 Found 416 hits with Last Name = 'yoshida' and Initial = 'n'
Found 416 hits with Last Name = 'yoshida' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50056419
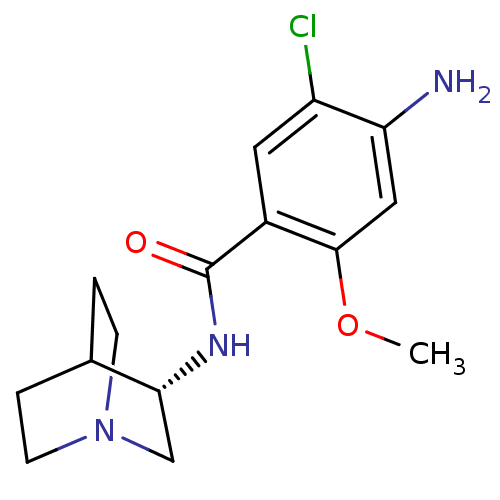
(4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl)-5-chloro-...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)N[C@@H]1CN2CCC1CC2 |wD:13.13,TLB:12:13:17.16:19.20,(11.11,-14.18,;11.11,-12.63,;9.77,-11.88,;8.43,-12.66,;7.09,-11.89,;5.75,-12.66,;7.09,-10.33,;5.76,-9.57,;8.43,-9.56,;9.75,-10.36,;11.09,-9.59,;11.11,-8.04,;12.44,-10.36,;13.77,-9.59,;14.8,-8.58,;16.63,-8.19,;17.94,-9.45,;16.9,-10.39,;15.57,-9.1,;15.88,-7.77,;16.88,-7.14,)| Show InChI InChI=1S/C15H20ClN3O2/c1-21-14-7-12(17)11(16)6-10(14)15(20)18-13-8-19-4-2-9(13)3-5-19/h6-7,9,13H,2-5,8,17H2,1H3,(H,18,20)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]GR-65630 binding to rat cortical membrane serotonin 5-hydroxytryptamine 3 receptor |
J Med Chem 46: 702-15 (2003)
Article DOI: 10.1021/jm020270n
BindingDB Entry DOI: 10.7270/Q25H7H06 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM82519
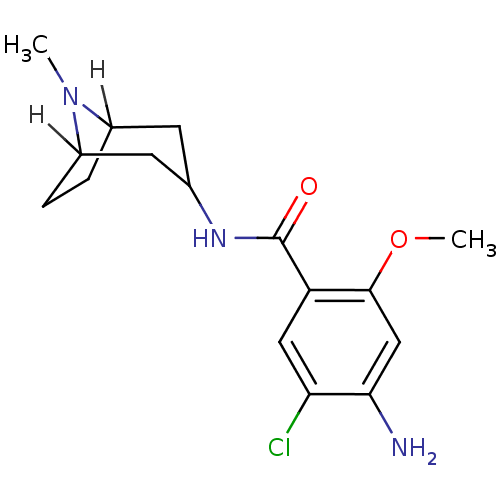
(4-amino-5-chloro-2-methoxy-N-(8-methyl-8-azabicycl...)Show SMILES [H]C12CCC([H])(CC(C1)NC(=O)c1cc(Cl)c(N)cc1OC)N2C Show InChI InChI=1S/C16H22ClN3O2/c1-20-10-3-4-11(20)6-9(5-10)19-16(21)12-7-13(17)14(18)8-15(12)22-2/h7-11H,3-6,18H2,1-2H3,(H,19,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]GR-65630 binding to rat cortical membrane serotonin 5-hydroxytryptamine 3 receptor |
J Med Chem 46: 702-15 (2003)
Article DOI: 10.1021/jm020270n
BindingDB Entry DOI: 10.7270/Q25H7H06 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM82519

