 Found 2753 hits with Last Name = 'zhou' and Initial = 'p'
Found 2753 hits with Last Name = 'zhou' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50109062
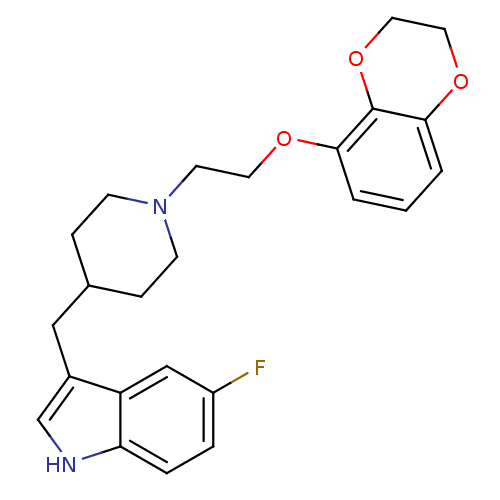
(3-{1-[2-(2,3-Dihydro-benzo[1,4]dioxin-5-yloxy)-eth...)Show SMILES Fc1ccc2[nH]cc(CC3CCN(CCOc4cccc5OCCOc45)CC3)c2c1 Show InChI InChI=1S/C24H27FN2O3/c25-19-4-5-21-20(15-19)18(16-26-21)14-17-6-8-27(9-7-17)10-11-28-22-2-1-3-23-24(22)30-13-12-29-23/h1-5,15-17,26H,6-14H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity of the compound for RB Serotonin transporter was determined in vitro by incubating compound |
Bioorg Med Chem Lett 12: 307-10 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G2M |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50493081
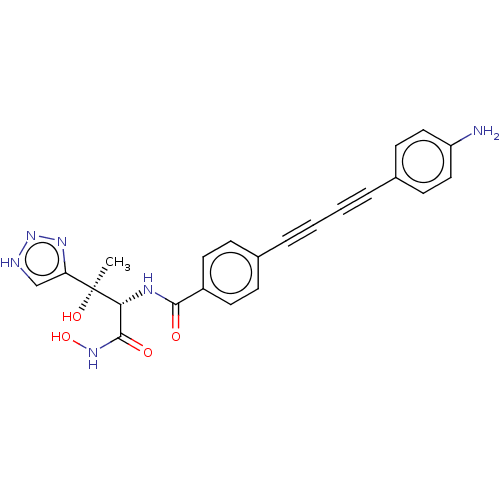
(CHEMBL2420205)Show SMILES C[C@@](O)([C@H](NC(=O)c1ccc(cc1)C#CC#Cc1ccc(N)cc1)C(=O)NO)c1c[nH]nn1 |r| Show InChI InChI=1S/C23H20N6O4/c1-23(32,19-14-25-29-27-19)20(22(31)28-33)26-21(30)17-10-6-15(7-11-17)4-2-3-5-16-8-12-18(24)13-9-16/h6-14,20,32-33H,24H2,1H3,(H,26,30)(H,28,31)(H,25,27,29)/t20-,23+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC enzyme using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetyl glucosamine and [gamma-32P] UDP-3-O-[(R)-3-hydroxymyristoy... |
J Med Chem 56: 6954-6966 (2013)
Article DOI: 10.1021/jm4007774
BindingDB Entry DOI: 10.7270/Q28K7D11 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50493080
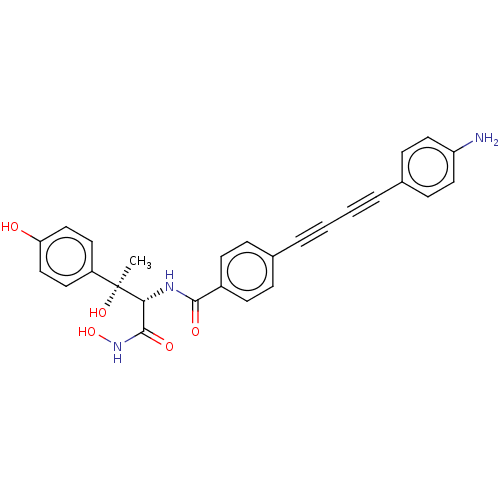
(CHEMBL2420203)Show SMILES C[C@@](O)([C@H](NC(=O)c1ccc(cc1)C#CC#Cc1ccc(N)cc1)C(=O)NO)c1ccc(O)cc1 |r| Show InChI InChI=1S/C27H23N3O5/c1-27(34,21-12-16-23(31)17-13-21)24(26(33)30-35)29-25(32)20-10-6-18(7-11-20)4-2-3-5-19-8-14-22(28)15-9-19/h6-17,24,31,34-35H,28H2,1H3,(H,29,32)(H,30,33)/t24-,27+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC enzyme using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetyl glucosamine and [gamma-32P] UDP-3-O-[(R)-3-hydroxymyristoy... |
J Med Chem 56: 6954-6966 (2013)
Article DOI: 10.1021/jm4007774
BindingDB Entry DOI: 10.7270/Q28K7D11 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50109058

