

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8987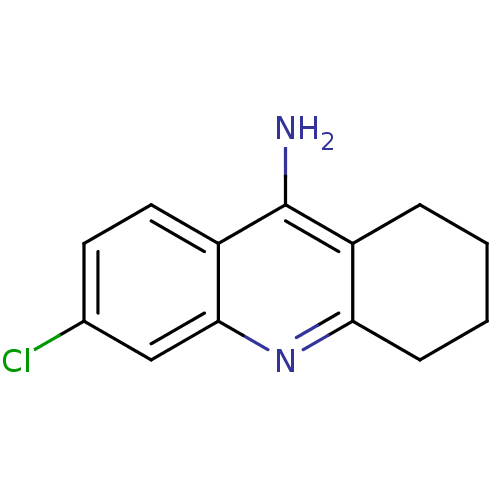 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.73 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31895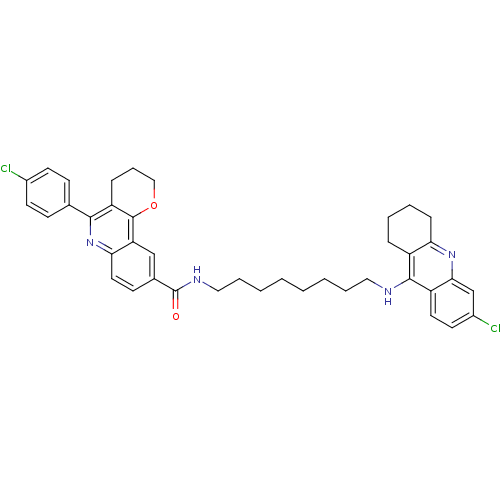 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 20....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.03 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8987 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.32 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31899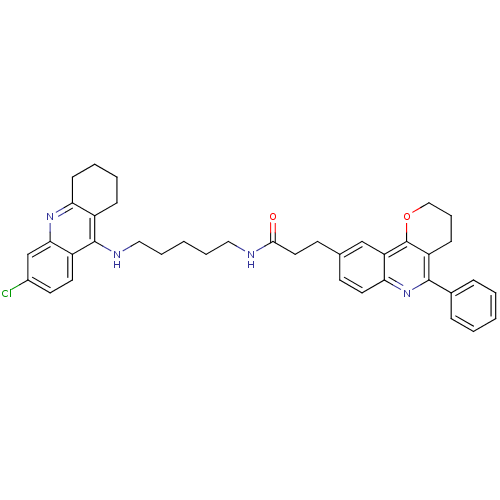 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 24....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.64 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of BuChE in horse serum using butyrylthiocholine as substrate by Ellman method | Eur J Med Chem 46: 2224-35 (2011) Article DOI: 10.1016/j.ejmech.2011.03.003 BindingDB Entry DOI: 10.7270/Q2X34XWC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by UV spectroscopic analysis | Eur J Med Chem 81: 350-8 (2014) Article DOI: 10.1016/j.ejmech.2014.04.075 BindingDB Entry DOI: 10.7270/Q23F4R6B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) Curated by ChEMBL | Assay Description Inhibition of BuChE in horse serum by Ellman method | J Med Chem 52: 7249-57 (2009) Article DOI: 10.1021/jm900628z BindingDB Entry DOI: 10.7270/Q2668DB1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrat... | Eur J Med Chem 130: 60-72 (2017) Article DOI: 10.1016/j.ejmech.2017.02.034 BindingDB Entry DOI: 10.7270/Q2JD5086 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 45: 6152-8 (2010) Article DOI: 10.1016/j.ejmech.2010.09.039 BindingDB Entry DOI: 10.7270/Q2KW5G84 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM31894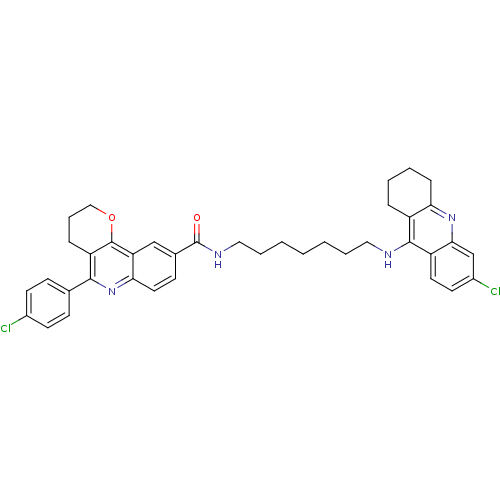 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 19....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM31895 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 20....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31900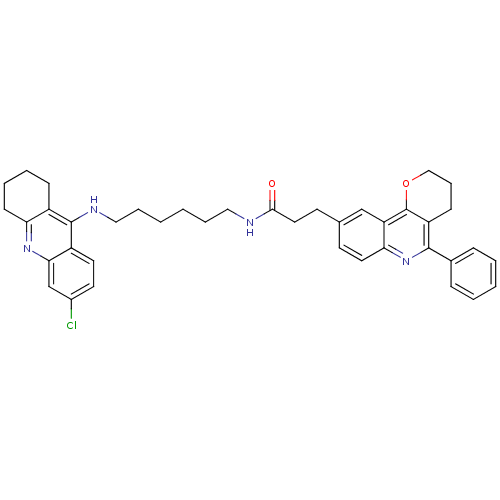 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 25....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.1 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31902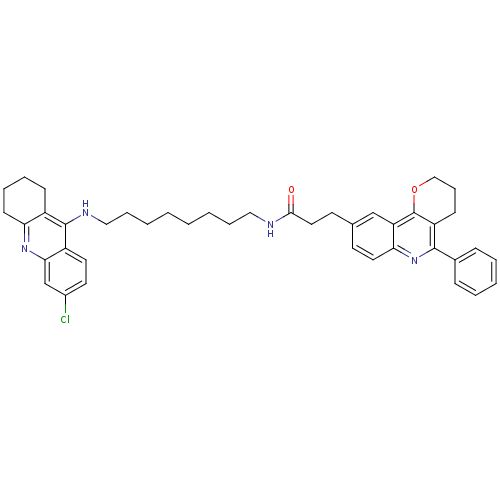 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 27....