(4-amino-5-chloro-2-methoxy-N-(8-methyl-8-azabicycl...)Show SMILES [H]C12CCC([H])(CC(C1)NC(=O)c1cc(Cl)c(N)cc1OC)N2C Show InChI InChI=1S/C16H22ClN3O2/c1-20-10-3-4-11(20)6-9(5-10)19-16(21)12-7-13(17)14(18)8-15(12)22-2/h7-11H,3-6,18H2,1-2H3,(H,19,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]GR-65630 binding to rat cortical membrane serotonin 5-hydroxytryptamine 3 receptor |
J Med Chem 46: 702-15 (2003)
Article DOI: 10.1021/jm020270n
BindingDB Entry DOI: 10.7270/Q25H7H06 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260267
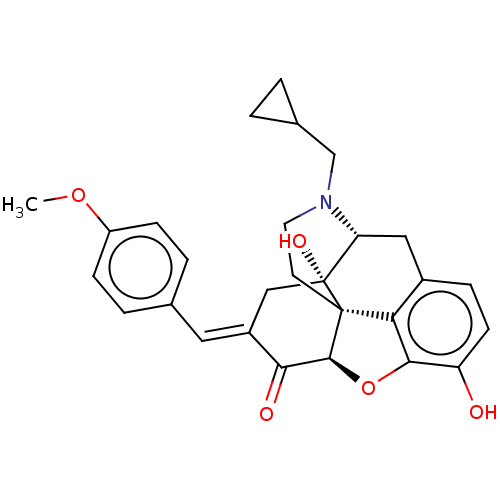
(CHEMBL2059382)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(OC)cc1)C2=O)ccc3O |r,TLB:32:5:9.14.15:17,THB:3:4:9.14.15:17| Show InChI InChI=1S/C28H29NO5/c1-33-20-7-4-16(5-8-20)12-19-14-28(32)22-13-18-6-9-21(30)25-23(18)27(28,26(34-25)24(19)31)10-11-29(22)15-17-2-3-17/h4-9,12,17,22,26,30,32H,2-3,10-11,13-15H2,1H3/b19-12+/t22-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260248
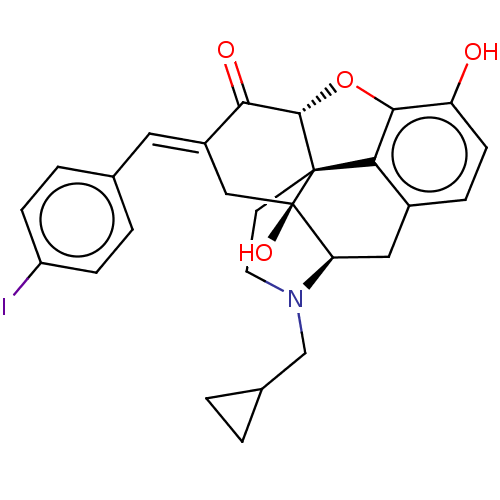
(CHEMBL4092119)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(I)cc1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26INO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50454798
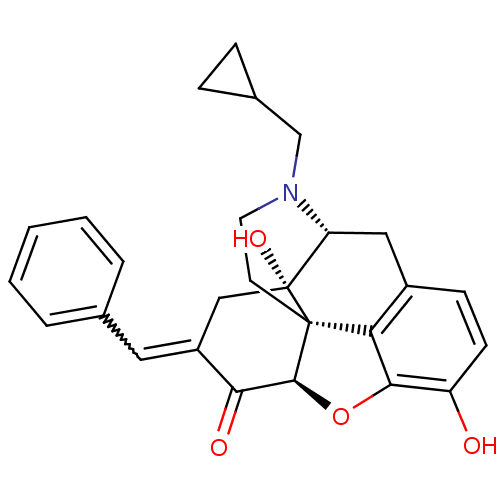
(7-Benzylidenenaltrexone | BNTX)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccccc1)C2=O)ccc3O |r,THB:10:9:17:6.5.4| Show InChI InChI=1S/C27H27NO4/c29-20-9-8-18-13-21-27(31)14-19(12-16-4-2-1-3-5-16)23(30)25-26(27,22(18)24(20)32-25)10-11-28(21)15-17-6-7-17/h1-5,8-9,12,17,21,25,29,31H,6-7,10-11,13-15H2/b19-12+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of Fibrinogen to thrombocyte alpha IIb beta-3 integrin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260257
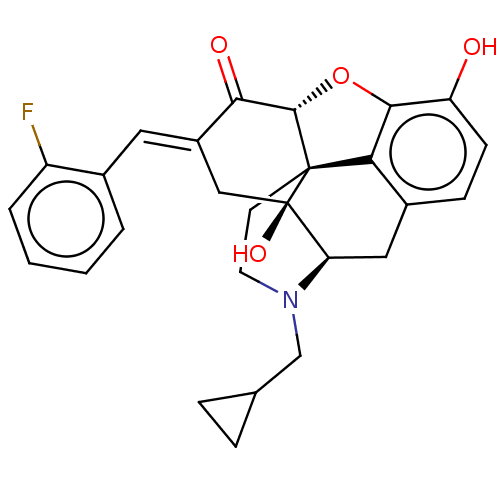
(CHEMBL4105306)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccccc1F)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26FNO4/c28-19-4-2-1-3-16(19)11-18-13-27(32)21-12-17-7-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-15-5-6-15/h1-4,7-8,11,15,21,25,30,32H,5-6,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260244
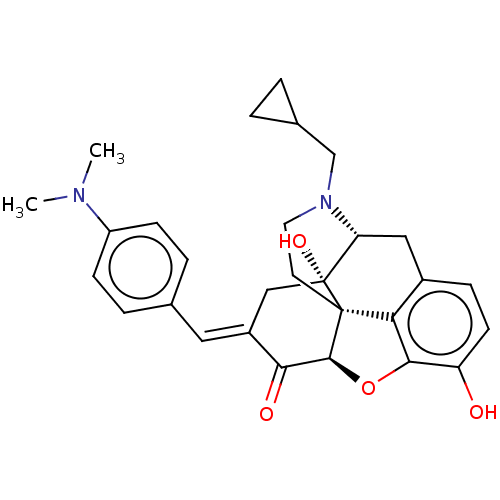
(CHEMBL3632690)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(cc1)N(C)C)C2=O)ccc3O |r,TLB:10:9:17:6.4.5| Show InChI InChI=1S/C29H32N2O4/c1-30(2)21-8-5-17(6-9-21)13-20-15-29(34)23-14-19-7-10-22(32)26-24(19)28(29,27(35-26)25(20)33)11-12-31(23)16-18-3-4-18/h5-10,13,18,23,27,32,34H,3-4,11-12,14-16H2,1-2H3/b20-13+/t23-,27+,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260242
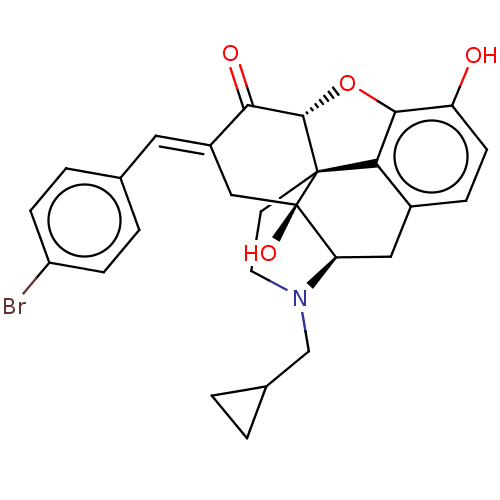
(CHEMBL4071324)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(Br)cc1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26BrNO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50260242

