

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM66082 ((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, UniversitÓ di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it. Curated by ChEMBL | Assay Description Inhibition of DHFR (unknown origin) | Eur J Med Chem 135: 467-478 (2017) Article DOI: 10.1016/j.ejmech.2017.04.070 BindingDB Entry DOI: 10.7270/Q2057JD0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50161776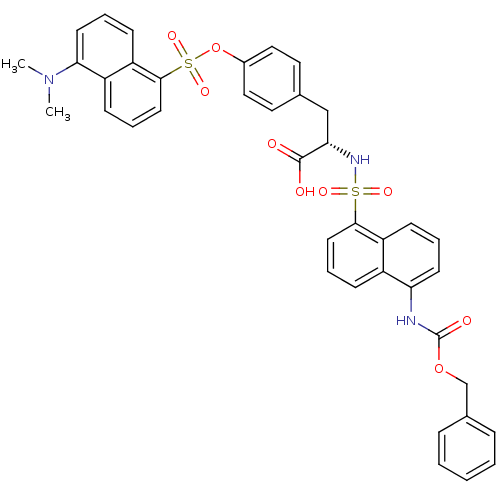 ((S)-2-(5-Benzyloxycarbonylamino-naphthalene-1-sulf...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibitory constant against thymidylate synthase from Lactobacillus casei | J Med Chem 48: 913-6 (2005) Article DOI: 10.1021/jm0491445 BindingDB Entry DOI: 10.7270/Q20R9NXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50161777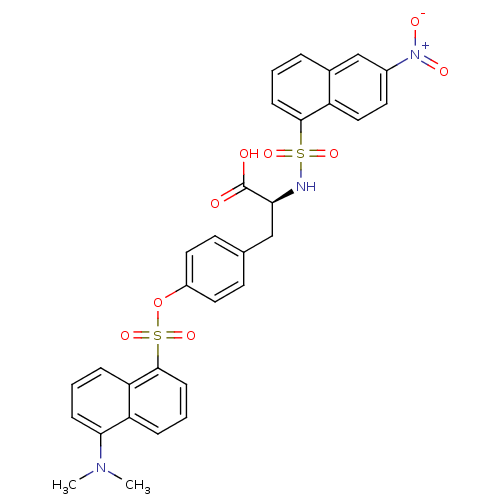 ((S)-3-[4-(5-Dimethylamino-naphthalene-1-sulfonylox...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibitory constant against thymidylate synthase from Escherichia coli | J Med Chem 48: 913-6 (2005) Article DOI: 10.1021/jm0491445 BindingDB Entry DOI: 10.7270/Q20R9NXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50161775 ((S)-2-(6-Amino-naphthalene-1-sulfonylamino)-3-[4-(...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibitory constant against thymidylate synthase from Escherichia coli | J Med Chem 48: 913-6 (2005) Article DOI: 10.1021/jm0491445 BindingDB Entry DOI: 10.7270/Q20R9NXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50161777 ((S)-3-[4-(5-Dimethylamino-naphthalene-1-sulfonylox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibitory constant against thymidylate synthase from Lactobacillus casei | J Med Chem 48: 913-6 (2005) Article DOI: 10.1021/jm0491445 BindingDB Entry DOI: 10.7270/Q20R9NXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50161775 ((S)-2-(6-Amino-naphthalene-1-sulfonylamino)-3-[4-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibitory constant against thymidylate synthase from Lactobacillus casei | J Med Chem 48: 913-6 (2005) Article DOI: 10.1021/jm0491445 BindingDB Entry DOI: 