(1H-4-indolyl 2-[4-(1H-3-indolyl)-1,2,3,6-tetrahydr...)Show SMILES C(CN1CCC(=CC1)c1c[nH]c2ccccc12)Oc1cccc2[nH]ccc12 |c:5| Show InChI InChI=1S/C23H23N3O/c1-2-5-21-18(4-1)20(16-25-21)17-9-12-26(13-10-17)14-15-27-23-7-3-6-22-19(23)8-11-24-22/h1-9,11,16,24-25H,10,12-15H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity of the compound for RB Serotonin transporter was determined in vitro by incubating compound |
Bioorg Med Chem Lett 12: 307-10 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G2M |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50074960

((R)-2-(3,4-Dimethoxy-5-propyl-phenyl)-4,5-dihydro-...)Show SMILES CCCc1cc(cc(OC)c1OC)C1=N[C@H](CO1)C(=O)NO |t:14| Show InChI InChI=1S/C15H20N2O5/c1-4-5-9-6-10(7-12(20-2)13(9)21-3)15-16-11(8-22-15)14(18)17-19/h6-7,11,19H,4-5,8H2,1-3H3,(H,17,18)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Proc Natl Acad Sci U S A 104: 18433-8 (2007)
Article DOI: 10.1073/pnas.0709412104
BindingDB Entry DOI: 10.7270/Q2SB48HR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50478376

(BB-78485 | CHEMBL261713)Show SMILES ONC(=O)[C@@H](Cc1ccc2ccccc2c1)NS(=O)(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C23H20N2O4S/c26-23(24-27)22(14-16-9-10-17-5-1-3-7-19(17)13-16)25-30(28,29)21-12-11-18-6-2-4-8-20(18)15-21/h1-13,15,22,25,27H,14H2,(H,24,26)/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Proc Natl Acad Sci U S A 104: 18433-8 (2007)
Article DOI: 10.1073/pnas.0709412104
BindingDB Entry DOI: 10.7270/Q2SB48HR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50150101
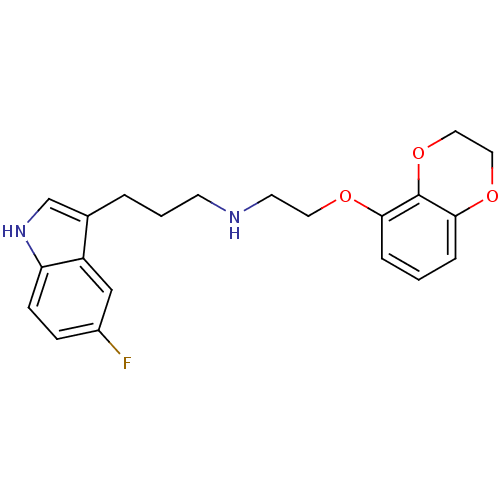
(CHEMBL419240 | [2-(2,3-Dihydro-benzo[1,4]dioxin-5-...)Show InChI InChI=1S/C21H23FN2O3/c22-16-6-7-18-17(13-16)15(14-24-18)3-2-8-23-9-10-25-19-4-1-5-20-21(19)27-12-11-26-20/h1,4-7,13-14,23-24H,2-3,8-12H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity against serotonin transporter in rat cortical tissues using radioligand [3H]-paroxetine |
J Med Chem 47: 3823-42 (2004)
Article DOI: 10.1021/jm0304010
BindingDB Entry DOI: 10.7270/Q2S46RDW |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50478376

(BB-78485 | CHEMBL261713)Show SMILES ONC(=O)[C@@H](Cc1ccc2ccccc2c1)NS(=O)(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C23H20N2O4S/c26-23(24-27)22(14-16-9-10-17-5-1-3-7-19(17)13-16)25-30(28,29)21-12-11-18-6-2-4-8-20(18)15-21/h1-13,15,22,25,27H,14H2,(H,24,26)/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC Q202W/G210S mutant |
Proc Natl Acad Sci U S A 104: 18433-8 (2007)