(CHEMBL4071324)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(Br)cc1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26BrNO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50260267

(CHEMBL2059382)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(OC)cc1)C2=O)ccc3O |r,TLB:32:5:9.14.15:17,THB:3:4:9.14.15:17| Show InChI InChI=1S/C28H29NO5/c1-33-20-7-4-16(5-8-20)12-19-14-28(32)22-13-18-6-9-21(30)25-23(18)27(28,26(34-25)24(19)31)10-11-29(22)15-17-2-3-17/h4-9,12,17,22,26,30,32H,2-3,10-11,13-15H2,1H3/b19-12+/t22-,26+,27+,28-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50280258

(4-Amino-N-(S)-1-aza-bicyclo[3.3.1]non-4-yl-5-chlor...)Show InChI InChI=1S/C16H22ClN3O2/c1-22-15-8-13(18)12(17)7-11(15)16(21)19-14-4-6-20-5-2-3-10(14)9-20/h7-8,10,14H,2-6,9,18H2,1H3,(H,19,21)/t10?,14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]GR-65630 binding to rat cortical membrane serotonin 5-hydroxytryptamine 3 receptor |
J Med Chem 46: 702-15 (2003)
Article DOI: 10.1021/jm020270n
BindingDB Entry DOI: 10.7270/Q25H7H06 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50260243

(CHEMBL4070277)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(Cl)cc1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26ClNO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260243

(CHEMBL4070277)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(Cl)cc1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26ClNO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260249

(CHEMBL4081053)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1cccc(F)c1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26FNO4/c28-19-3-1-2-16(11-19)10-18-13-27(32)21-12-17-6-7-20(30)24-22(17)26(27,25(33-24)23(18)31)8-9-29(21)14-15-4-5-15/h1-3,6-7,10-11,15,21,25,30,32H,4-5,8-9,12-14H2/b18-10+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50260249

(CHEMBL4081053)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1cccc(F)c1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26FNO4/c28-19-3-1-2-16(11-19)10-18-13-27(32)21-12-17-6-7-20(30)24-22(17)26(27,25(33-24)23(18)31)8-9-29(21)14-15-4-5-15/h1-3,6-7,10-11,15,21,25,30,32H,4-5,8-9,12-14H2/b18-10+/t21-,25+,26+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of Fibrinogen to thrombocyte alpha IIb beta-3 integrin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50454798

