

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50230827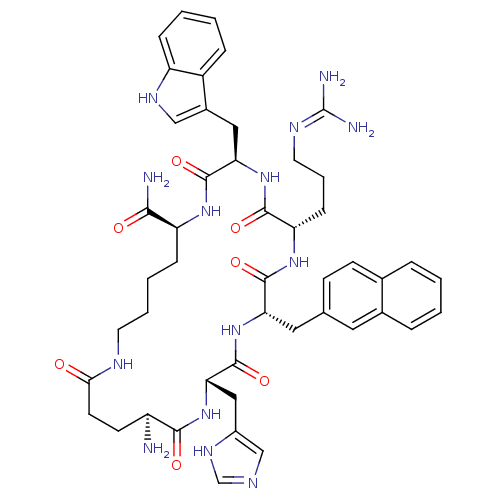 (CHEMBL253364 | MK-9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I][Nle4,D-Phe7]alpha-MSH from human MC3 receptor expressed in HEK293 cells | J Med Chem 51: 187-95 (2008) Article DOI: 10.1021/jm070461w BindingDB Entry DOI: 10.7270/Q2V125N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of human AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition by Lineweaver-B... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469009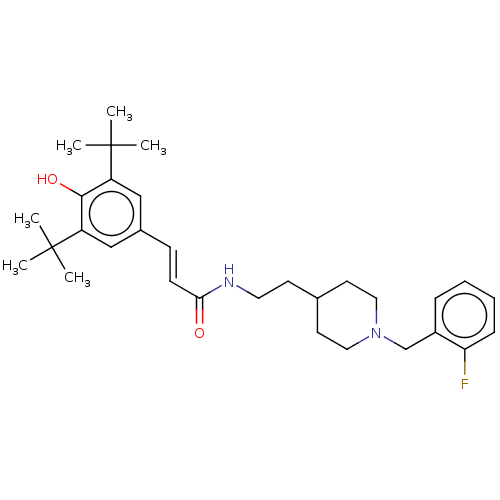 (CHEMBL4292766) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of human AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition by Lineweaver-B... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469008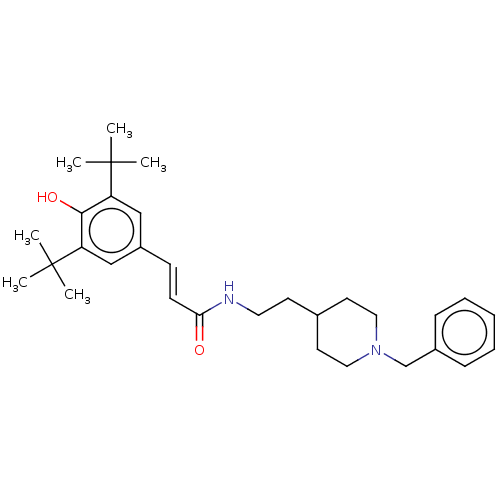 (CHEMBL4283390) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of human AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition by Lineweaver-B... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50193833 (CHEMBL220018 | N-[(S)-1-((3S,8aS)-3-benzyl-hexahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from human MC5R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 5462-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.015 BindingDB Entry DOI: 10.7270/Q2XD11BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50193829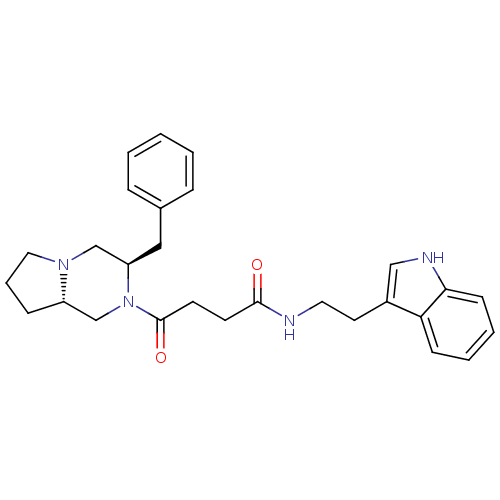 (CHEMBL425442 | N-(2-(1H-indol-3-yl)ethyl)-4-((3R,8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from human MC5R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 5462-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.015 BindingDB Entry DOI: 10.7270/Q2XD11BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50184359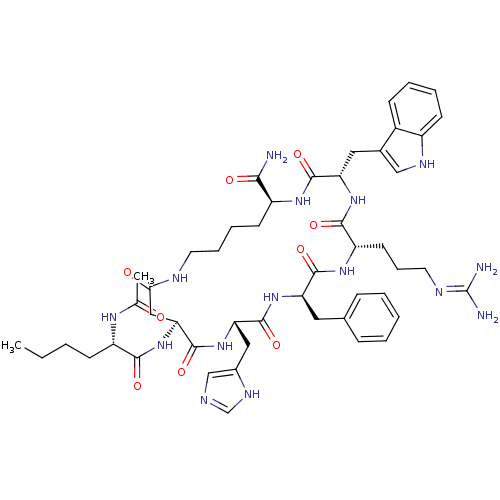 ((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from human MC1R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 5462-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.015 