Article DOI: 10.1073/pnas.0709412104
BindingDB Entry DOI: 10.7270/Q2SB48HR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50102006
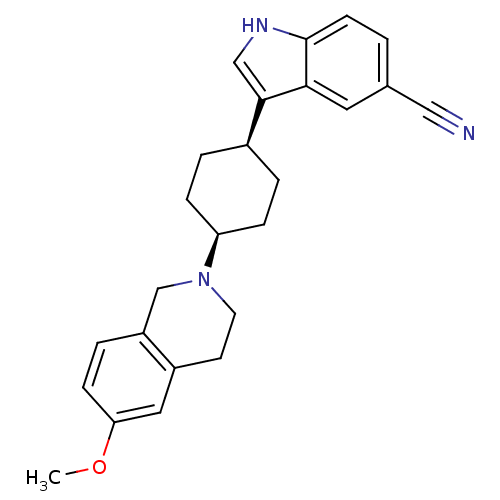
(3-[4-(6-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-c...)Show SMILES COc1ccc2CN(CCc2c1)[C@H]1CC[C@H](CC1)c1c[nH]c2ccc(cc12)C#N |r,wU:12.13,15.20,(10.58,5.27,;9.25,6.04,;7.91,5.27,;7.91,3.73,;6.57,2.97,;5.25,3.74,;3.93,2.97,;2.59,3.75,;2.59,5.28,;3.93,6.05,;5.26,5.28,;6.59,6.04,;1.26,2.98,;1.26,1.44,;-.07,.67,;-1.41,1.45,;-1.41,2.97,;-.08,3.75,;-2.74,.67,;-2.89,-.87,;-4.41,-1.2,;-5.19,.14,;-6.69,.45,;-7.18,1.91,;-6.15,3.07,;-4.64,2.76,;-4.16,1.3,;-6.63,4.53,;-7.11,5.99,)| Show InChI InChI=1S/C25H27N3O/c1-29-22-8-5-20-16-28(11-10-19(20)13-22)21-6-3-18(4-7-21)24-15-27-25-9-2-17(14-26)12-23(24)25/h2,5,8-9,12-13,15,18,21,27H,3-4,6-7,10-11,16H2,1H3/t18-,21+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro for its binding affinity for serotonin transporter RB5-HT-T in rat cortical membranes |
Bioorg Med Chem Lett 11: 1885-8 (2001)
BindingDB Entry DOI: 10.7270/Q2GM86K9 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50109057
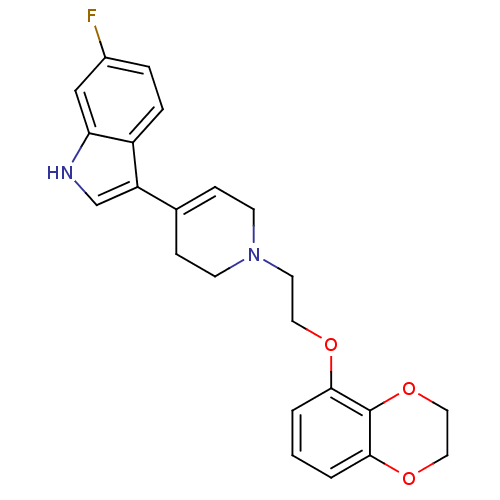
(3-{1-[2-(2,3-Dihydro-benzo[1,4]dioxin-5-yloxy)-eth...)Show SMILES Fc1ccc2c(c[nH]c2c1)C1=CCN(CCOc2cccc3OCCOc23)CC1 |t:12| Show InChI InChI=1S/C23H23FN2O3/c24-17-4-5-18-19(15-25-20(18)14-17)16-6-8-26(9-7-16)10-11-27-21-2-1-3-22-23(21)29-13-12-28-22/h1-6,14-15,25H,7-13H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity of the compound for RB Serotonin transporter was determined in vitro by incubating compound |
Bioorg Med Chem Lett 12: 307-10 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G2M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50109055
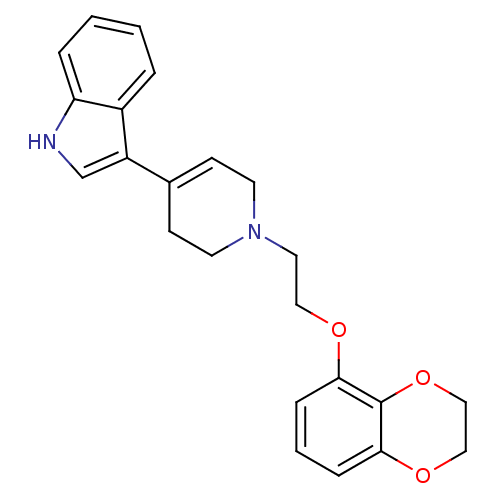
(3-{1-[2-(2,3-Dihydro-benzo[1,4]dioxin-5-yloxy)-eth...)Show SMILES C(CN1CCC(=CC1)c1c[nH]c2ccccc12)Oc1cccc2OCCOc12 |c:5| Show InChI InChI=1S/C23H24N2O3/c1-2-5-20-18(4-1)19(16-24-20)17-8-10-25(11-9-17)12-13-26-21-6-3-7-22-23(21)28-15-14-27-22/h1-8,16,24H,9-15H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity of the compound for RB Serotonin transporter was determined in vitro by incubating compound |
Bioorg Med Chem Lett 12: 307-10 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G2M |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50109058