(7-Benzylidenenaltrexone | BNTX)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccccc1)C2=O)ccc3O |r,THB:10:9:17:6.5.4| Show InChI InChI=1S/C27H27NO4/c29-20-9-8-18-13-21-27(31)14-19(12-16-4-2-1-3-5-16)23(30)25-26(27,22(18)24(20)32-25)10-11-28(21)15-17-6-7-17/h1-5,8-9,12,17,21,25,29,31H,6-7,10-11,13-15H2/b19-12+/t21-,25+,26+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50132693
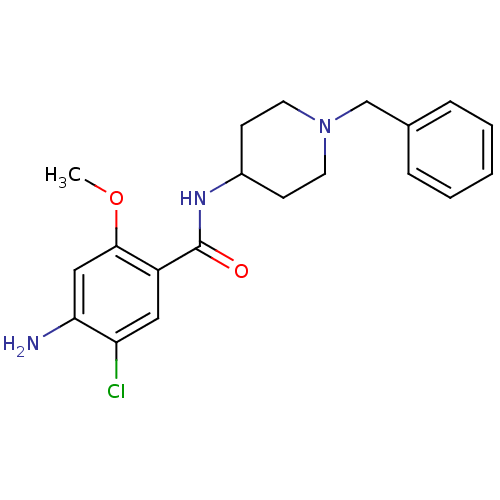
(4-Amino-N-(1-benzyl-piperidin-4-yl)-5-chloro-2-met...)Show InChI InChI=1S/C20H24ClN3O2/c1-26-19-12-18(22)17(21)11-16(19)20(25)23-15-7-9-24(10-8-15)13-14-5-3-2-4-6-14/h2-6,11-12,15H,7-10,13,22H2,1H3,(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 in rat striatum using [3H]spiperone as radioligand |
J Med Chem 46: 702-15 (2003)
Article DOI: 10.1021/jm020270n
BindingDB Entry DOI: 10.7270/Q25H7H06 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50260257

(CHEMBL4105306)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccccc1F)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26FNO4/c28-19-4-2-1-3-16(19)11-18-13-27(32)21-12-17-7-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-15-5-6-15/h1-4,7-8,11,15,21,25,30,32H,5-6,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of Fibrinogen to thrombocyte alpha IIb beta-3 integrin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50176892
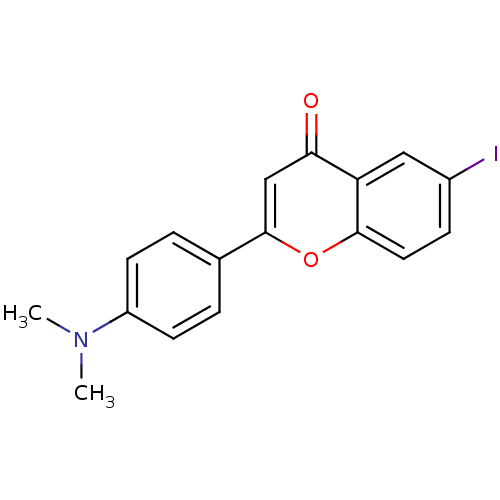
(6-iodo-4'-dimethyaminoflavone | CHEMBL375934)Show InChI InChI=1S/C17H14INO2/c1-19(2)13-6-3-11(4-7-13)17-10-15(20)14-9-12(18)5-8-16(14)21-17/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]6-iodo-4'-dimethyaminofl avone from beta amyloid (1-40) protein aggregates |
J Med Chem 48: 7253-60 (2005)
Article DOI: 10.1021/jm050635e
BindingDB Entry DOI: 10.7270/Q2H70FC0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50005836

(4-Amino-5-chloro-N-{1-[3-(4-fluoro-phenoxy)-propyl...)Show SMILES COC1CN(CCCOc2ccc(F)cc2)CCC1NC(=O)c1cc(Cl)c(N)cc1OC Show InChI InChI=1S/C23H29ClFN3O4/c1-30-21-13-19(26)18(24)12-17(21)23(29)27-20-8-10-28(14-22(20)31-2)9-3-11-32-16-6-4-15(25)5-7-16/h4-7,12-13,20,22H,3,8-11,14,26H2,1-2H3,(H,27,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]GR-113808 binding to guinea pig striatum 5-hydroxytryptamine 4 receptor |
J Med Chem 46: 702-15 (2003)
Article DOI: 10.1021/jm020270n
BindingDB Entry DOI: 10.7270/Q25H7H06 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50176892

(6-iodo-4'-dimethyaminoflavone | CHEMBL375934)Show InChI InChI=1S/C17H14INO2/c1-19(2)13-6-3-11(4-7-13)17-10-15(20)14-9-12(18)5-8-16(14)21-17/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]6-iodo-4'-dimethyaminofl avone from beta amyloid (1-42) protein aggregates |
J Med Chem 48: 7253-60 (2005)
Article DOI: 10.1021/jm050635e
BindingDB Entry DOI: 10.7270/Q2H70FC0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50260244