10.7270/Q20R9NXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM18796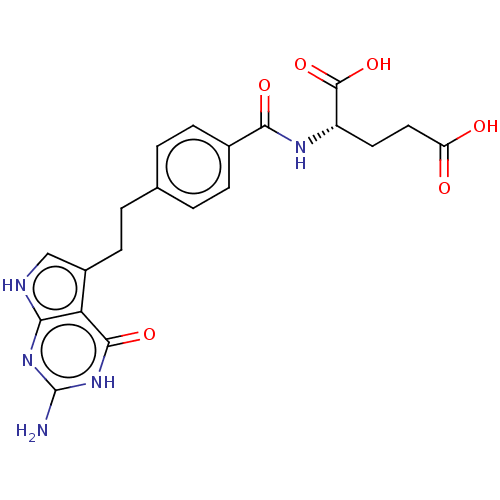 ((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Theoretical Studies gGmbH Curated by ChEMBL | Assay Description Inhibition of thymidin synthase | J Med Chem 53: 6539-49 (2010) Article DOI: 10.1021/jm901869w BindingDB Entry DOI: 10.7270/Q24J0F9S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50161780 ((S)-2-(4-Benzyloxycarbonylamino-naphthalene-1-sulf...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibitory constant against thymidylate synthase from Escherichia coli | J Med Chem 48: 913-6 (2005) Article DOI: 10.1021/jm0491445 BindingDB Entry DOI: 10.7270/Q20R9NXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50161778 ((S)-3-[4-(5-Dimethylamino-naphthalene-1-sulfonylox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibitory constant against human thymidylate synthase | J Med Chem 48: 913-6 (2005) Article DOI: 10.1021/jm0491445 BindingDB Entry DOI: 10.7270/Q20R9NXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50161778 ((S)-3-[4-(5-Dimethylamino-naphthalene-1-sulfonylox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibitory constant against thymidylate synthase from Lactobacillus casei | J Med Chem 48: 913-6 (2005) Article DOI: 10.1021/jm0491445 BindingDB Entry DOI: 10.7270/Q20R9NXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50161780 ((S)-2-(4-Benzyloxycarbonylamino-naphthalene-1-sulf...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibitory constant against thymidylate synthase from Lactobacillus casei | J Med Chem 48: 913-6 (2005) Article DOI: 10.1021/jm0491445 BindingDB Entry DOI: 10.7270/Q20R9NXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50161777 ((S)-3-[4-(5-Dimethylamino-naphthalene-1-sulfonylox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibitory constant against human thymidylate synthase | J Med Chem 48: 913-6 (2005) Article DOI: 10.1021/jm0491445 BindingDB Entry DOI: 10.7270/Q20R9NXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18796 ((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Theoretical Studies gGmbH Curated by ChEMBL | Assay Description Inhibition of DHFR | J Med Chem 53: 6539-49 (2010) Article DOI: 10.1021/jm901869w BindingDB Entry DOI: 10.7270/Q24J0F9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50161775 ((S)-2-(6-Amino-naphthalene-1-sulfonylamino)-3-[4-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibitory constant against human thymidylate synthase | J Med Chem 48: 913-6 (2005) Article DOI: 10.1021/jm0491445 BindingDB Entry DOI: 10.7270/Q20R9NXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50161776 ((S)-2-(5-Benzyloxycarbonylamino-naphthalene-1-sulf...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibitory constant against thymidylate synthase from Escherichia coli | J Med Chem 48: 913-6 (2005) Article DOI: 10.1021/jm0491445 BindingDB Entry DOI: 10.7270/Q20R9NXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50225373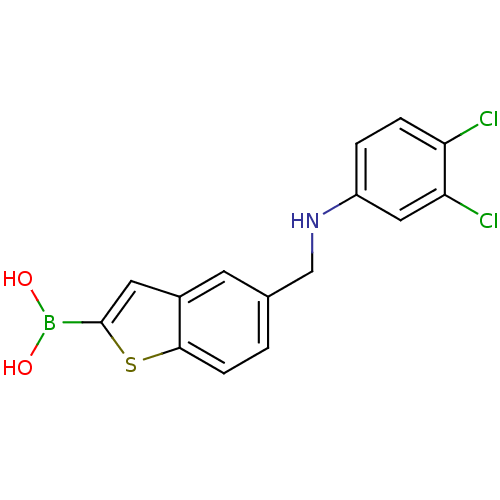 (CHEMBL396872 | pinacol 5-[(3,4-dichlorophenylamino...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-lactamase | J Med Chem 50: 5644-54 (2007) Article DOI: 10.1021/jm070643q BindingDB Entry DOI: 10.7270/Q21N80W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50433811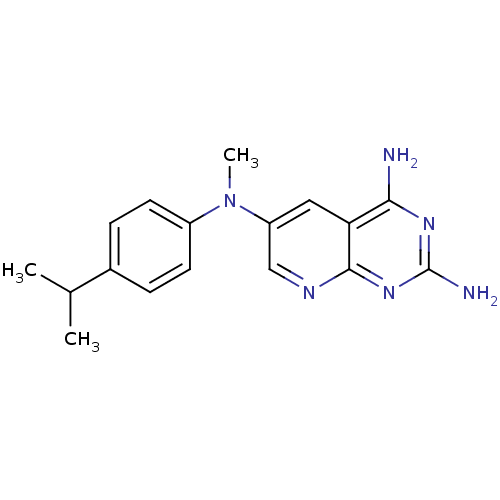 (CHEMBL2382332) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, UniversitÓ di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it. Curated by ChEMBL | Assay Description Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysis | Eur J Med Chem 135: 467-478 (2017) Article DOI: 10.1016/j.ejmech.2017.04.070 BindingDB Entry DOI: 10.7270/Q2057JD0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50161780 ((S)-2-(4-Benzyloxycarbonylamino-naphthalene-1-sulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibitory constant against human thymidylate synthase | J Med Chem 48: 913-6 (2005) Article DOI: 10.1021/jm0491445 BindingDB Entry DOI: 10.7270/Q20R9NXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50161776 ((S)-2-(5-Benzyloxycarbonylamino-naphthalene-1-sulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibitory constant against human thymidylate synthase | J Med Chem 48: 913-6 (2005) Article DOI: 10.1021/jm0491445 BindingDB Entry DOI: 10.7270/Q20R9NXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50022238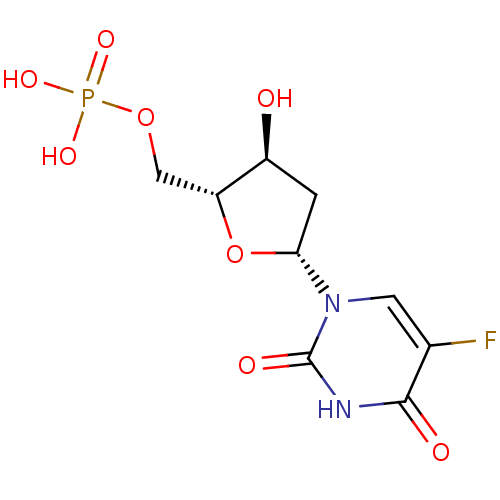 ((R)-5-Fluoro-1-((4S,5R)-4-hydroxy-5-methylphosphat...