(1H-4-indolyl 2-[4-(1H-3-indolyl)-1,2,3,6-tetrahydr...)Show SMILES C(CN1CCC(=CC1)c1c[nH]c2ccccc12)Oc1cccc2[nH]ccc12 |c:5| Show InChI InChI=1S/C23H23N3O/c1-2-5-21-18(4-1)20(16-25-21)17-9-12-26(13-10-17)14-15-27-23-7-3-6-22-19(23)8-11-24-22/h1-9,11,16,24-25H,10,12-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Alpha-1 adrenergic receptor was determined by the displacement of [3H]prazosin |
Bioorg Med Chem Lett 12: 307-10 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G2M |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483252

(CHEMBL1643369)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#CC#Cc1ccc(N)cc1)C(=O)NO |r| Show InChI InChI=1S/C21H19N3O4/c1-14(25)19(21(27)24-28)23-20(26)17-10-6-15(7-11-17)4-2-3-5-16-8-12-18(22)13-9-16/h6-14,19,25,28H,22H2,1H3,(H,23,26)(H,24,27)/t14-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem 19: 852-60 (2011)
Article DOI: 10.1016/j.bmc.2010.12.017
BindingDB Entry DOI: 10.7270/Q2DV1NQM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483252

(CHEMBL1643369)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#CC#Cc1ccc(N)cc1)C(=O)NO |r| Show InChI InChI=1S/C21H19N3O4/c1-14(25)19(21(27)24-28)23-20(26)17-10-6-15(7-11-17)4-2-3-5-16-8-12-18(22)13-9-16/h6-14,19,25,28H,22H2,1H3,(H,23,26)(H,24,27)/t14-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem 19: 852-60 (2011)
Article DOI: 10.1016/j.bmc.2010.12.017
BindingDB Entry DOI: 10.7270/Q2DV1NQM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228676
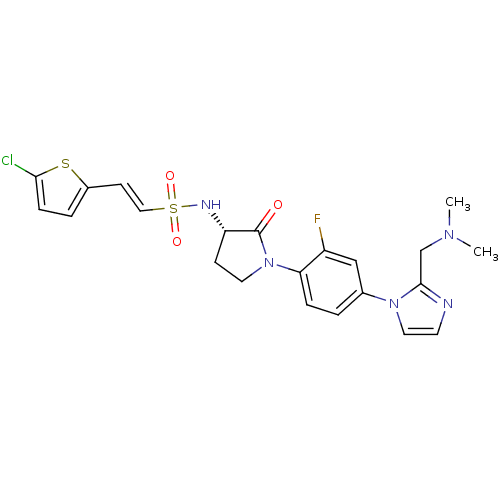
((S)-2-(5-chlorothiophen-2-yl)-N-(1-(4-(2-((dimethy...)Show SMILES CN(C)Cc1nccn1-c1ccc(N2CC[C@H](NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 Show InChI InChI=1S/C22H23ClFN5O3S2/c1-27(2)14-21-25-9-11-28(21)15-3-5-19(17(24)13-15)29-10-7-18(22(29)30)26-34(31,32)12-8-16-4-6-20(23)33-16/h3-6,8-9,11-13,18,26H,7,10,14H2,1-2H3/b12-8+/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by fluorescence assay |
Bioorg Med Chem Lett 20: 618-22 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.077
BindingDB Entry DOI: 10.7270/Q24M94NN |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50306153
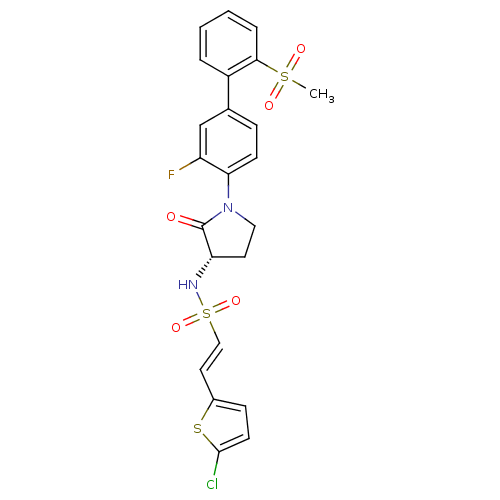