(CHEMBL3632690)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(cc1)N(C)C)C2=O)ccc3O |r,TLB:10:9:17:6.4.5| Show InChI InChI=1S/C29H32N2O4/c1-30(2)21-8-5-17(6-9-21)13-20-15-29(34)23-14-19-7-10-22(32)26-24(19)28(29,27(35-26)25(20)33)11-12-31(23)16-18-3-4-18/h5-10,13,18,23,27,32,34H,3-4,11-12,14-16H2,1-2H3/b20-13+/t23-,27+,28+,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260258

(CHEMBL4089193)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(cc1)C#N)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C28H26N2O4/c29-14-17-3-1-16(2-4-17)11-20-13-28(33)22-12-19-7-8-21(31)25-23(19)27(28,26(34-25)24(20)32)9-10-30(22)15-18-5-6-18/h1-4,7-8,11,18,22,26,31,33H,5-6,9-10,12-13,15H2/b20-11+/t22-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50260248

(CHEMBL4092119)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(I)cc1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26INO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50260247
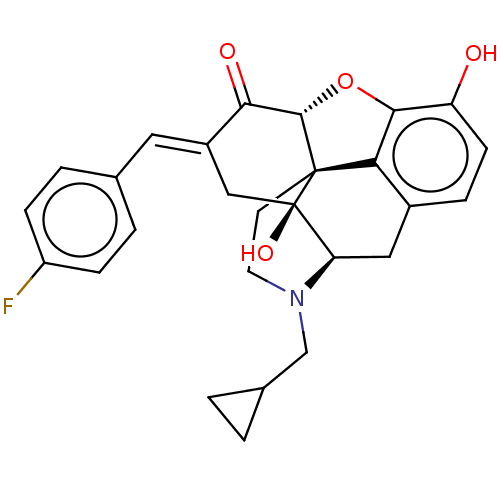
(CHEMBL2059381)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(F)cc1)C2=O)ccc3O |r,TLB:31:5:9.14.15:17,THB:3:4:9.14.15:17| Show InChI InChI=1S/C27H26FNO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of Fibrinogen to thrombocyte alpha IIb beta-3 integrin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50005833
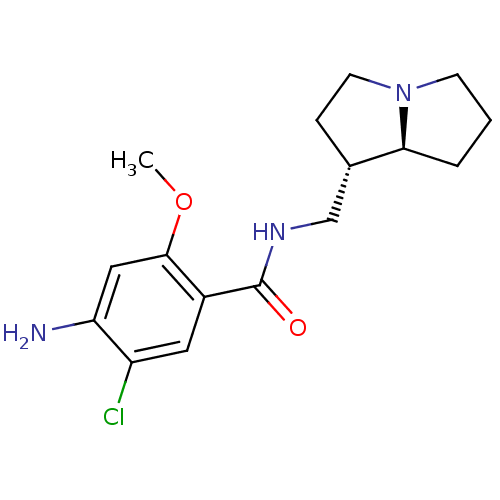
((exo)4-Amino-5-chloro-N-(hexahydro-pyrrolizin-1-yl...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC[C@@H]1CCN2CCC[C@@H]12 Show InChI InChI=1S/C16H22ClN3O2/c1-22-15-8-13(18)12(17)7-11(15)16(21)19-9-10-4-6-20-5-2-3-14(10)20/h7-8,10,14H,2-6,9,18H2,1H3,(H,19,21)/t10-,14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]GR-113808 binding to guinea pig striatum 5-hydroxytryptamine 4 receptor |
J Med Chem 46: 702-15 (2003)
Article DOI: 10.1021/jm020270n
BindingDB Entry DOI: 10.7270/Q25H7H06 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50176896
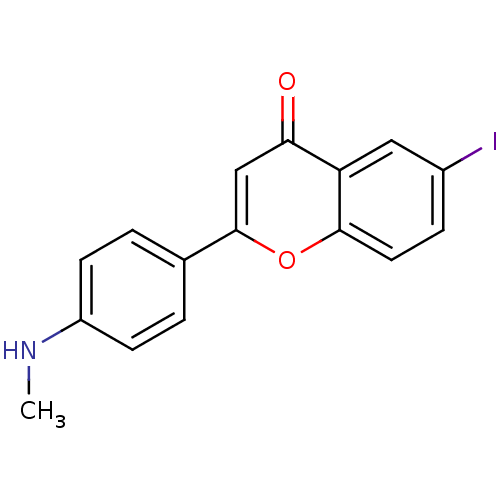
(6-iodo-4'-methylaminoflavone | CHEMBL224643)Show InChI InChI=1S/C16H12INO2/c1-18-12-5-2-10(3-6-12)16-9-14(19)13-8-11(17)4-7-15(13)20-16/h2-9,18H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]6-iodo-4'-dimethyaminofl avone from beta amyloid (1-40) protein aggregates |
J Med Chem 48: 7253-60 (2005)
Article DOI: 10.1021/jm050635e
BindingDB Entry DOI: 10.7270/Q2H70FC0 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50123801