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Perugia Curated by ChEMBL | Assay Description Inhibition of human thymidylate synthase expressed in Escherichia coli incubated for 1 hr by spectrophotometry | J Med Chem 55: 10272-6 (2012) Article DOI: 10.1021/jm300850v BindingDB Entry DOI: 10.7270/Q2P2708R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50161778 ((S)-3-[4-(5-Dimethylamino-naphthalene-1-sulfonylox...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibitory constant against thymidylate synthase from Escherichia coli | J Med Chem 48: 913-6 (2005) Article DOI: 10.1021/jm0491445 BindingDB Entry DOI: 10.7270/Q20R9NXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM26139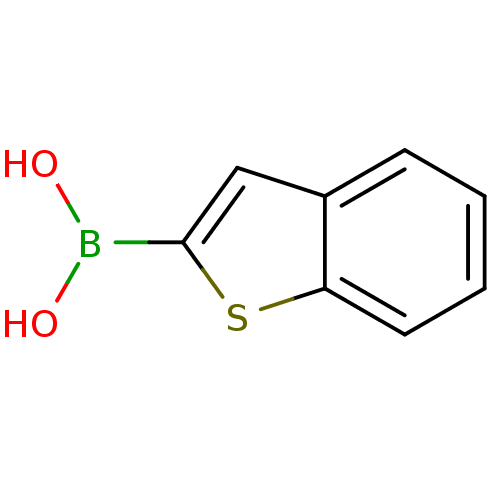 (1-benzothiophen-2-ylboranediol | 1-benzothiophen-2...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-lactamase | J Med Chem 50: 5644-54 (2007) Article DOI: 10.1021/jm070643q BindingDB Entry DOI: 10.7270/Q21N80W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50225393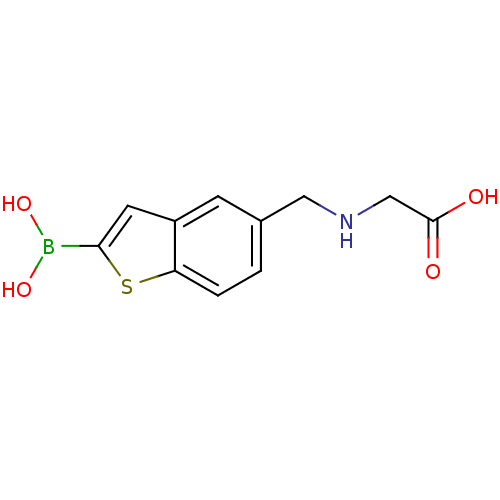 (CHEMBL235526 | pinacol 5-[(carboxymethylamino)meth...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-lactamase | J Med Chem 50: 5644-54 (2007) Article DOI: 10.1021/jm070643q BindingDB Entry DOI: 10.7270/Q21N80W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50225381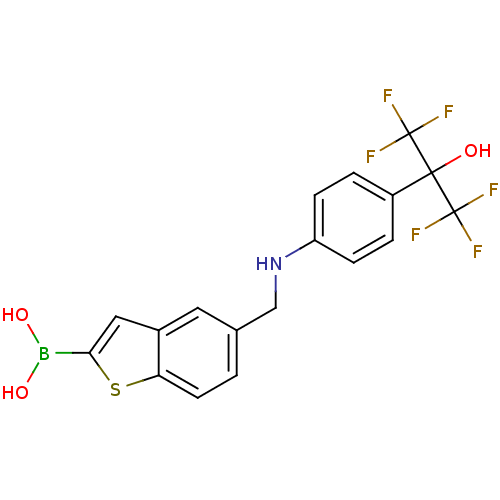 (CHEMBL235293 | pinacol5-{[4-(1-hydroxy-2,2,2-trifl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-lactamase | J Med Chem 50: 5644-54 (2007) Article DOI: 10.1021/jm070643q BindingDB Entry DOI: 10.7270/Q21N80W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50123588 (CHEMBL297258) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Competitive inhibition of human FolD dehydrogenase activity | J Med Chem 58: 7938-48 (2015) Article DOI: 10.1021/acs.jmedchem.5b00687 BindingDB Entry DOI: 10.7270/Q2SN0BSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50225376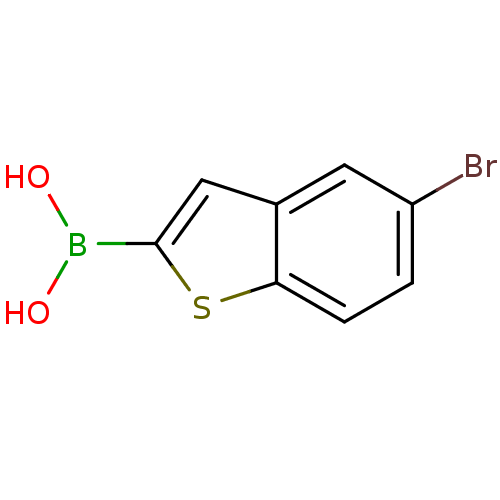 (CHEMBL397522 | pinacol 5-bromomethylbenzo[b]thioph...