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31901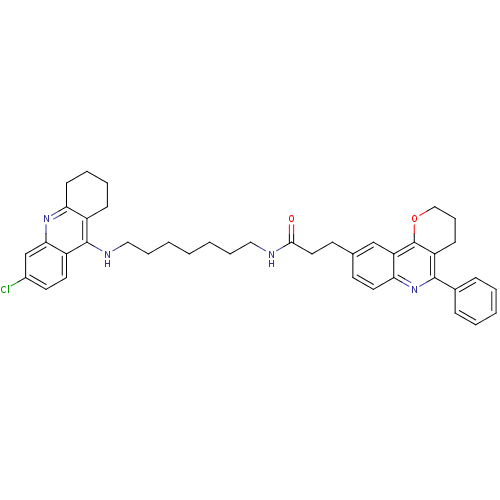 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 26....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31898 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 23....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16.6 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31894 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 19....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18.3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31893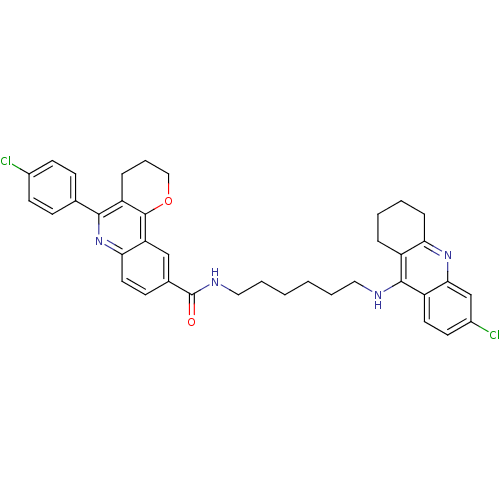 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 18....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19.2 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM31893 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 18....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20.4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM31899 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 24....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23.9 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM31896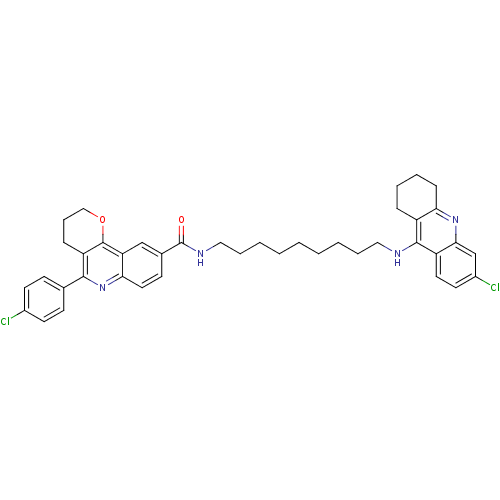 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 21....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31896 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 21....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24.9 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM31901 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 26....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29.9 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM31900 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 25....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30.8 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM31902 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 27....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34.1 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocyte by Ellman method | J Med Chem 52: 7249-57 (2009) Article DOI: 10.1021/jm900628z BindingDB Entry DOI: 10.7270/Q2668DB1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine as substrate by Ellman method | Eur J Med Chem 46: 2224-35 (2011) Article DOI: 10.1016/j.ejmech.2011.03.003 BindingDB Entry DOI: 10.7270/Q2X34XWC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's method | Eur J Med Chem 45: 6152-8 (2010) Article DOI: 