

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073270 (CHEMBL333781 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073266 (CHEMBL119898 | {2-[(1S,3S,4S)-1-Benzyl-4-((2S,3S)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073253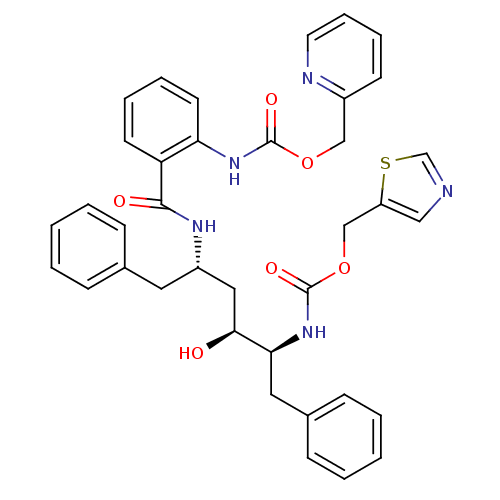 (CHEMBL278935 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073250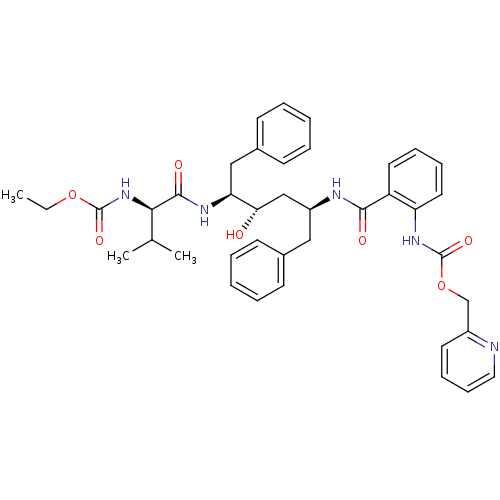 (CHEMBL333420 | {2-[(1S,3S,4S)-1-Benzyl-4-((R)-2-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073269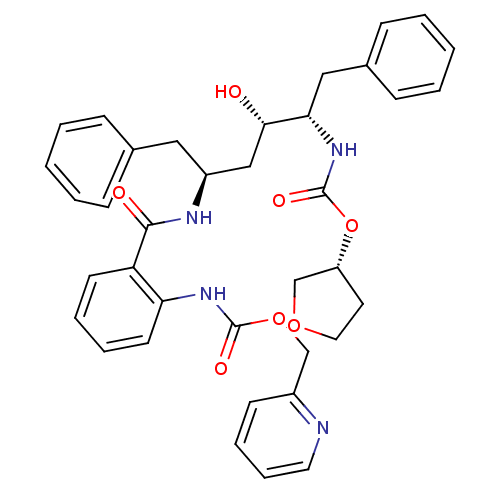 ((2-{(1S,3S,4S)-1-Benzyl-3-hydroxy-5-phenyl-4-[(R)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073252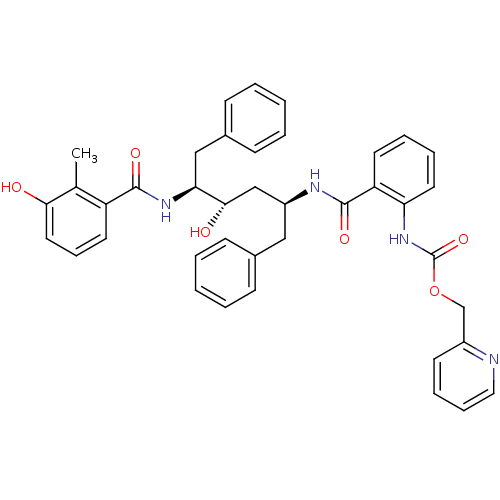 (CHEMBL324157 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073265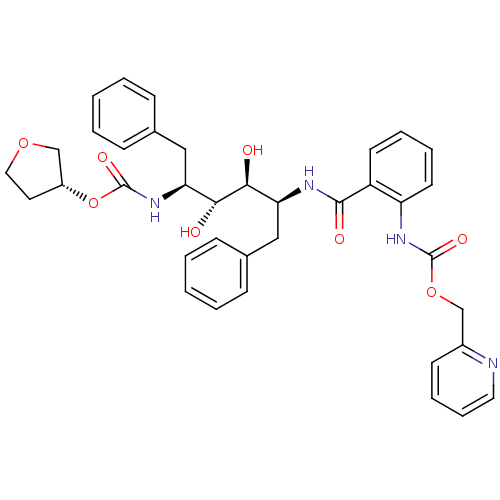 ((2-{(1S,2S,3R,4S)-1-Benzyl-2,3-dihydroxy-5-phenyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073254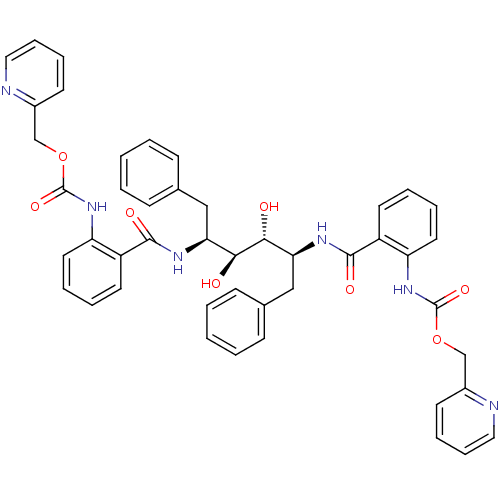 (Anthranilamide derivative | CHEMBL408110) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073268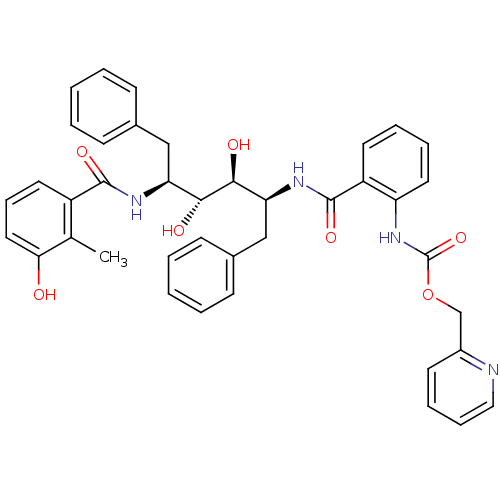 (CHEMBL331294 | {2-[(1S,2S,3R,4S)-1-Benzyl-2,3-dihy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073263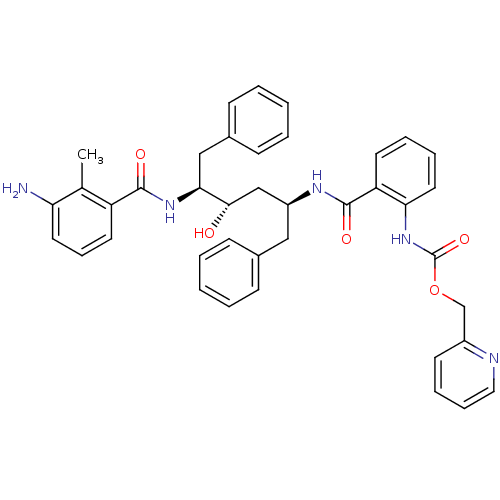 (CHEMBL117629 | {2-[(1S,3S,4S)-4-(3-Amino-2-methyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073244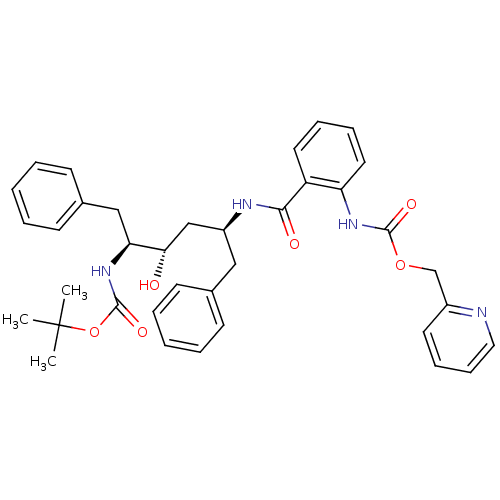 (CHEMBL332825 | [2-((1S,3S,4S)-1-Benzyl-4-tert-buto...