(4-Amino-N-[2-(benzyl-methyl-amino)-ethyl]-5-chloro...)Show InChI InChI=1S/C18H22ClN3O2/c1-22(12-13-6-4-3-5-7-13)9-8-21-18(23)14-10-15(19)16(20)11-17(14)24-2/h3-7,10-11H,8-9,12,20H2,1-2H3,(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 in rat striatum using [3H]spiperone as radioligand |
J Med Chem 46: 702-15 (2003)
Article DOI: 10.1021/jm020270n
BindingDB Entry DOI: 10.7270/Q25H7H06 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50260267

(CHEMBL2059382)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(OC)cc1)C2=O)ccc3O |r,TLB:32:5:9.14.15:17,THB:3:4:9.14.15:17| Show InChI InChI=1S/C28H29NO5/c1-33-20-7-4-16(5-8-20)12-19-14-28(32)22-13-18-6-9-21(30)25-23(18)27(28,26(34-25)24(19)31)10-11-29(22)15-17-2-3-17/h4-9,12,17,22,26,30,32H,2-3,10-11,13-15H2,1H3/b19-12+/t22-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50260244

(CHEMBL3632690)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(cc1)N(C)C)C2=O)ccc3O |r,TLB:10:9:17:6.4.5| Show InChI InChI=1S/C29H32N2O4/c1-30(2)21-8-5-17(6-9-21)13-20-15-29(34)23-14-19-7-10-22(32)26-24(19)28(29,27(35-26)25(20)33)11-12-31(23)16-18-3-4-18/h5-10,13,18,23,27,32,34H,3-4,11-12,14-16H2,1-2H3/b20-13+/t23-,27+,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50176894
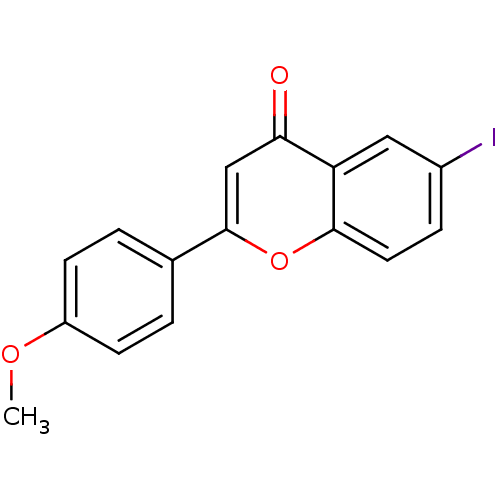
(6-iodo-4'-methoxyflavone | CHEMBL224064)Show InChI InChI=1S/C16H11IO3/c1-19-12-5-2-10(3-6-12)16-9-14(18)13-8-11(17)4-7-15(13)20-16/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]6-iodo-4'-dimethyaminofl avone from beta amyloid (1-40) protein aggregates |
J Med Chem 48: 7253-60 (2005)
Article DOI: 10.1021/jm050635e
BindingDB Entry DOI: 10.7270/Q2H70FC0 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50176896

(6-iodo-4'-methylaminoflavone | CHEMBL224643)Show InChI InChI=1S/C16H12INO2/c1-18-12-5-2-10(3-6-12)16-9-14(19)13-8-11(17)4-7-15(13)20-16/h2-9,18H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]6-iodo-4'-dimethyaminofl avone from beta amyloid (1-42) protein aggregates |
J Med Chem 48: 7253-60 (2005)
Article DOI: 10.1021/jm050635e
BindingDB Entry DOI: 10.7270/Q2H70FC0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50260258

(CHEMBL4089193)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(cc1)C#N)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C28H26N2O4/c29-14-17-3-1-16(2-4-17)11-20-13-28(33)22-12-19-7-8-21(31)25-23(19)27(28,26(34-25)24(20)32)9-10-30(22)15-18-5-6-18/h1-4,7-8,11,18,22,26,31,33H,5-6,9-10,12-13,15H2/b20-11+/t22-,26+,27+,28-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50454798

(7-Benzylidenenaltrexone | BNTX)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccccc1)C2=O)ccc3O |r,THB:10:9:17:6.5.4| Show InChI InChI=1S/C27H27NO4/c29-20-9-8-18-13-21-27(31)14-19(12-16-4-2-1-3-5-16)23(30)25-26(27,22(18)24(20)32-25)10-11-28(21)15-17-6-7-17/h1-5,8-9,12,17,21,25,29,31H,6-7,10-11,13-15H2/b19-12+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260247

(CHEMBL2059381)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(F)cc1)C2=O)ccc3O |r,TLB:31:5:9.14.15:17,THB:3:4:9.14.15:17| Show InChI InChI=1S/C27H26FNO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50260257