10.1016/j.ejmech.2010.09.039 BindingDB Entry DOI: 10.7270/Q2KW5G84 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) Curated by ChEMBL | Assay Description Inhibition of human BuChE in serum | J Med Chem 52: 7249-57 (2009) Article DOI: 10.1021/jm900628z BindingDB Entry DOI: 10.7270/Q2668DB1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM31898 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 23....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48.1 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31897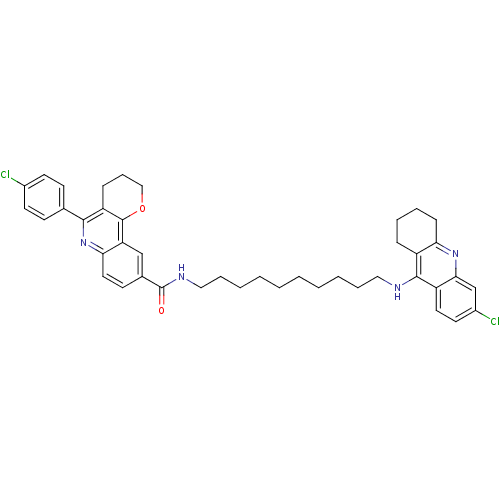 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 22....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50311996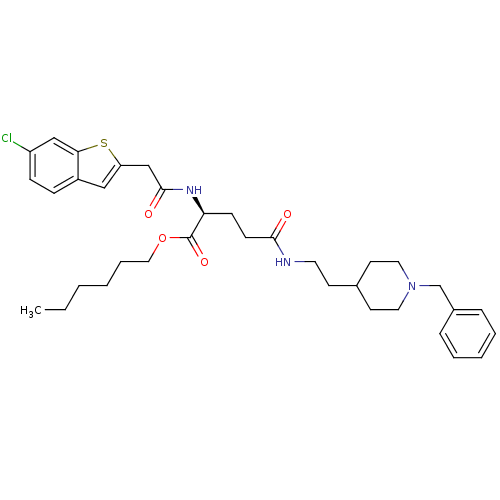 ((S)-hexyl 5-(2-(1-benzylpiperidin-4-yl)ethylamino)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) Curated by ChEMBL | Assay Description Inhibition of BuChE in horse serum by Ellman method | J Med Chem 52: 7249-57 (2009) Article DOI: 10.1021/jm900628z BindingDB Entry DOI: 10.7270/Q2668DB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50311996 ((S)-hexyl 5-(2-(1-benzylpiperidin-4-yl)ethylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) Curated by ChEMBL | Assay Description Inhibition of human BuChE in serum | J Med Chem 52: 7249-57 (2009) Article DOI: 10.1021/jm900628z BindingDB Entry DOI: 10.7270/Q2668DB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM31897 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 22....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93.7 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50311996 ((S)-hexyl 5-(2-(1-benzylpiperidin-4-yl)ethylamino)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) Curated by ChEMBL | Assay Description Inhibition of human AChE in erythrocyte | J Med Chem 52: 7249-57 (2009) Article DOI: 10.1021/jm900628z BindingDB Entry DOI: 10.7270/Q2668DB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50264655 (CHEMBL4102571) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 mins... | Eur J Med Chem 130: 60-72 (2017) Article DOI: 10.1016/j.ejmech.2017.02.034 BindingDB Entry DOI: 10.7270/Q2JD5086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50311999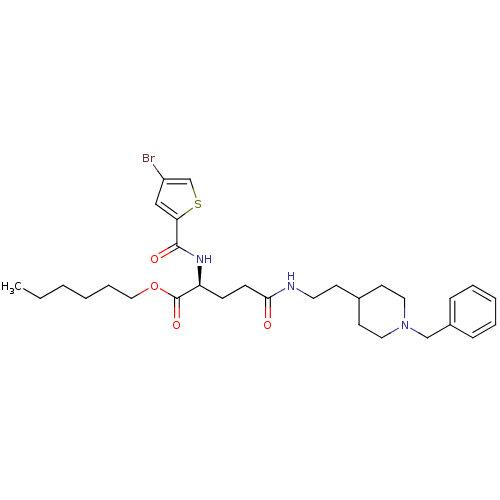 ((S)-hexyl 5-(2-(1-benzylpiperidin-4-yl)ethylamino)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) Curated by ChEMBL | Assay Description Inhibition of human AChE in erythrocyte | J Med Chem 52: 7249-57 (2009) Article DOI: 10.1021/jm900628z BindingDB Entry DOI: 10.7270/Q2668DB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM31900 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 25....