((S)-2-(5-chlorothiophen-2-yl)-N-(1-(3-fluoro-2'-(m...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(N2CC[C@H](NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 |r| Show InChI InChI=1S/C23H20ClFN2O5S3/c1-34(29,30)21-5-3-2-4-17(21)15-6-8-20(18(25)14-15)27-12-10-19(23(27)28)26-35(31,32)13-11-16-7-9-22(24)33-16/h2-9,11,13-14,19,26H,10,12H2,1H3/b13-11+/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by fluorescence assay |
Bioorg Med Chem Lett 20: 618-22 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.077
BindingDB Entry DOI: 10.7270/Q24M94NN |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228676

((S)-2-(5-chlorothiophen-2-yl)-N-(1-(4-(2-((dimethy...)Show SMILES CN(C)Cc1nccn1-c1ccc(N2CC[C@H](NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 Show InChI InChI=1S/C22H23ClFN5O3S2/c1-27(2)14-21-25-9-11-28(21)15-3-5-19(17(24)13-15)29-10-7-18(22(29)30)26-34(31,32)12-8-16-4-6-20(23)33-16/h3-6,8-9,11-13,18,26H,7,10,14H2,1-2H3/b12-8+/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 18: 28-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.019
BindingDB Entry DOI: 10.7270/Q29024PS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374259
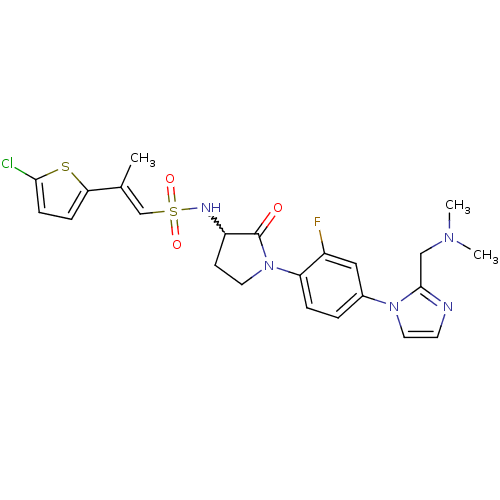
(CHEMBL257741)Show SMILES CN(C)Cc1nccn1-c1ccc(N2CCC(NS(=O)(=O)\C=C(/C)c3ccc(Cl)s3)C2=O)c(F)c1 |w:16.17| Show InChI InChI=1S/C23H25ClFN5O3S2/c1-15(20-6-7-21(24)34-20)14-35(32,33)27-18-8-10-30(23(18)31)19-5-4-16(12-17(19)25)29-11-9-26-22(29)13-28(2)3/h4-7,9,11-12,14,18,27H,8,10,13H2,1-3H3/b15-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 18: 28-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.019
BindingDB Entry DOI: 10.7270/Q29024PS |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50109060
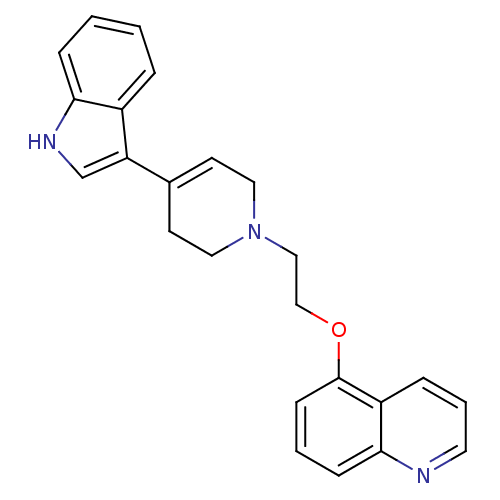
(5-{2-[4-(1H-Indol-3-yl)-3,6-dihydro-2H-pyridin-1-y...)Show SMILES C(CN1CCC(=CC1)c1c[nH]c2ccccc12)Oc1cccc2ncccc12 |c:5| Show InChI InChI=1S/C24H23N3O/c1-2-7-22-19(5-1)21(17-26-22)18-10-13-27(14-11-18)15-16-28-24-9-3-8-23-20(24)6-4-12-25-23/h1-10,12,17,26H,11,13-16H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity of the compound for RB Serotonin transporter was determined in vitro by incubating compound |
Bioorg Med Chem Lett 12: 307-10 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G2M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50316949
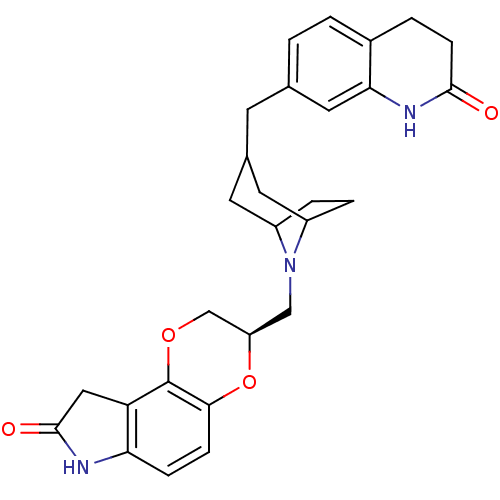