(CHEMBL4105306)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccccc1F)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26FNO4/c28-19-4-2-1-3-16(19)11-18-13-27(32)21-12-17-7-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-15-5-6-15/h1-4,7-8,11,15,21,25,30,32H,5-6,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69,593 from human kappa opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50176894

(6-iodo-4'-methoxyflavone | CHEMBL224064)Show InChI InChI=1S/C16H11IO3/c1-19-12-5-2-10(3-6-12)16-9-14(18)13-8-11(17)4-7-15(13)20-16/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]6-iodo-4'-dimethyaminofl avone from beta amyloid (1-42) protein aggregates |
J Med Chem 48: 7253-60 (2005)
Article DOI: 10.1021/jm050635e
BindingDB Entry DOI: 10.7270/Q2H70FC0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50280258

(4-Amino-N-(S)-1-aza-bicyclo[3.3.1]non-4-yl-5-chlor...)Show InChI InChI=1S/C16H22ClN3O2/c1-22-15-8-13(18)12(17)7-11(15)16(21)19-14-4-6-20-5-2-3-10(14)9-20/h7-8,10,14H,2-6,9,18H2,1H3,(H,19,21)/t10?,14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]GR-113808 binding to guinea pig striatum 5-hydroxytryptamine 4 receptor |
J Med Chem 46: 702-15 (2003)
Article DOI: 10.1021/jm020270n
BindingDB Entry DOI: 10.7270/Q25H7H06 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM82519

(4-amino-5-chloro-2-methoxy-N-(8-methyl-8-azabicycl...)Show SMILES [H]C12CCC([H])(CC(C1)NC(=O)c1cc(Cl)c(N)cc1OC)N2C Show InChI InChI=1S/C16H22ClN3O2/c1-20-10-3-4-11(20)6-9(5-10)19-16(21)12-7-13(17)14(18)8-15(12)22-2/h7-11H,3-6,18H2,1-2H3,(H,19,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]GR-113808 binding to guinea pig striatum 5-hydroxytryptamine 4 receptor |
J Med Chem 46: 702-15 (2003)
Article DOI: 10.1021/jm020270n
BindingDB Entry DOI: 10.7270/Q25H7H06 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50260249

(CHEMBL4081053)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1cccc(F)c1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26FNO4/c28-19-3-1-2-16(11-19)10-18-13-27(32)21-12-17-6-7-20(30)24-22(17)26(27,25(33-24)23(18)31)8-9-29(21)14-15-4-5-15/h1-3,6-7,10-11,15,21,25,30,32H,4-5,8-9,12-14H2/b18-10+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69,593 from human kappa opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM94630
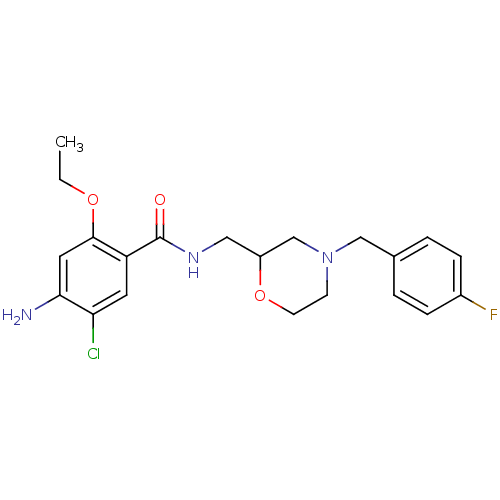
(4-Amino-5-chloro-2-ethoxy-N-[4-(4-fluoro-benzyl)-m...)Show SMILES CCOc1cc(N)c(Cl)cc1C(=O)NCC1CN(Cc2ccc(F)cc2)CCO1 Show InChI InChI=1S/C21H25ClFN3O3/c1-2-28-20-10-19(24)18(22)9-17(20)21(27)25-11-16-13-26(7-8-29-16)12-14-3-5-15(23)6-4-14/h3-6,9-10,16H,2,7-8,11-13,24H2,1H3,(H,25,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 69.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]GR-113808 binding to guinea pig striatum 5-hydroxytryptamine 4 receptor |
J Med Chem 46: 702-15 (2003)
Article DOI: 10.1021/jm020270n
BindingDB Entry DOI: 10.7270/Q25H7H06 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50260242