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 218 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona | Assay Description BChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using BChE from human serum and butyrylthiocholine as substrat... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM31901 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 26....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 234 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona | Assay Description BChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using BChE from human serum and butyrylthiocholine as substrat... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50311995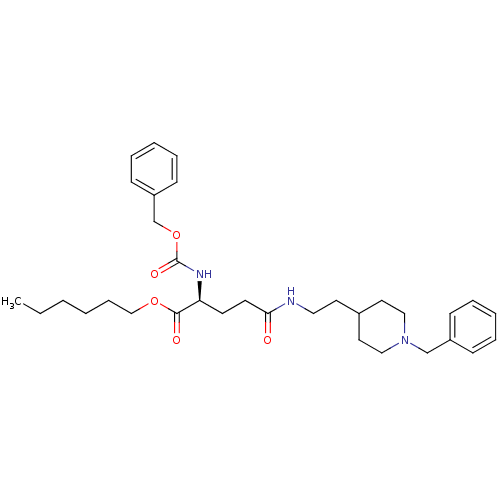 ((S)-hexyl 2-(benzyloxycarbonylamino)-5-(2-(1-benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) Curated by ChEMBL | Assay Description Inhibition of human AChE in erythrocyte | J Med Chem 52: 7249-57 (2009) Article DOI: 10.1021/jm900628z BindingDB Entry DOI: 10.7270/Q2668DB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50312000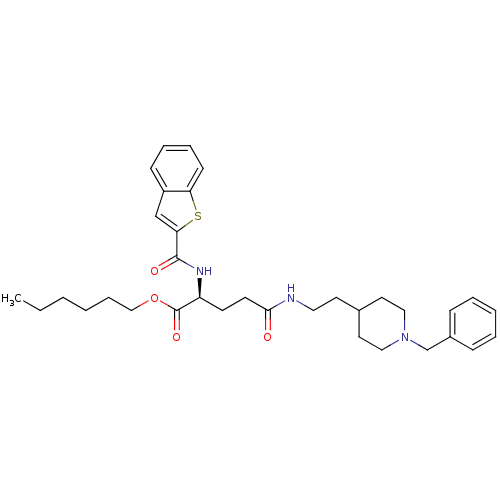 ((S)-hexyl 2-(benzo[b]thiophene-2-carboxamido)-5-(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) Curated by ChEMBL | Assay Description Inhibition of human AChE in erythrocyte | J Med Chem 52: 7249-57 (2009) Article DOI: 10.1021/jm900628z BindingDB Entry DOI: 10.7270/Q2668DB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM31899 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 24....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona | Assay Description BChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using BChE from human serum and butyrylthiocholine as substrat... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50264683 (CHEMBL4081699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 mins... | Eur J Med Chem 130: 60-72 (2017) Article DOI: 10.1016/j.ejmech.2017.02.034 BindingDB Entry DOI: 10.7270/Q2JD5086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50312002 ((S)-hexyl 5-(2-(1-benzylpiperidin-4-yl)ethylamino)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) Curated by ChEMBL | Assay