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073262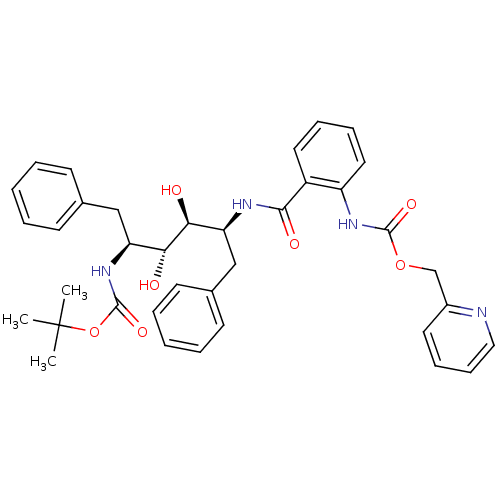 (CHEMBL333290 | [2-((1S,2S,3R,4S)-1-Benzyl-4-tert-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073248 (CHEMBL119745 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073243 ((2-{(1S,2S,3R,4S)-1-Benzyl-2,3-dihydroxy-4-[(isoxa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073249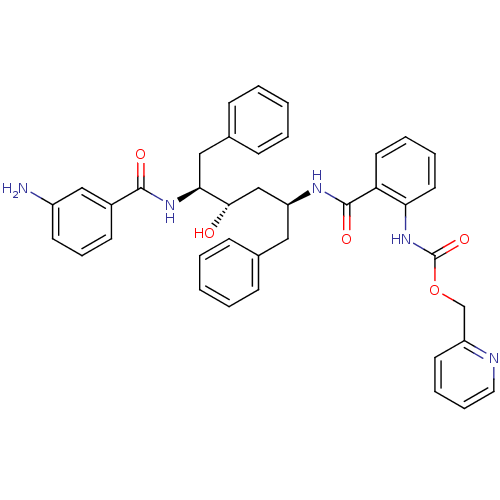 (CHEMBL331199 | {2-[(1S,3S,4S)-4-(3-Amino-benzoylam...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073259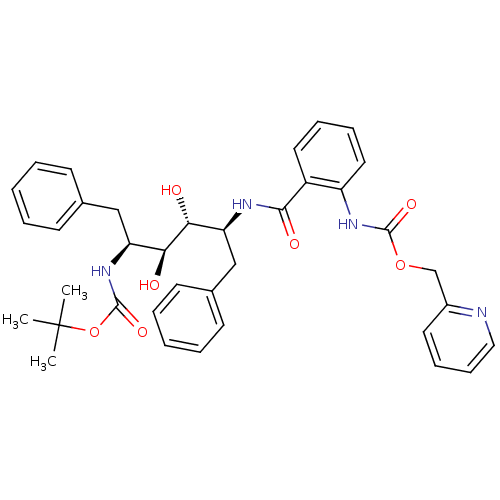 (CHEMBL333407 | [2-((1S,2R,3S,4S)-1-Benzyl-4-tert-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073261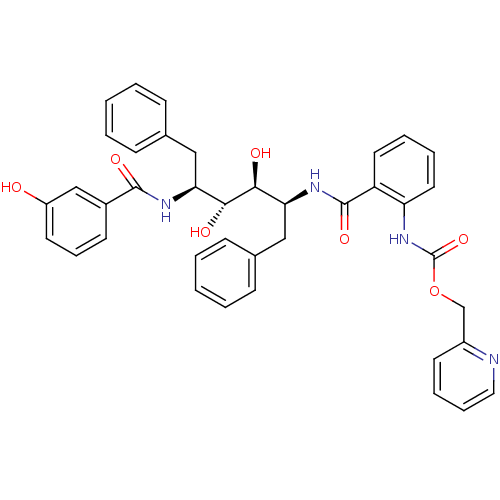 (CHEMBL116482 | {2-[(1S,2S,3R,4S)-1-Benzyl-2,3-dihy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073251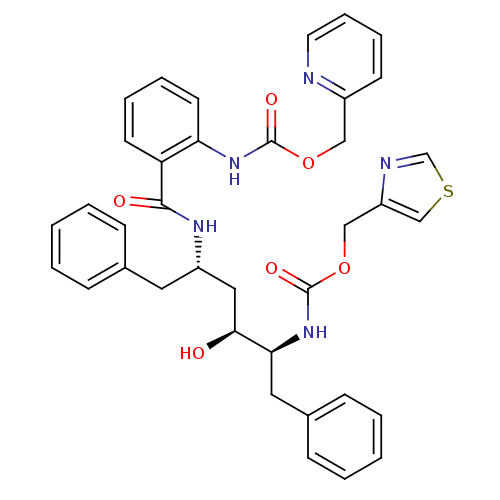 (CHEMBL118396 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073247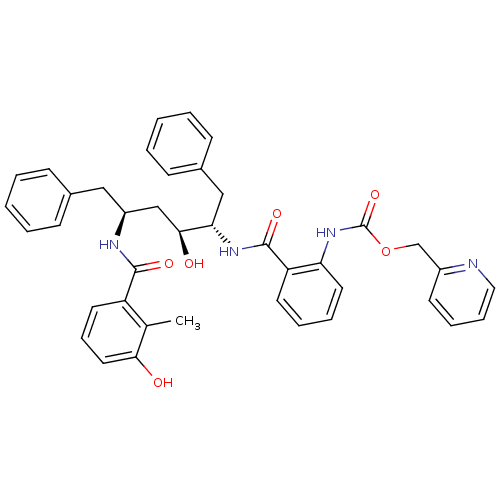 (CHEMBL118513 | {2-[(1S,2S,4S)-1-Benzyl-2-hydroxy-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073246 (CHEMBL333392 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073256 (CHEMBL122006 | [2-((1S,2S,4S)-1-Benzyl-4-tert-buto...