BindingDB Entry DOI: 10.7270/Q2XD11BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50027084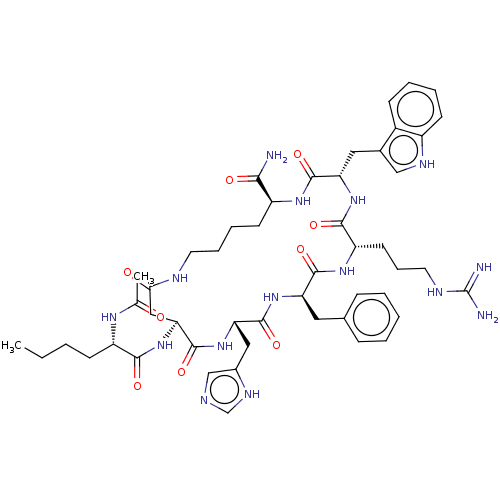 (Melatonan) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I][Nle4,D-Phe7]alpha-MSH from human MC1 receptor expressed in HEK293 cells | J Med Chem 51: 187-95 (2008) Article DOI: 10.1021/jm070461w BindingDB Entry DOI: 10.7270/Q2V125N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50193831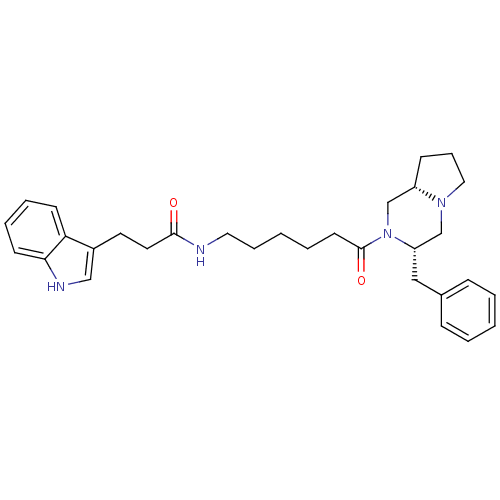 (CHEMBL218558 | N-(6-((3S,8aS)-3-benzyl-hexahydropy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from human MC5R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 5462-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.015 BindingDB Entry DOI: 10.7270/Q2XD11BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50193834 (CHEMBL218361 | N-(2-(1H-indol-3-yl)ethyl)-4-((3S,8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from human MC5R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 5462-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.015 BindingDB Entry DOI: 10.7270/Q2XD11BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50193830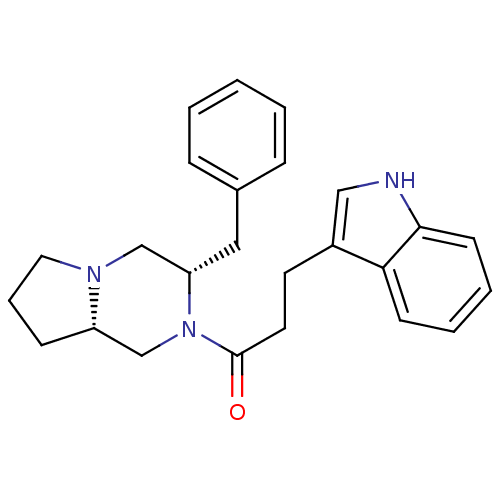 (1-((3S,8aS)-3-benzyl-hexahydropyrrolo[1,2-a]pyrazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from human MC5R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 5462-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.015 BindingDB Entry DOI: 10.7270/Q2XD11BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50409078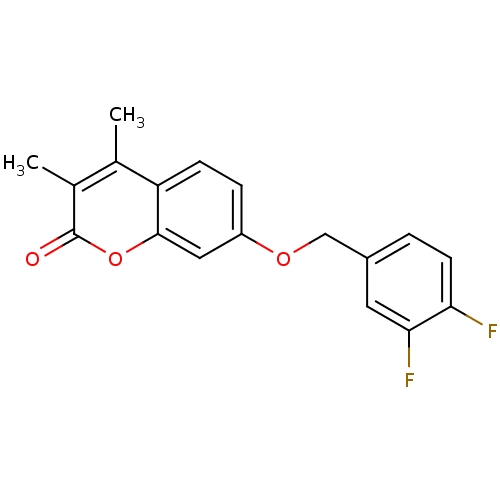 (CHEMBL325761) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of rat brain mitochondrial MAOB using kynuramine as substrate assessed as decrease in 4-hydroxyquinoline production by spectrophotometry | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50027084 (Melatonan) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I][Nle4,D-Phe7]alpha-MSH from human MC4 receptor expressed in HEK293 cells | J Med Chem 51: 187-95 (2008) Article DOI: 10.1021/jm070461w BindingDB Entry DOI: 10.7270/Q2V125N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50193836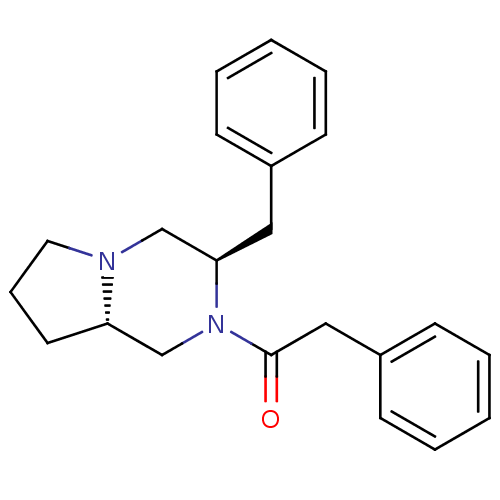 (1-((3R,8aS)-3-benzyl-hexahydropyrrolo[1,2-a]pyrazi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from human MC4R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 5462-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.015 BindingDB Entry DOI: 10.7270/Q2XD11BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50193832 (1-((3R,8aS)-3-benzyl-hexahydropyrrolo[1,2-a]pyrazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from human MC3R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 5462-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.015 BindingDB Entry DOI: 10.7270/Q2XD11BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50193836 (1-((3R,8aS)-3-benzyl-hexahydropyrrolo[1,2-a]pyrazi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from human MC1R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 5462-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.015 BindingDB Entry DOI: 10.7270/Q2XD11BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50199097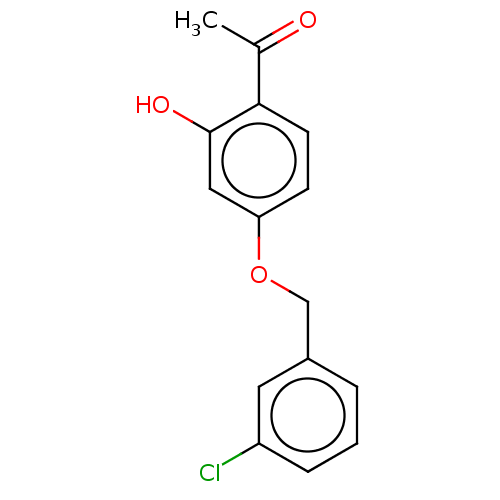 (CHEMBL3911291) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using kynuramine as substrate assessed as decrease in 4-hydroxyquinoline production incubated for 20 mins by fl... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50027084 (Melatonan) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I][Nle4,D-Phe7]alpha-MSH from human MC3 receptor expressed in HEK293 cells | J Med Chem 51: 187-95 (2008) Article DOI: 10.1021/jm070461w BindingDB Entry DOI: 10.7270/Q2V125N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50121268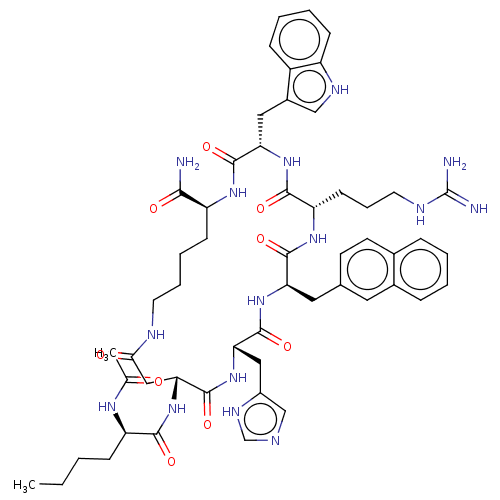 (21-(2-Acetylamino-hexanoylamino)-7-[3-(diaminometh...