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-lactamase | J Med Chem 50: 5644-54 (2007) Article DOI: 10.1021/jm070643q BindingDB Entry DOI: 10.7270/Q21N80W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM18771 ((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIMORE | Assay Description TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... | J Med Chem 49: 5958-68 (2006) Article DOI: 10.1021/jm051187d BindingDB Entry DOI: 10.7270/Q2S180R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50225371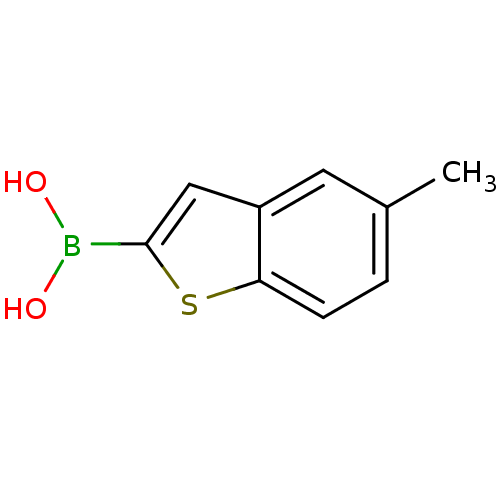 (5-methyl-benzo[b]thiophen-2-ylboronic acid | CHEMB...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-lactamase | J Med Chem 50: 5644-54 (2007) Article DOI: 10.1021/jm070643q BindingDB Entry DOI: 10.7270/Q21N80W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50225379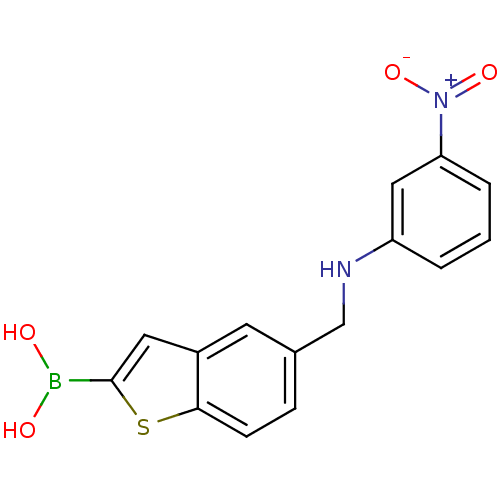 (CHEMBL235292 | pinacol 5-[(3-nitrophenylamino)meth...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-lactamase | J Med Chem 50: 5644-54 (2007) Article DOI: 10.1021/jm070643q BindingDB Entry DOI: 10.7270/Q21N80W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50225378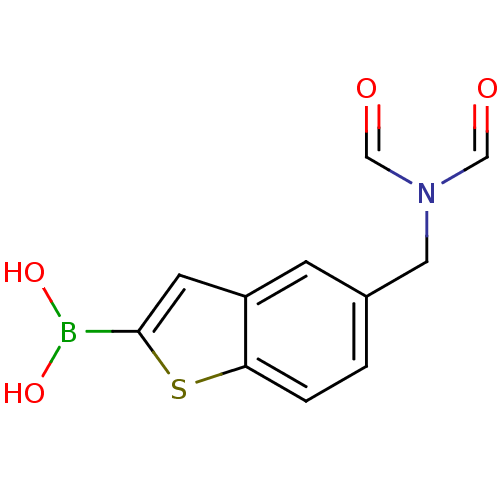 (CHEMBL235073 | pinacol 5-diformylaminomethylbenzo[...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-lactamase | J Med Chem 50: 5644-54 (2007) Article DOI: 10.1021/jm070643q BindingDB Entry DOI: 10.7270/Q21N80W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50225392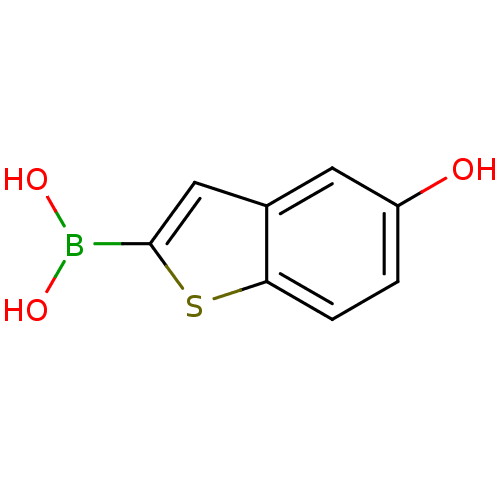 (5-hydroxymethylbenzo[b]thiophen-2-ylboronic acid |...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-lactamase | J Med Chem 50: 5644-54 (2007) Article DOI: 10.1021/jm070643q BindingDB Entry DOI: 10.7270/Q21N80W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50225380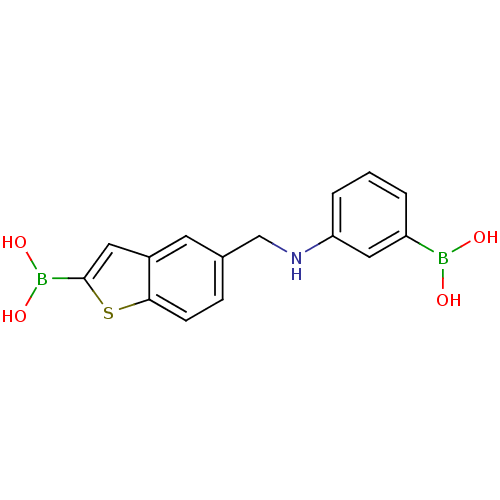 (CHEMBL235308 | pinacol 5-[(phenylamino3-boronicaci...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-lactamase | J Med Chem 50: 5644-54 (2007) Article DOI: 10.1021/jm070643q BindingDB Entry DOI: 10.7270/Q21N80W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM18771 ((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIMORE | Assay Description TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... | J Med Chem 49: 5958-68 (2006) Article DOI: 10.1021/jm051187d BindingDB Entry DOI: 10.7270/Q2S180R0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM18771 ((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 60 | -40.