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073267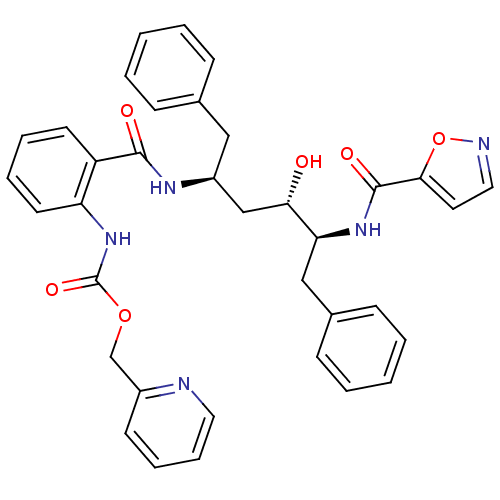 ((2-{(1S,3S,4S)-1-Benzyl-3-hydroxy-4-[(isoxazole-5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073264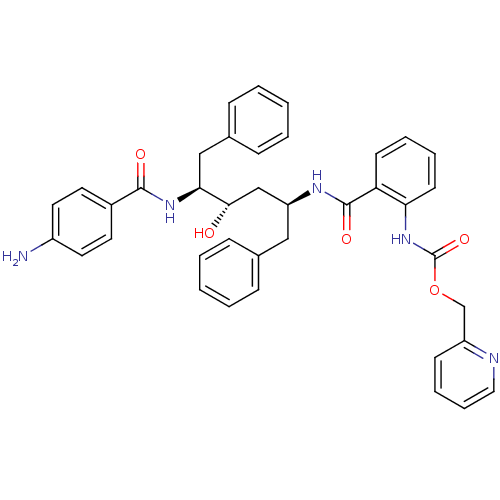 (CHEMBL118531 | {2-[(1S,3S,4S)-4-(4-Amino-benzoylam...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073257 (CHEMBL325499 | {2-[(1S,2R,3S,4S)-1-Benzyl-2,3-dihy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073255 ((2-{(1S,3S,4S)-1-Benzyl-3-hydroxy-5-phenyl-4-[(thi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073258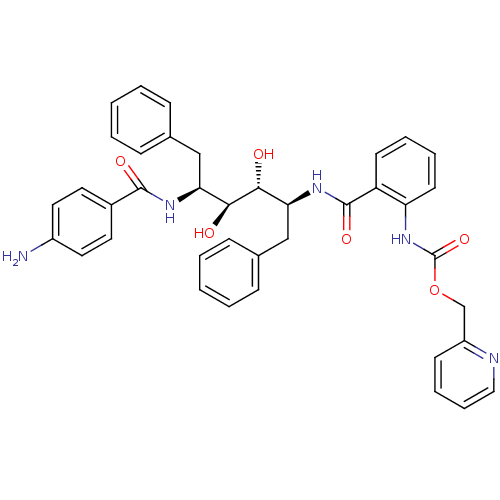 (CHEMBL118221 | {2-[(1S,2R,3S,4S)-4-(4-Amino-benzoy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073245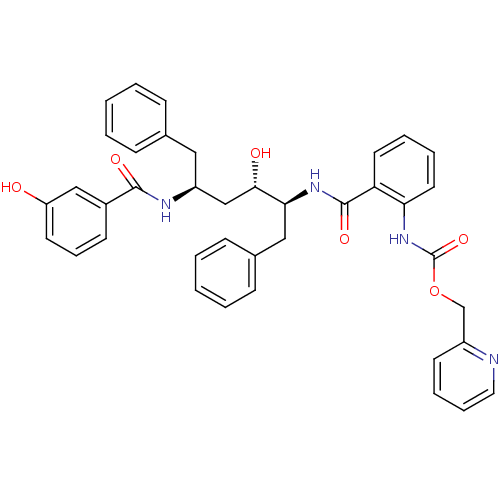 (CHEMBL118362 | {2-[(1S,2S,4S)-1-Benzyl-2-hydroxy-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50471917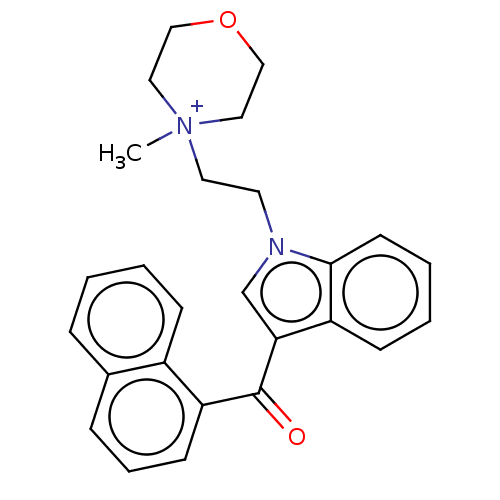 (CHEMBL105602) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from Cannabinoid receptor 1 of rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032571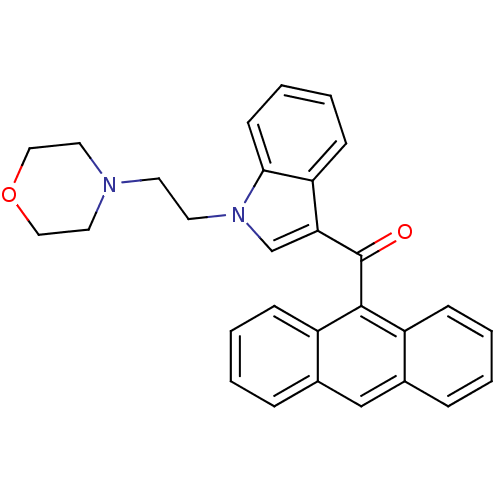 (Anthracen-9-yl-[1-(2-morpholin-4-yl-ethyl)-1H-indo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Cannabinoid receptor 1 binding affinity by measuring its ability to displace [3H]WIN-55212-2 in rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032617 (CHEMBL310539 | [2,6-Dimethyl-1-(2-morpholin-4-yl-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.76E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Cannabinoid receptor 1 binding affinity by measuring its ability to displace [3H]WIN-55212-2 in rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032563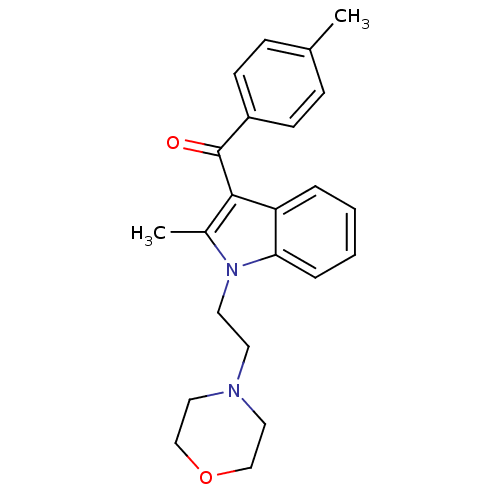 (CHEMBL275242 | [2-Methyl-1-(2-morpholin-4-yl-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Cannabinoid receptor 1 binding affinity by measuring its ability to displace [3H]WIN-55212-2 in rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032591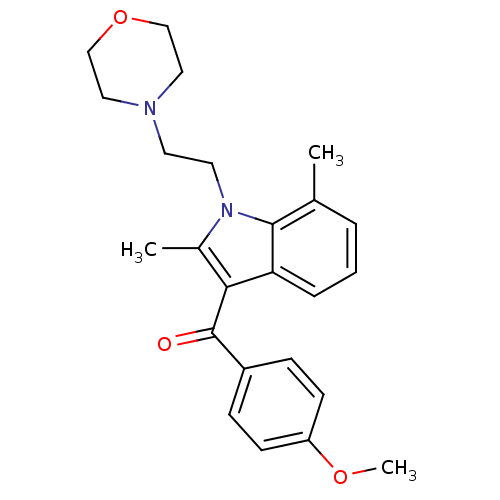 (CHEMBL310538 | [2,7-Dimethyl-1-(2-morpholin-4-yl-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Cannabinoid receptor 1 binding affinity by measuring its ability to displace [3H]WIN-55212-2 in rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032541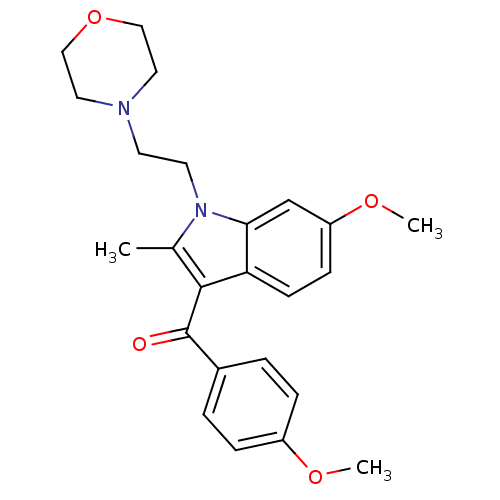 (CHEMBL310751 | [6-Methoxy-2-methyl-1-(2-morpholin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.71E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Cannabinoid receptor 1 binding affinity by measuring its ability to displace [3H]WIN-55212-2 in rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032614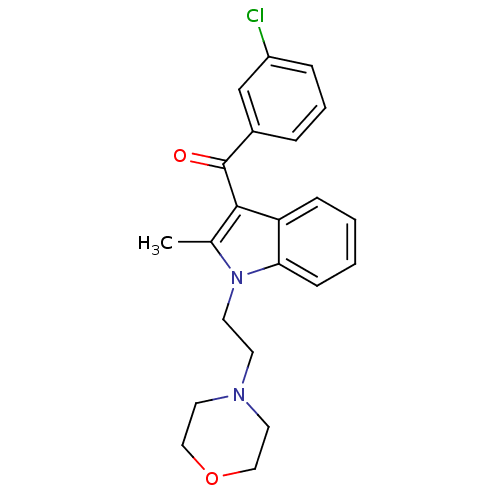 ((3-Chloro-phenyl)-[2-methyl-1-(2-morpholin-4-yl-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.55E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Cannabinoid receptor 1 binding affinity by measuring its ability to displace [3H]WIN-55212-2 in rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50471922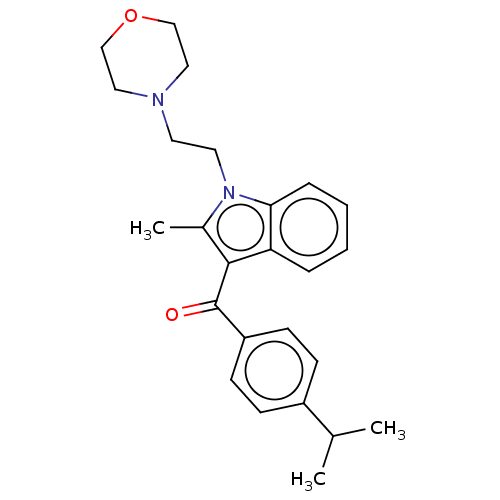 (CHEMBL441060) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.12E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Cannabinoid receptor 1 binding affinity by measuring its ability to displace [3H]WIN-55212-2 in rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50368231 (CHEMBL337134) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.12E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from Cannabinoid receptor 1 of rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032566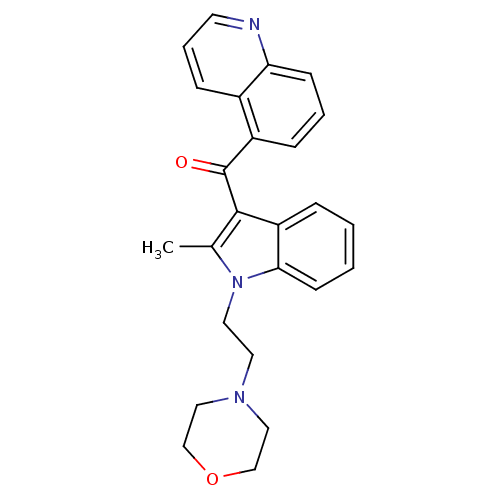 (CHEMBL78710 | [2-Methyl-1-(2-morpholin-4-yl-ethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.51E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Cannabinoid receptor 1 binding affinity by measuring its ability to displace [3H]WIN-55212-2 in rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032576 ((2-Methoxy-phenyl)-[2-methyl-1-(2-morpholin-4-yl-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.58E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Cannabinoid receptor 