(CHEMBL4071324)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(Br)cc1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26BrNO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50176895
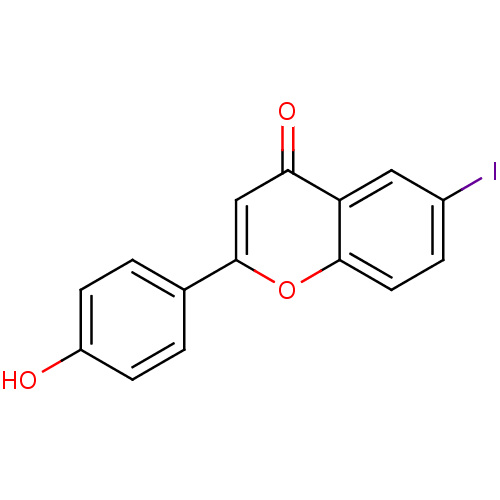
(6-iodo-4'-hydroxyflavone | CHEMBL388844)Show InChI InChI=1S/C15H9IO3/c16-10-3-6-14-12(7-10)13(18)8-15(19-14)9-1-4-11(17)5-2-9/h1-8,17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 72.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]6-iodo-4'-dimethyaminofl avone from beta amyloid (1-40) protein aggregates |
J Med Chem 48: 7253-60 (2005)
Article DOI: 10.1021/jm050635e
BindingDB Entry DOI: 10.7270/Q2H70FC0 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50176895

(6-iodo-4'-hydroxyflavone | CHEMBL388844)Show InChI InChI=1S/C15H9IO3/c16-10-3-6-14-12(7-10)13(18)8-15(19-14)9-1-4-11(17)5-2-9/h1-8,17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 77.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]6-iodo-4'-dimethyaminofl avone from beta amyloid (1-42) protein aggregates |
J Med Chem 48: 7253-60 (2005)
Article DOI: 10.1021/jm050635e
BindingDB Entry DOI: 10.7270/Q2H70FC0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50260243

(CHEMBL4070277)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(Cl)cc1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26ClNO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260262
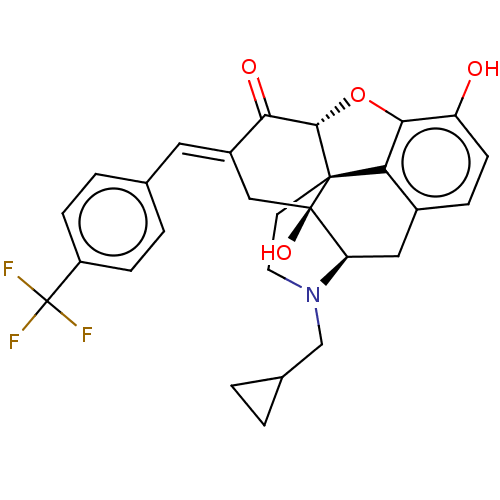
(CHEMBL4061882)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(cc1)C(F)(F)F)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C28H26F3NO4/c29-28(30,31)19-6-3-15(4-7-19)11-18-13-27(35)21-12-17-5-8-20(33)24-22(17)26(27,25(36-24)23(18)34)9-10-32(21)14-16-1-2-16/h3-8,11,16,21,25,33,35H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50005836

(4-Amino-5-chloro-N-{1-[3-(4-fluoro-phenoxy)-propyl...)Show SMILES COC1CN(CCCOc2ccc(F)cc2)CCC1NC(=O)c1cc(Cl)c(N)cc1OC Show InChI InChI=1S/C23H29ClFN3O4/c1-30-21-13-19(26)18(24)12-17(21)23(29)27-20-8-10-28(14-22(20)31-2)9-3-11-32-16-6-4-15(25)5-7-16/h4-7,12-13,20,22H,3,8-11,14,26H2,1-2H3,(H,27,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 94.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]GR-65630 binding to rat cortical membrane serotonin 5-hydroxytryptamine 3 receptor |
J Med Chem 46: 702-15 (2003)
Article DOI: 10.1021/jm020270n
BindingDB Entry DOI: 10.7270/Q25H7H06 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260263

(CHEMBL4090365)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccccc1)C2=O)ccc3OC |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C28H29NO4/c1-32-21-10-9-19-14-22-28(31)15-20(13-17-5-3-2-4-6-17)24(30)26-27(28,23(19)25(21)33-26)11-12-29(22)16-18-7-8-18/h2-6,9-10,13,18,22,26,31H,7-8,11-12,14-16H2,1H3/b20-13+/t22-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50132693

(4-Amino-N-(1-benzyl-piperidin-4-yl)-5-chloro-2-met...)Show InChI InChI=1S/C20H24ClN3O2/c1-26-19-12-18(22)17(21)11-16(19)20(25)23-15-7-9-24(10-8-15)13-14-5-3-2-4-6-14/h2-6,11-12,15H,7-10,13,22H2,1H3,(H,23,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]GR-113808 binding to guinea pig striatum 5-hydroxytryptamine 4 receptor |
J Med Chem 46: 702-15 (2003)
Article DOI: 10.1021/jm020270n
BindingDB Entry DOI: 10.7270/Q25H7H06 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data