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
UNIMORE | Assay Description TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... | J Med Chem 49: 5958-68 (2006) Article DOI: 10.1021/jm051187d BindingDB Entry DOI: 10.7270/Q2S180R0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM39849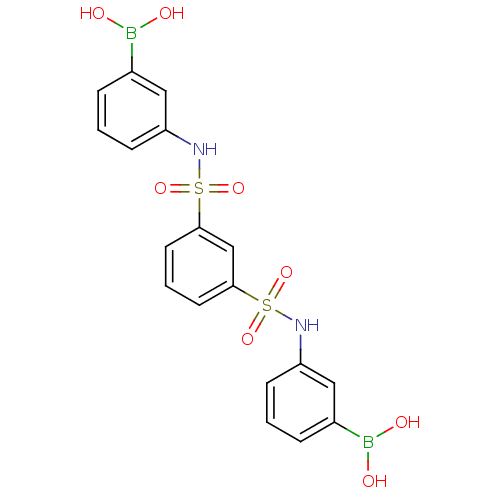 (Amide and sulfonamide derivatives, 33) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Inhibition assay against beta lactamase enzymes. | Chem Biol 8: 593-611 (2001) Article DOI: 10.1016/S1074-5521(01)00034-5 BindingDB Entry DOI: 10.7270/Q27M06BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50405066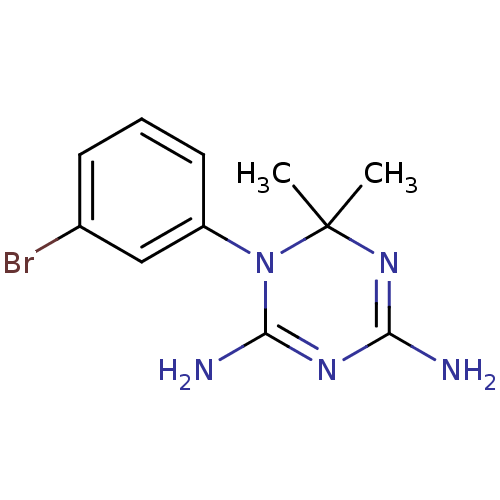 (CHEMBL268088) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, UniversitÓ di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it. Curated by ChEMBL | Assay Description Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysis | Eur J Med Chem 135: 467-478 (2017) Article DOI: 10.1016/j.ejmech.2017.04.070 BindingDB Entry DOI: 10.7270/Q2057JD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50115616 (3-(4-BENZENESULFONYL-THIOPHENE-2-SULFONYLAMINO)-PH...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Inhibition assay against beta lactamase enzymes. | Chem Biol 8: 593-611 (2001) Article DOI: 10.1016/S1074-5521(01)00034-5 BindingDB Entry DOI: 10.7270/Q27M06BZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM39856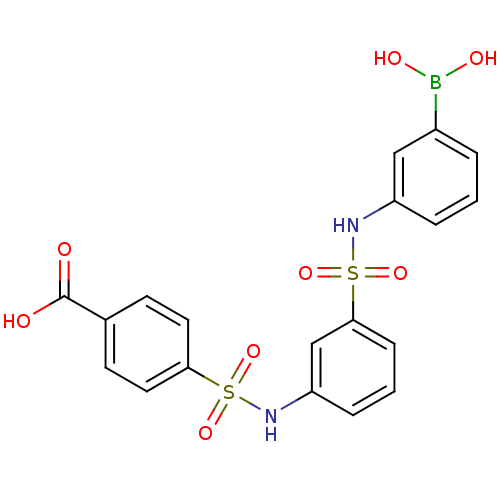 (Amide and sulfonamide derivatives, 41) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Inhibition assay against beta lactamase enzymes. | Chem Biol 8: 593-611 (2001) Article DOI: 10.1016/S1074-5521(01)00034-5 BindingDB Entry DOI: 10.7270/Q27M06BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50225383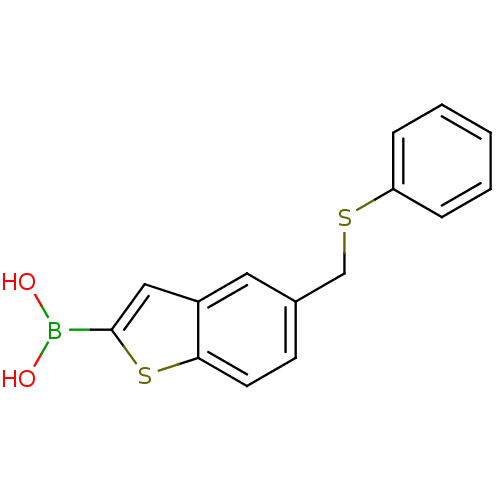 (CHEMBL236203 | pinacol 5-phenylsulphanylmethylbenz...