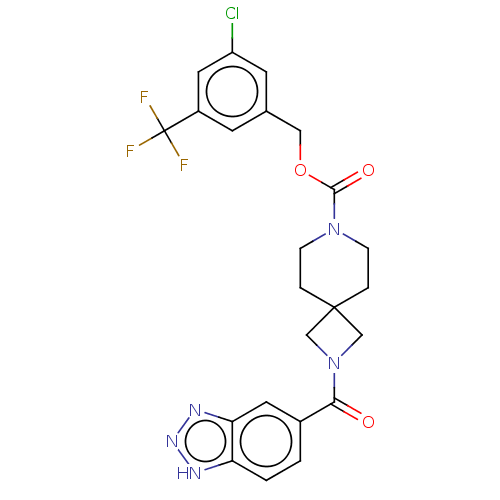 (US10633384, Example 12.44 | US9493486, 12.44) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258504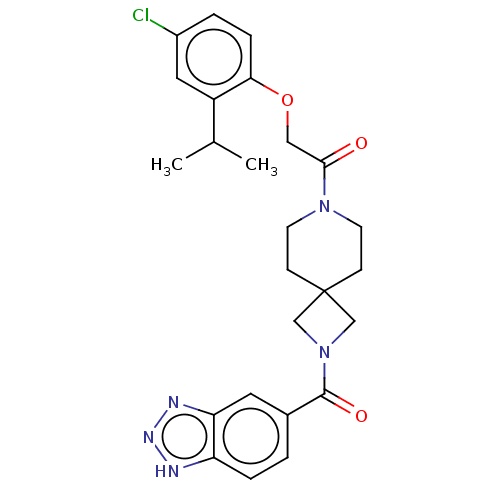 (US10633384, Example 1.26 | US9493486, 1.26) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258582 (US10633384, Example 12.44 | US9493486, 12.44) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258504 (US10633384, Example 1.26 | US9493486, 1.26) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258591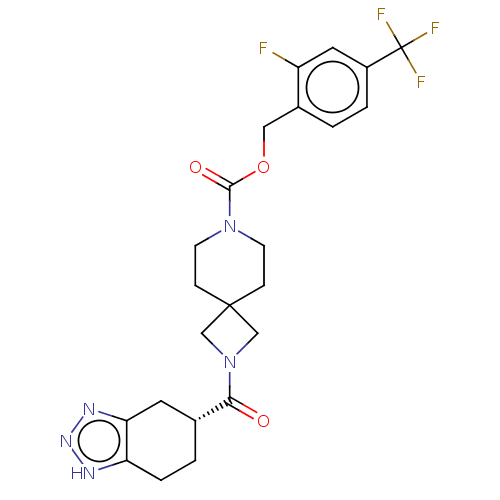 (BDBM440745 | US9493486, 16B) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258591 (BDBM440745 | US9493486, 16B) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258572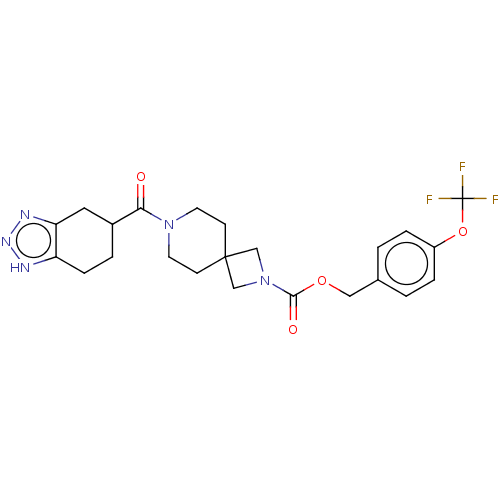 (US10633384, Example 12.34 | US9493486, 12.34) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258572 (US10633384, Example 12.34 | US9493486, 12.34) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM371829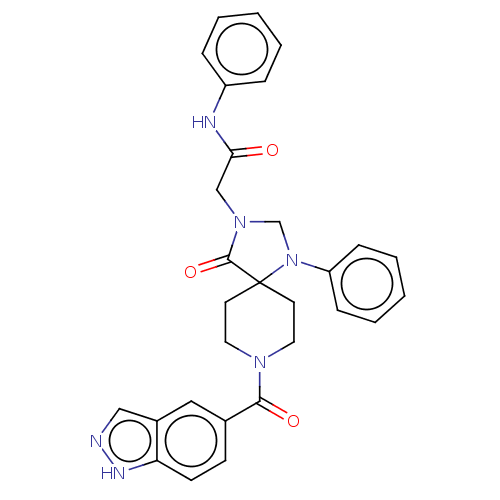 (2-(8-(1H-Indazole-5-carbonyl)-4-oxo-1-phenyl-1,3,8...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epithelial discoidin domain-containing receptor 1 (Homo sapiens (Human)) | BDBM371829 (2-(8-(1H-Indazole-5-carbonyl)-4-oxo-1-phenyl-1,3,8...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | US Patent US10435407 (2019) BindingDB Entry DOI: 10.7270/Q2MP55M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM371835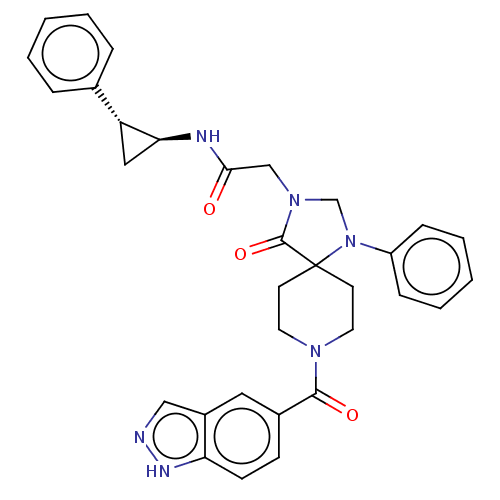 ((rac, trans)-2-(8-(1H-Indazole-5-carbonyl)-4-oxo-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epithelial discoidin domain-containing receptor 1 (Homo sapiens (Human)) | BDBM371835 ((rac, trans)-2-(8-(1H-Indazole-5-carbonyl)-4-oxo-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | US Patent US10435407 (2019) BindingDB Entry DOI: 10.7270/Q2MP55M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258502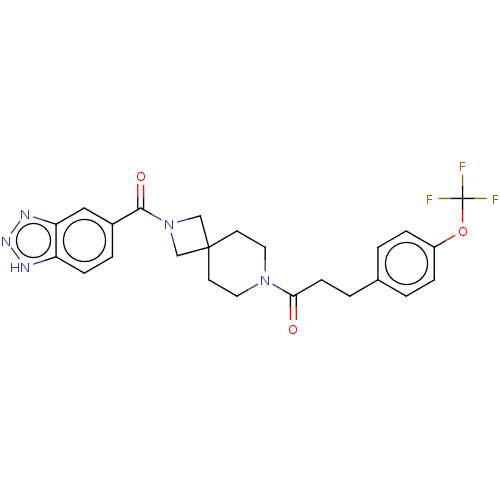 (US10633384, Example 1.24 | US9493486, 1.24) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258502 (US10633384, Example 1.24 | US9493486, 1.24) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epithelial discoidin domain-containing receptor 1 (Homo sapiens (Human)) | BDBM372088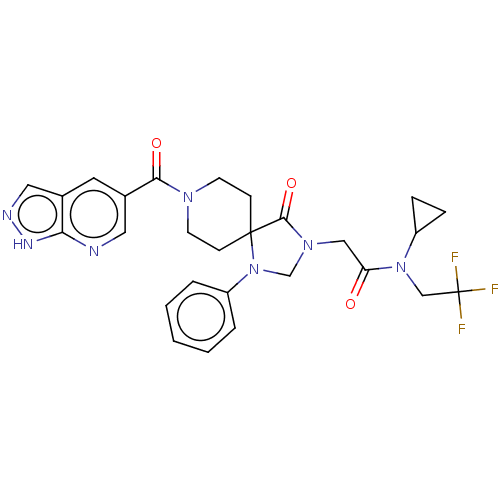 (N-Cyclopropyl-2-[4-oxo-1-phenyl-8-(1H-pyrazolo[3,4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | US Patent US10435407 (2019) BindingDB Entry DOI: 10.7270/Q2MP55M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM372088 (N-Cyclopropyl-2-[4-oxo-1-phenyl-8-(1H-pyrazolo[3,4...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epithelial discoidin domain-containing receptor 1 (Homo sapiens (Human)) | BDBM371821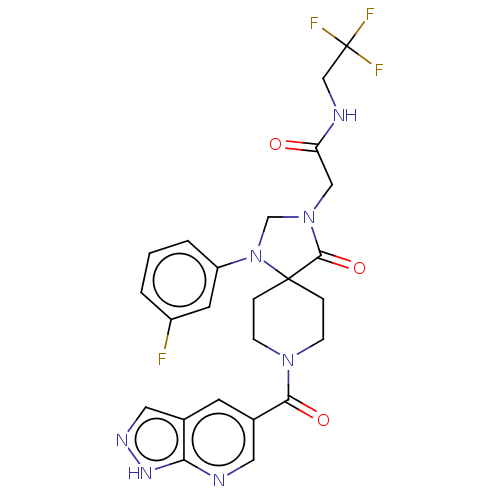 (2-[1-(3-Fluoro-phenyl)-4-oxo-8-(1H-pyrazolo[3,4-b]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | US Patent US10435407 (2019) BindingDB Entry DOI: 10.7270/Q2MP55M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM371821 (2-[1-(3-Fluoro-phenyl)-4-oxo-8-(1H-pyrazolo[3,4-b]...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM372102 (2-(1-(3-Chlorophenyl)-4-oxo-8-(1H-pyrazolo[3,4-b]p...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epithelial discoidin domain-containing receptor 1 (Homo sapiens (Human)) | BDBM372102 (2-(1-(3-Chlorophenyl)-4-oxo-8-(1H-pyrazolo[3,4-b]p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | US Patent US10435407 (2019) BindingDB Entry DOI: 10.7270/Q2MP55M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1492 total ) | Next | Last >> |