1 binding affinity by measuring its ability to displace [3H]WIN-55212-2 in rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50213819 (CHEMBL421497) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.82E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Cannabinoid receptor 1 binding affinity by measuring its ability to displace [3H]WIN-55212-2 in rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032572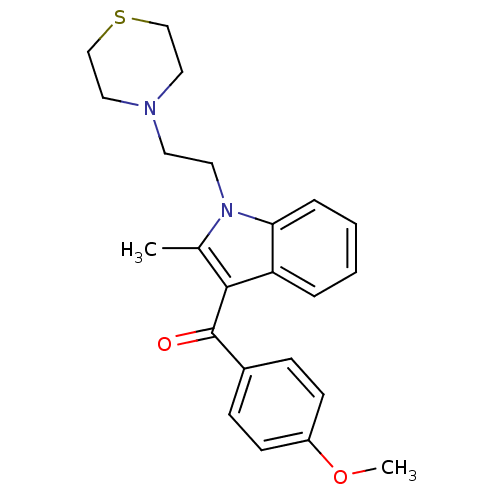 ((4-Methoxy-phenyl)-[2-methyl-1-(2-thiomorpholin-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.04E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Cannabinoid receptor 1 binding affinity by measuring its ability to displace [3H]WIN-55212-2 in rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032553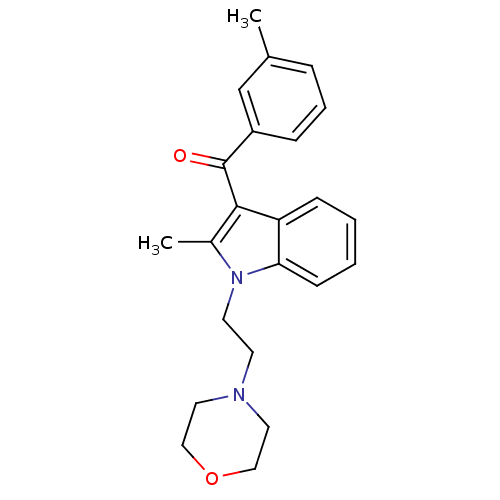 (CHEMBL12914 | [2-Methyl-1-(2-morpholin-4-yl-ethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.09E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Cannabinoid receptor 1 binding affinity by measuring its ability to displace [3H]WIN-55212-2 in rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50009862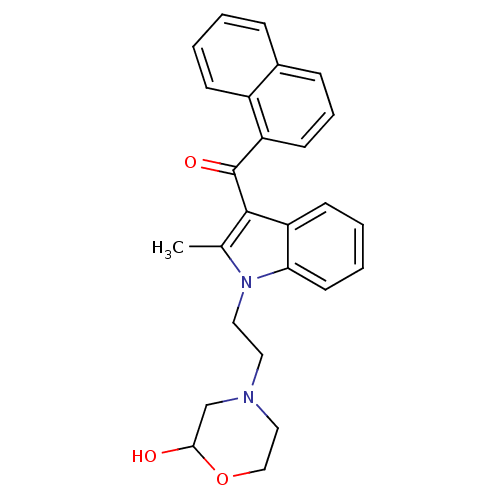 (CHEMBL12566 | {1-[2-(2-Hydroxy-morpholin-4-yl)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.24E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from Cannabinoid receptor 1 of rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032546 (CHEMBL78701 | [7-Fluoro-2-methyl-1-(2-morpholin-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.24E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Cannabinoid receptor 1 binding affinity by measuring its ability to displace [3H]WIN-55212-2 in rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032586 (CHEMBL310741 | [6-Bromo-2-methyl-1-(2-morpholin-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.45E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Cannabinoid receptor 1 binding affinity by measuring its ability to displace [3H]WIN-55212-2 in rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032619 (CHEMBL309700 | [2-Methyl-1-(2-morpholin-4-yl-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.51E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Cannabinoid receptor 1 binding affinity by measuring its ability to displace [3H]WIN-55212-2 in rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50471921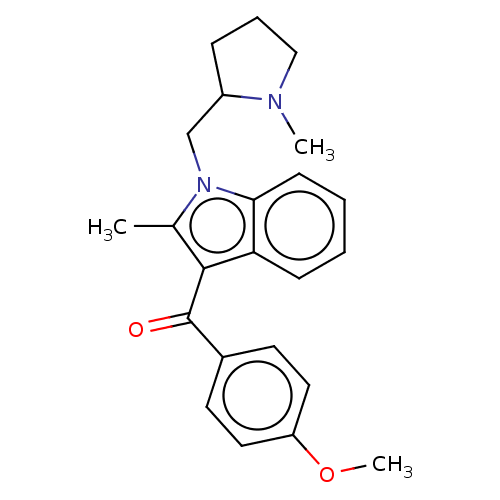 (CHEMBL302991) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.57E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Cannabinoid receptor 1 binding affinity by measuring its ability to displace [3H]WIN-55212-2 in rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032618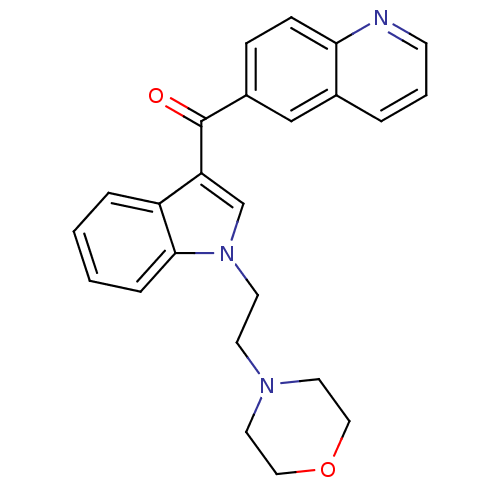 (CHEMBL78992 | [1-(2-Morpholin-4-yl-ethyl)-1H-indol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.57E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Cannabinoid receptor 1 binding affinity by measuring its ability to displace [3H]WIN-55212-2 in rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50009868 ((4-Methoxy-phenyl)-[1-(2-morpholin-4-yl-ethyl)-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.82E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from Cannabinoid receptor 1 of rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032551 (Benzofuran-2-yl-[2-methyl-1-(2-morpholin-4-yl-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.02E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Cannabinoid receptor 1 binding affinity by measuring its ability to displace [3H]WIN-55212-2 in rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50009868 ((4-Methoxy-phenyl)-[1-(2-morpholin-4-yl-ethyl)-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.24E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Cannabinoid receptor 1 binding affinity by measuring its ability to displace [3H]WIN-55212-2 in rat forebrain membranes | J Med Chem 41: 4521-32 (1998) Article DOI: 10.1021/jm980305c BindingDB Entry DOI: 10.7270/Q2XD14F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 271 total ) | Next | Last >> |