Description Inhibition of human AChE in erythrocyte | J Med Chem 52: 7249-57 (2009) Article DOI: 10.1021/jm900628z BindingDB Entry DOI: 10.7270/Q2668DB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50312001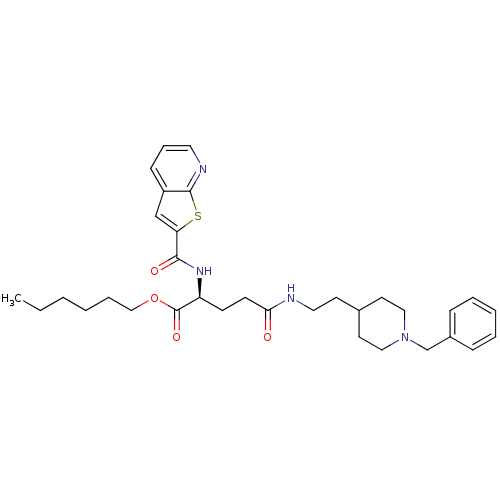 ((S)-hexyl 5-(2-(1-benzylpiperidin-4-yl)ethylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocyte by Ellman method | J Med Chem 52: 7249-57 (2009) Article DOI: 10.1021/jm900628z BindingDB Entry DOI: 10.7270/Q2668DB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50312000 ((S)-hexyl 2-(benzo[b]thiophene-2-carboxamido)-5-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) Curated by ChEMBL | Assay Description Inhibition of human BuChE in serum | J Med Chem 52: 7249-57 (2009) Article DOI: 10.1021/jm900628z BindingDB Entry DOI: 10.7270/Q2668DB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM31895 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 20....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 331 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description BChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using BChE from human serum and butyrylthiocholine as substrat... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) Curated by ChEMBL | Assay Description Inhibition of human AChE in erythrocyte | J Med Chem 52: 7249-57 (2009) Article DOI: 10.1021/jm900628z BindingDB Entry DOI: 10.7270/Q2668DB1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50312002 ((S)-hexyl 5-(2-(1-benzylpiperidin-4-yl)ethylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) Curated by ChEMBL | Assay Description Inhibition of human BuChE in serum | J Med Chem 52: 7249-57 (2009) Article DOI: 10.1021/jm900628z BindingDB Entry DOI: 10.7270/Q2668DB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50264652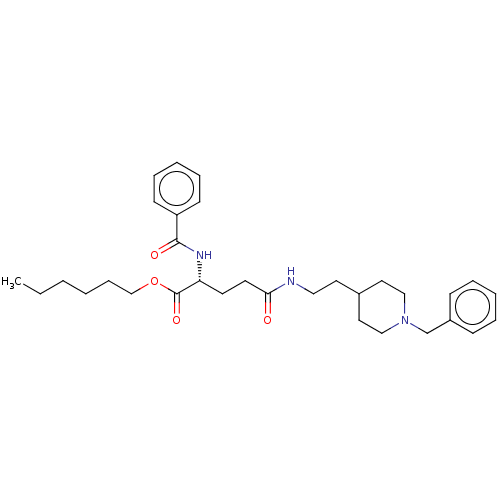 (CHEMBL4079599) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 mins... | Eur J Med Chem 130: 60-72 (2017) Article DOI: 10.1016/j.ejmech.2017.02.034 BindingDB Entry DOI: 10.7270/Q2JD5086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50264682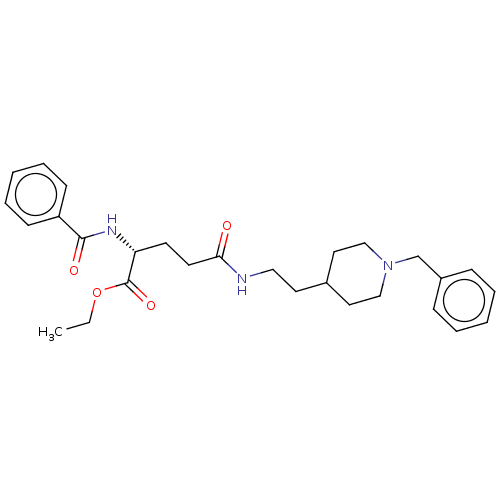 (CHEMBL4059766) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 mins... | Eur J Med Chem 130: 60-72 (2017) Article DOI: 10.1016/j.ejmech.2017.02.034 BindingDB Entry DOI: 10.7270/Q2JD5086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 174 total ) | Next | Last >> |