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-lactamase | J Med Chem 50: 5644-54 (2007) Article DOI: 10.1021/jm070643q BindingDB Entry DOI: 10.7270/Q21N80W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50115616 (3-(4-BENZENESULFONYL-THIOPHENE-2-SULFONYLAMINO)-PH...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Apparent inhibition constant against Escherichia coli AmpC beta-lactamase | Bioorg Med Chem Lett 14: 3979-83 (2004) Article DOI: 10.1016/j.bmcl.2004.05.054 BindingDB Entry DOI: 10.7270/Q2XW4J8P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50225389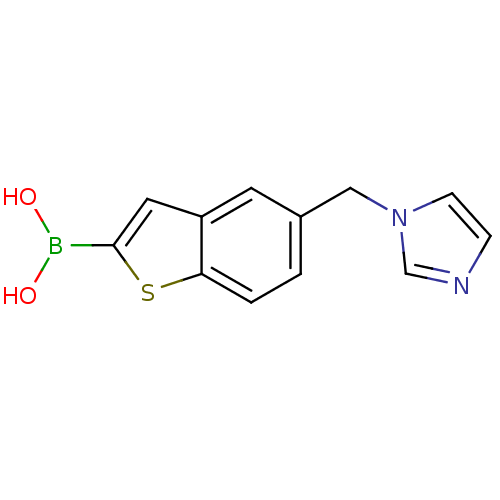 (5-imidazol-1-yl-methylbenzo[b]thiophen-2-ylboronic...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-lactamase | J Med Chem 50: 5644-54 (2007) Article DOI: 10.1021/jm070643q BindingDB Entry DOI: 10.7270/Q21N80W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50225377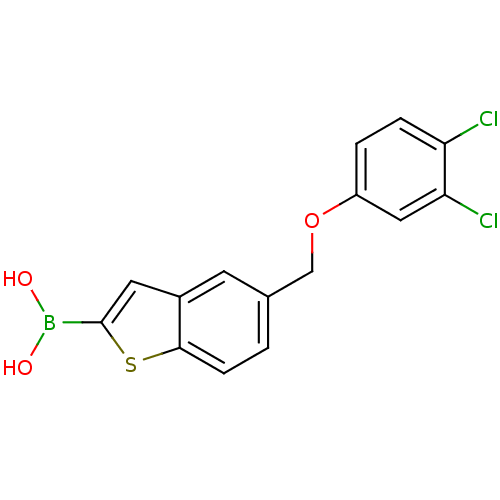 (CHEMBL237390 | pinacol 5-(3,4-dichlorophenoxymethy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-lactamase | J Med Chem 50: 5644-54 (2007) Article DOI: 10.1021/jm070643q BindingDB Entry DOI: 10.7270/Q21N80W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50251158 (2-[(3,4,5-Trimethoxy-phenyl)amino]-3-phenyl-5,7-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari Curated by ChEMBL | Assay Description Inhibition of human dihydrofolate reductase | Eur J Med Chem 43: 189-203 (2008) Article DOI: 10.1016/j.ejmech.2007.03.035 BindingDB Entry DOI: 10.7270/Q27P8Z43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM39819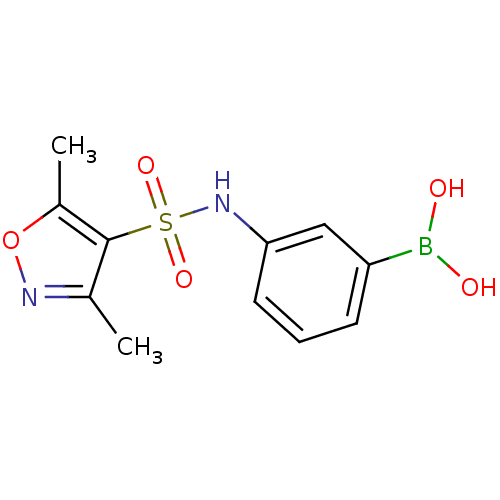 (Amide and sulfonamide derivatives, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Inhibition assay against beta lactamase enzymes. | Chem Biol 8: 593-611 (2001) Article DOI: 10.1016/S1074-5521(01)00034-5 BindingDB Entry DOI: 10.7270/Q27M06BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50090054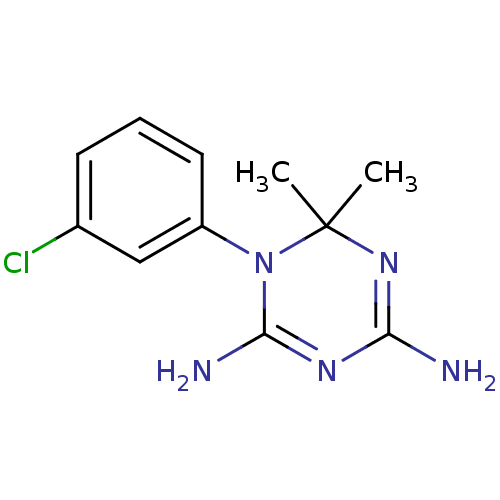 (1-(3-Chloro-phenyl)-6,6-dimethyl-1,6-dihydro-[1,3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, UniversitÓ di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it. Curated by ChEMBL | Assay