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I][Nle4,D-Phe7]alpha-MSH from human MC4 receptor expressed in HEK293 cells | J Med Chem 51: 187-95 (2008) Article DOI: 10.1021/jm070461w BindingDB Entry DOI: 10.7270/Q2V125N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50184359 ((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from human MC4R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 5462-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.015 BindingDB Entry DOI: 10.7270/Q2XD11BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50184359 ((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from human MC3R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 5462-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.015 BindingDB Entry DOI: 10.7270/Q2XD11BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50193835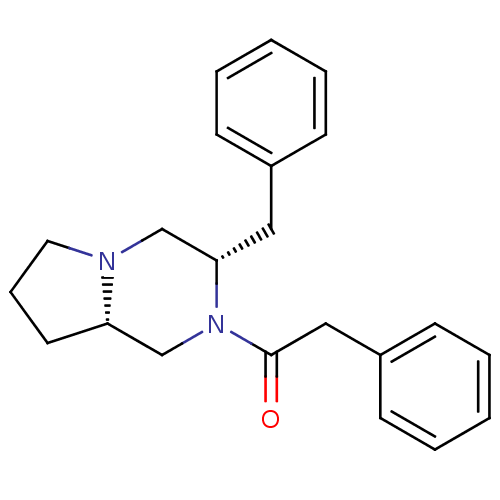 (1-((3S,8aS)-3-benzyl-hexahydropyrrolo[1,2-a]pyrazi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from human MC1R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 5462-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.015 BindingDB Entry DOI: 10.7270/Q2XD11BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50184359 ((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC4R expressed in HEK293 cells after 40 mins by Wallac 1470 gamma counting | Bioorg Med Chem Lett 21: 3099-102 (2011) Article DOI: 10.1016/j.bmcl.2011.03.019 BindingDB Entry DOI: 10.7270/Q2BC3ZVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50184359 ((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK293 cells after 40 mins by Wallac 1470 gamma counting | Bioorg Med Chem Lett 21: 3099-102 (2011) Article DOI: 10.1016/j.bmcl.2011.03.019 BindingDB Entry DOI: 10.7270/Q2BC3ZVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50199101 (CHEMBL3920226) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using kynuramine as substrate assessed as decrease in 4-hydroxyquinoline production incubated for 20 mins by fl... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50199094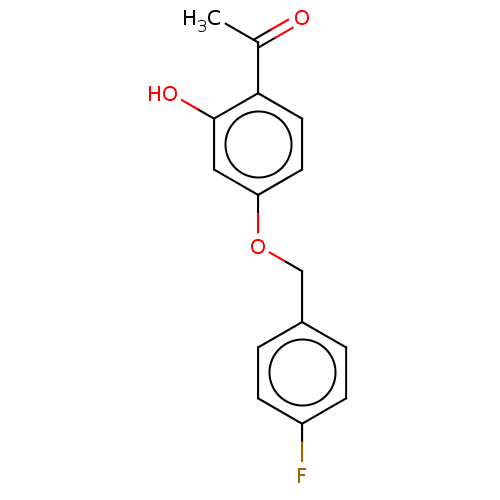 (CHEMBL3948027) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using kynuramine as substrate assessed as decrease in 4-hydroxyquinoline production incubated for 20 mins by fl... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50282510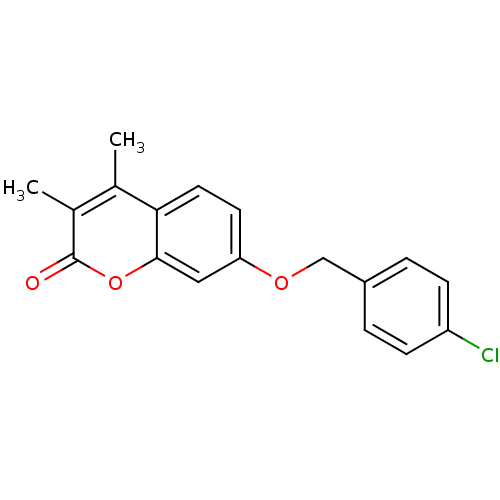 (7-(4-Chloro-benzyloxy)-3,4-dimethyl-chromen-2-one ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of rat brain mitochondrial MAOB using kynuramine as substrate assessed as decrease in 4-hydroxyquinoline production by spectrophotometry | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50409097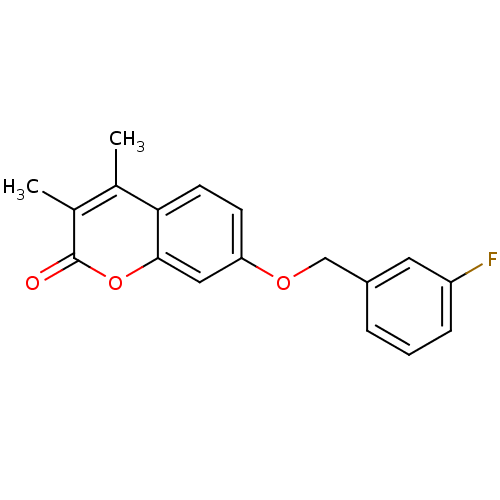 (CHEMBL108697) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of rat brain mitochondrial MAOB using kynuramine as substrate assessed as decrease in 4-hydroxyquinoline production by spectrophotometry | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50121268 (21-(2-Acetylamino-hexanoylamino)-7-[3-(diaminometh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I][Nle4,D-Phe7]alpha-MSH from human MC3 receptor expressed in HEK293 cells | J Med Chem 51: 187-95 (2008) Article DOI: 10.1021/jm070461w BindingDB Entry DOI: 10.7270/Q2V125N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50193832 (1-((3R,8aS)-3-benzyl-hexahydropyrrolo[1,2-a]pyrazi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from human MC1R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 5462-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.015 BindingDB Entry DOI: 10.7270/Q2XD11BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50184359 ((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC5R expressed in HEK293 cells after 40 mins by Wallac 1470 gamma counting | Bioorg Med Chem Lett 21: 3099-102 (2011) Article DOI: 10.1016/j.bmcl.2011.03.019 BindingDB Entry DOI: 10.7270/Q2BC3ZVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50409078 (CHEMBL325761) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50409097 (CHEMBL108697) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50193836 (1-((3R,8aS)-3-benzyl-hexahydropyrrolo[1,2-a]pyrazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from human MC3R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 5462-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.015 BindingDB Entry DOI: 10.7270/Q2XD11BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50199103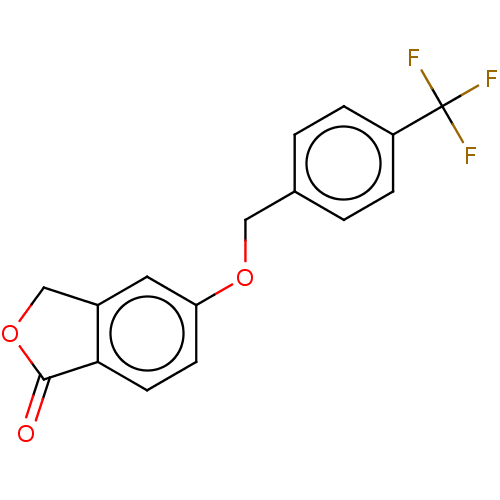 (CHEMBL3938837) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50282510 (7-(4-Chloro-benzyloxy)-3,4-dimethyl-chromen-2-one ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50184359 ((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from human MC5R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 5462-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.015 BindingDB Entry DOI: 10.7270/Q2XD11BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50017427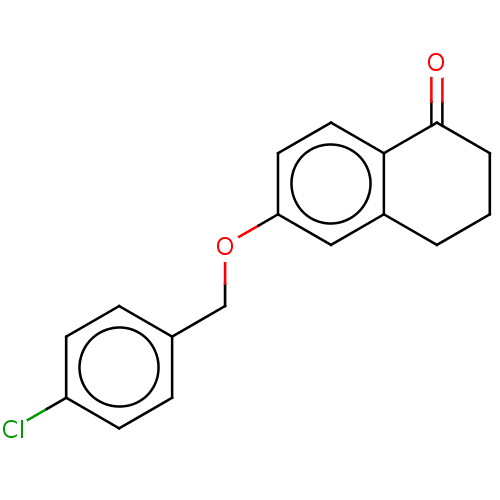 (CHEMBL3288295) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50027084 (Melatonan) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I][Nle4,D-Phe7]alpha-MSH from human MC5 receptor expressed in HEK293 cells | J Med Chem 51: 187-95 (2008) Article DOI: 10.1021/jm070461w BindingDB Entry DOI: 10.7270/Q2V125N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM10989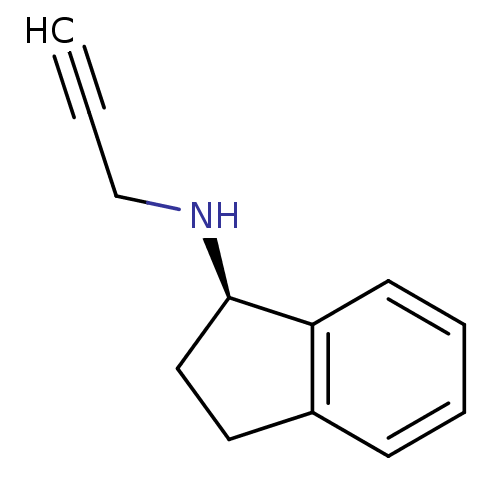 ((1R)-N-(prop-2-yn-1-yl)-2,3-dihydro-1H-inden-1-ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50184359 ((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC3R expressed in HEK293 cells after 40 mins by Wallac 1470 gamma counting | Bioorg Med Chem Lett 21: 3099-102 (2011) Article DOI: 10.1016/j.bmcl.2011.03.019 BindingDB Entry DOI: 10.7270/Q2BC3ZVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM92663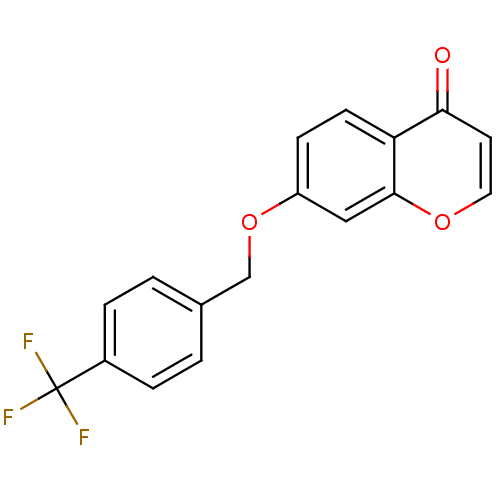 (C7-substituted chromone derivative, 3n) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B expressed in insect cell microsomes using kynuramine as substrate assessed as decrease in 4-hydroxyquinoline pr... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50199104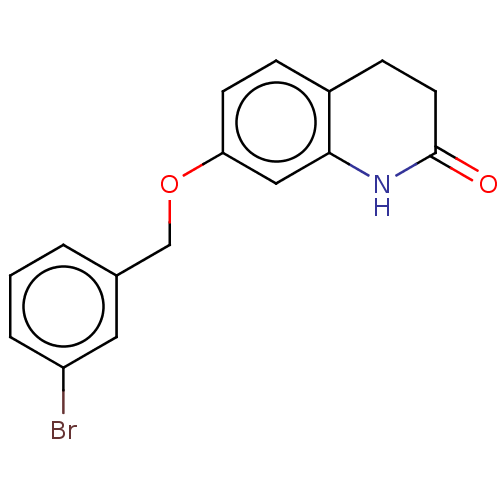 (CHEMBL2430707) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50172756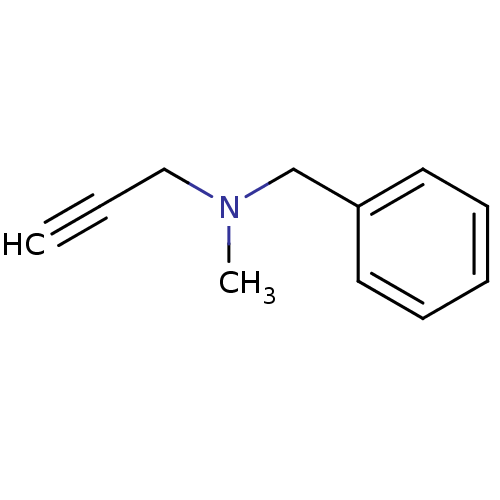 (Benzyl-methyl-prop-2-ynyl-amine | CHEMBL673 | Euto...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of MAO-B (unknown origin) | Eur J Med Chem 138: 715-728 (2017) Article DOI: 10.1016/j.ejmech.2017.07.008 BindingDB Entry DOI: 10.7270/Q2S46VKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50199106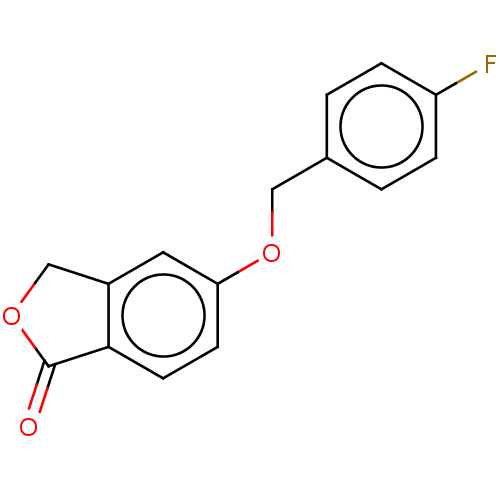 (CHEMBL3929816) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 180... | Eur J Med Chem 139: 68-83 (2017) Article DOI: 10.1016/j.ejmech.2017.07.077 BindingDB Entry DOI: 10.7270/Q2Q52S78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50230824 (CHEMBL398665 | c[CO-(CH2)3-CO-Pro-D-Nal(2)-Arg-Trp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I][Nle4,D-Phe7]alpha-MSH from human MC3 receptor expressed in HEK293 cells | J Med Chem 51: 187-95 (2008) Article DOI: 10.1021/jm070461w BindingDB Entry DOI: 10.7270/Q2V125N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50199095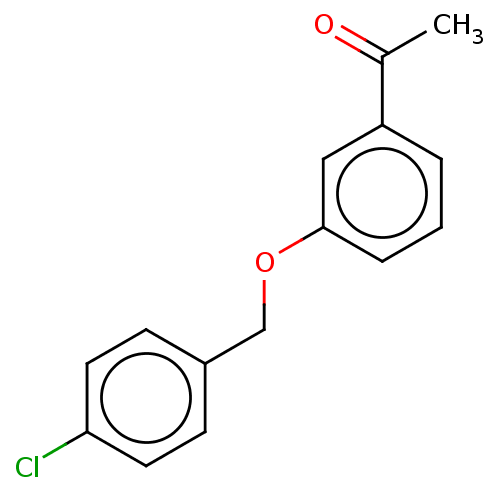 (CHEMBL3771110) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50172756 (Benzyl-methyl-prop-2-ynyl-amine | CHEMBL673 | Euto...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of MAO-A (unknown origin) | Eur J Med Chem 138: 715-728 (2017) Article DOI: 10.1016/j.ejmech.2017.07.008 BindingDB Entry DOI: 10.7270/Q2S46VKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50230816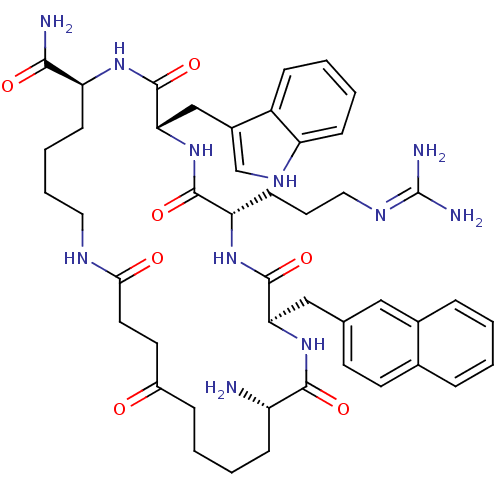 (CHEMBL267265 | c[CO-(CH2)2-CO-Nle-D-Nal(2)-Arg-Trp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I][Nle4,D-Phe7]alpha-MSH from human MC3 receptor expressed in HEK293 cells | J Med Chem 51: 187-95 (2008) Article DOI: 10.1021/jm070461w BindingDB Entry DOI: 10.7270/Q2V125N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 682 total ) | Next | Last >> |