((3R)-3-((3-((2-oxo-1,2,3,4-tetrahydroquinolin-7-yl...)Show SMILES O=C1Cc2c(N1)ccc1O[C@H](CN3C4CCC3CC(Cc3ccc5CCC(=O)Nc5c3)C4)COc21 |r| Show InChI InChI=1S/C28H31N3O4/c32-26-8-3-18-2-1-16(12-24(18)30-26)9-17-10-19-4-5-20(11-17)31(19)14-21-15-34-28-22-13-27(33)29-23(22)6-7-25(28)35-21/h1-2,6-7,12,17,19-21H,3-5,8-11,13-15H2,(H,29,33)(H,30,32)/t17?,19?,20?,21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram form human SRET by liquid scintillation counting |
Bioorg Med Chem Lett 20: 2983-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.105
BindingDB Entry DOI: 10.7270/Q2VH5P0Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338686

((R/S)-3-chloro-N-((3S)-1-(1-(methylamino)-2,3-dihy...)Show SMILES CNC1CCc2cc(ccc12)N1CC[C@H](NS(=O)(=O)c2ccc3c(Cl)c[nH]c3c2)C1=O |r| Show InChI InChI=1S/C22H23ClN4O3S/c1-24-19-7-2-13-10-14(3-5-16(13)19)27-9-8-20(22(27)28)26-31(29,30)15-4-6-17-18(23)12-25-21(17)11-15/h3-6,10-12,19-20,24-26H,2,7-9H2,1H3/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50074960

((R)-2-(3,4-Dimethoxy-5-propyl-phenyl)-4,5-dihydro-...)Show SMILES CCCc1cc(cc(OC)c1OC)C1=N[C@H](CO1)C(=O)NO |t:14| Show InChI InChI=1S/C15H20N2O5/c1-4-5-9-6-10(7-12(20-2)13(9)21-3)15-16-11(8-22-15)14(18)17-19/h6-7,11,19H,4-5,8H2,1-3H3,(H,17,18)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC Q202W/G210S mutant |
Proc Natl Acad Sci U S A 104: 18433-8 (2007)
Article DOI: 10.1073/pnas.0709412104
BindingDB Entry DOI: 10.7270/Q2SB48HR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50150096
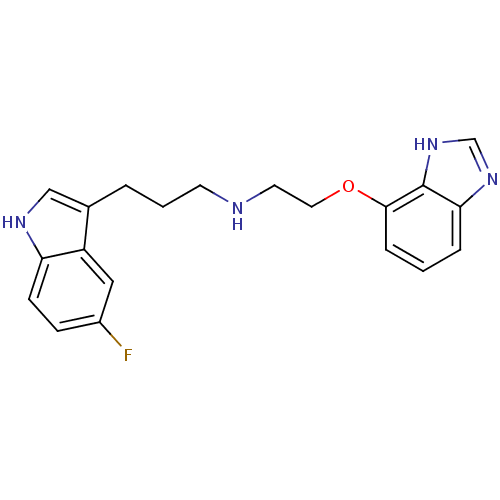
(CHEMBL330827 | [2-(1H-Benzoimidazol-4-yloxy)-ethyl...)Show InChI InChI=1S/C20H21FN4O/c21-15-6-7-17-16(11-15)14(12-23-17)3-2-8-22-9-10-26-19-5-1-4-18-20(19)25-13-24-18/h1,4-7,11-13,22-23H,2-3,8-10H2,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity against serotonin transporter in rat cortical tissues using radioligand [3H]-paroxetine |
J Med Chem 47: 3823-42 (2004)
Article DOI: 10.1021/jm0304010
BindingDB Entry DOI: 10.7270/Q2S46RDW |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50150103
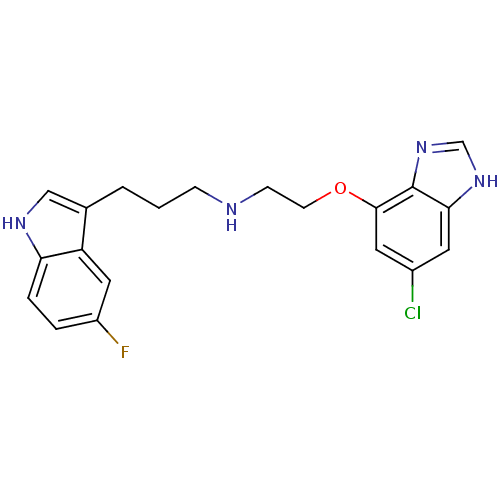
(CHEMBL338520 | [2-(6-Chloro-1H-benzoimidazol-4-ylo...)Show SMILES Fc1ccc2[nH]cc(CCCNCCOc3cc(Cl)cc4[nH]cnc34)c2c1 Show InChI InChI=1S/C20H20ClFN4O/c21-14-8-18-20(26-12-25-18)19(9-14)27-7-6-23-5-1-2-13-11-24-17-4-3-15(22)10-16(13)17/h3-4,8-12,23-24H,1-2,5-7H2,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity against serotonin transporter in rat cortical tissues using radioligand [3H]-paroxetine |