Description Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysis | Eur J Med Chem 135: 467-478 (2017) Article DOI: 10.1016/j.ejmech.2017.04.070 BindingDB Entry DOI: 10.7270/Q2057JD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50090069 (1-(3,4-Dichloro-phenyl)-6,6-dimethyl-1,6-dihydro-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, UniversitÓ di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it. Curated by ChEMBL | Assay Description Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysis | Eur J Med Chem 135: 467-478 (2017) Article DOI: 10.1016/j.ejmech.2017.04.070 BindingDB Entry DOI: 10.7270/Q2057JD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM39820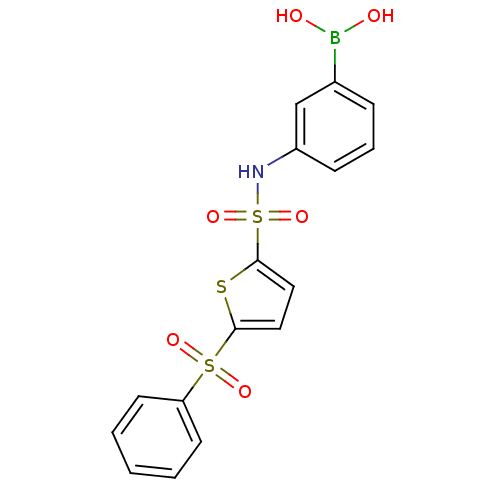 (Amide and sulfonamide derivatives, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Inhibition assay against beta lactamase enzymes. | Chem Biol 8: 593-611 (2001) Article DOI: 10.1016/S1074-5521(01)00034-5 BindingDB Entry DOI: 10.7270/Q27M06BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50291793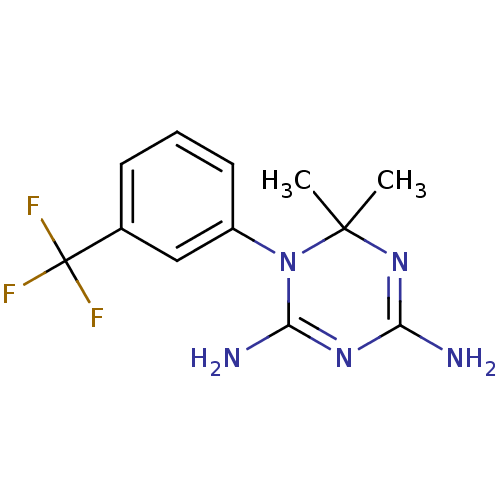 (6,6-Dimethyl-1-(3-trifluoromethyl-phenyl)-1,6-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, UniversitÓ di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it. Curated by ChEMBL | Assay Description Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysis | Eur J Med Chem 135: 467-478 (2017) Article DOI: 10.1016/j.ejmech.2017.04.070 BindingDB Entry DOI: 10.7270/Q2057JD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM39856 (Amide and sulfonamide derivatives, 41) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Inhibition assay against beta lactamase enzymes. | Chem Biol 8: 593-611 (2001) Article DOI: 10.1016/S1074-5521(01)00034-5 BindingDB Entry DOI: 10.7270/Q27M06BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50225374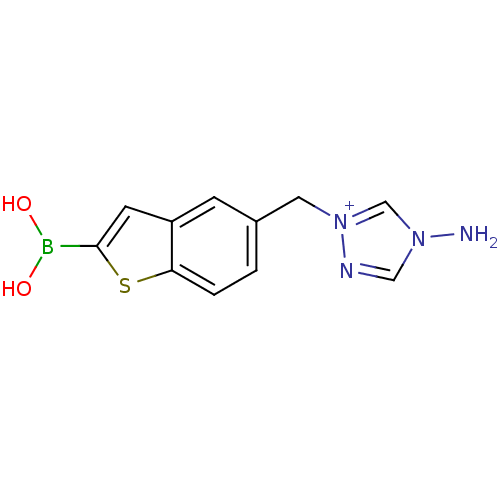 (5 [(1,2,4-triazol-1-ylmethyl]benzo[b]thiophen-2-yl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-lactamase | J Med Chem 50: 5644-54 (2007) Article DOI: 10.1021/jm070643q BindingDB Entry DOI: 10.7270/Q21N80W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1196 total ) | Next | Last >> |