J Med Chem 47: 3823-42 (2004)
Article DOI: 10.1021/jm0304010
BindingDB Entry DOI: 10.7270/Q2S46RDW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374264

(CHEMBL257863)Show SMILES Fc1cc(ccc1N1CCC(NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O)-n1ccnc1CN1CCCC1 |w:10.11| Show InChI InChI=1S/C24H25ClFN5O3S2/c25-22-6-4-18(35-22)8-14-36(33,34)28-20-7-12-31(24(20)32)21-5-3-17(15-19(21)26)30-13-9-27-23(30)16-29-10-1-2-11-29/h3-6,8-9,13-15,20,28H,1-2,7,10-12,16H2/b14-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 18: 28-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.019
BindingDB Entry DOI: 10.7270/Q29024PS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50158032
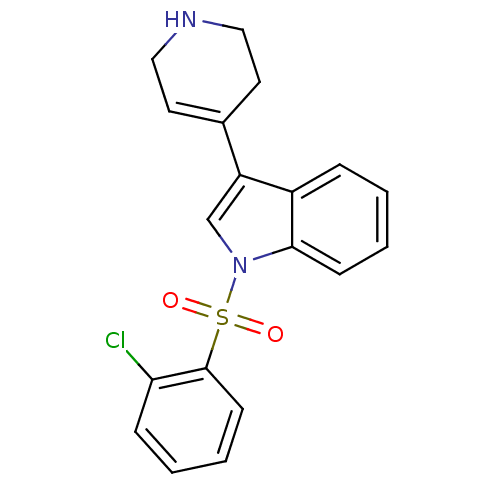
(1-(2-Chloro-benzenesulfonyl)-3-(1,2,3,6-tetrahydro...)Show SMILES Clc1ccccc1S(=O)(=O)n1cc(C2=CCNCC2)c2ccccc12 |t:14| Show InChI InChI=1S/C19H17ClN2O2S/c20-17-6-2-4-8-19(17)25(23,24)22-13-16(14-9-11-21-12-10-14)15-5-1-3-7-18(15)22/h1-9,13,21H,10-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity against cloned human 5-hydroxytryptamine 6 receptor expressed in HeLa cells using [3H]-LSD |
Bioorg Med Chem Lett 15: 379-83 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.064
BindingDB Entry DOI: 10.7270/Q20G3JNQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374262
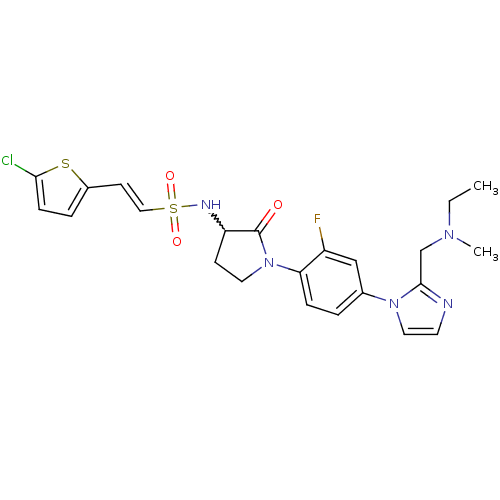
(CHEMBL272779)Show SMILES CCN(C)Cc1nccn1-c1ccc(N2CCC(NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 |w:17.18| Show InChI InChI=1S/C23H25ClFN5O3S2/c1-3-28(2)15-22-26-10-12-29(22)16-4-6-20(18(25)14-16)30-11-8-19(23(30)31)27-35(32,33)13-9-17-5-7-21(24)34-17/h4-7,9-10,12-14,19,27H,3,8,11,15H2,1-2H3/b13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 18: 28-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.019
BindingDB Entry DOI: 10.7270/Q29024PS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM294039
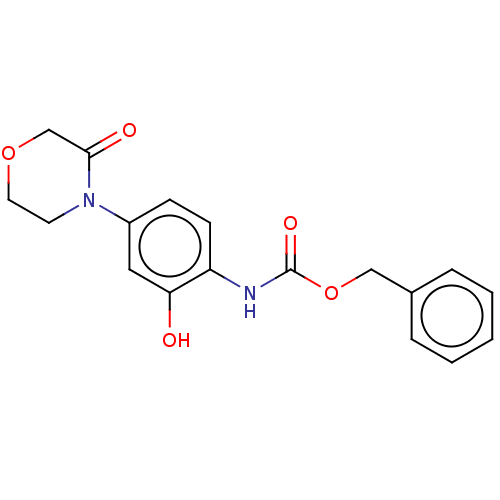
(Process 1 | US10106557, Compound 1)Show InChI InChI=1S/C18H18N2O5/c21-16-10-14(20-8-9-24-12-17(20)22)6-7-15(16)19-18(23)25-11-13-4-2-1-3-5-13/h1-7,10,21H,8-9,11-12H2,(H,19,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
NORTH CHINA PHARMACEUTICAL NEW DRUG R&D CO., LTD.
US Patent
| Assay Description
The activity of the compound to be tested against prothrombinase was determined by the production of thrombin. In summary, 12.5 μL human factor ... |
US Patent US10106557 (2018)
BindingDB Entry DOI: 10.7270/Q2X63Q0W |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50150105
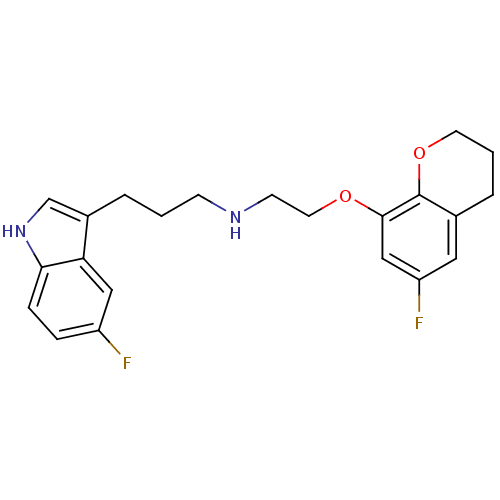
(CHEMBL124069 | [2-(6-Fluoro-chroman-8-yloxy)-ethyl...)Show SMILES Fc1cc2CCCOc2c(OCCNCCCc2c[nH]c3ccc(F)cc23)c1 Show InChI InChI=1S/C22H24F2N2O2/c23-17-5-6-20-19(12-17)16(14-26-20)3-1-7-25-8-10-27-21-13-18(24)11-15-4-2-9-28-22(15)21/h5-6,11-14,25-26H,1-4,7-10H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity against serotonin transporter in rat cortical tissues using radioligand [3H]-paroxetine |
J Med Chem 47: 3823-42 (2004)
Article DOI: 10.1021/jm0304010
BindingDB Entry DOI: 10.7270/Q2S46RDW |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50150082
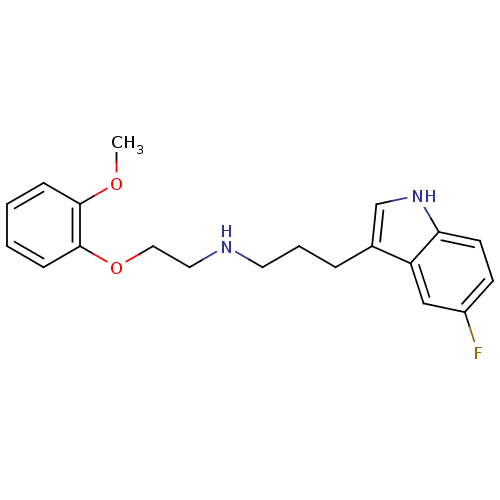
(CHEMBL126484 | [3-(5-Fluoro-1H-indol-3-yl)-propyl]...)Show InChI InChI=1S/C20H23FN2O2/c1-24-19-6-2-3-7-20(19)25-12-11-22-10-4-5-15-14-23-18-9-8-16(21)13-17(15)18/h2-3,6-9,13-14,22-23H,4-5,10-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity against serotonin transporter in rat cortical tissues using radioligand [3H]-paroxetine |
J Med Chem 47: 3823-42 (2004)
Article DOI: 10.1021/jm0304010
BindingDB Entry DOI: 10.7270/Q2S46RDW |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50150099

(CHEMBL339490 | [2-(7-Fluoro-2,3-dihydro-benzo[1,4]...)Show SMILES Fc1ccc2[nH]cc(CCCNCCOc3cc(F)cc4OCCOc34)c2c1 Show InChI InChI=1S/C21H22F2N2O3/c22-15-3-4-18-17(10-15)14(13-25-18)2-1-5-24-6-7-26-19-11-16(23)12-20-21(19)28-9-8-27-20/h3-4,10-13,24-25H,1-2,5-9H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity against serotonin transporter in rat cortical tissues using radioligand [3H]-paroxetine |
J Med Chem 47: 3823-42 (2004)
Article DOI: 10.1021/jm0304010
BindingDB Entry DOI: 10.7270/Q2S46RDW |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50200120

(CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C24H27N3O5/c1-17(28)22(24(30)26-31)25-23(29)21-10-8-19(9-11-21)3-2-18-4-6-20(7-5-18)16-27-12-14-32-15-13-27/h4-11,17,22,28,31H,12-16H2,1H3,(H,25,29)(H,26,30)/t17-,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Proc Natl Acad Sci U S A 104: 18433-8 (2007)
Article DOI: 10.1073/pnas.0709412104
BindingDB Entry DOI: 10.7270/Q2SB48HR |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50200120

(CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C24H27N3O5/c1-17(28)22(24(30)26-31)25-23(29)21-10-8-19(9-11-21)3-2-18-4-6-20(7-5-18)16-27-12-14-32-15-13-27/h4-11,17,22,28,31H,12-16H2,1H3,(H,25,29)(H,26,30)/t17-,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC enzyme |
J Med Chem 56: 6954-6966 (2013)
Article DOI: 10.1021/jm4007774
BindingDB Entry DOI: 10.7270/Q28K7D11 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374270
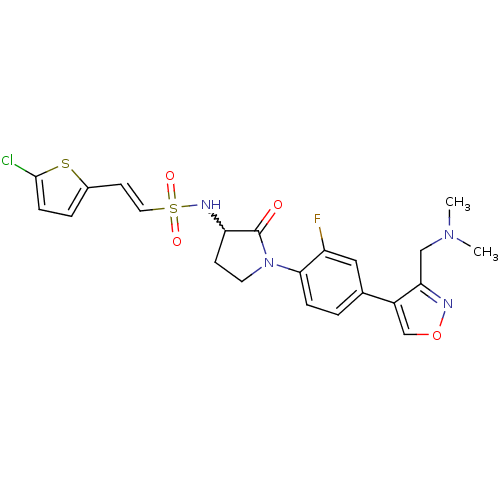
(CHEMBL271015)Show SMILES CN(C)Cc1nocc1-c1ccc(N2CCC(NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 |w:16.17| Show InChI InChI=1S/C22H22ClFN4O4S2/c1-27(2)12-19-16(13-32-25-19)14-3-5-20(17(24)11-14)28-9-7-18(22(28)29)26-34(30,31)10-8-15-4-6-21(23)33-15/h3-6,8,10-11,13,18,26H,7,9,12H2,1-2H3/b10-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 18: 28-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.019
BindingDB Entry DOI: 10.7270/Q29024PS |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219760
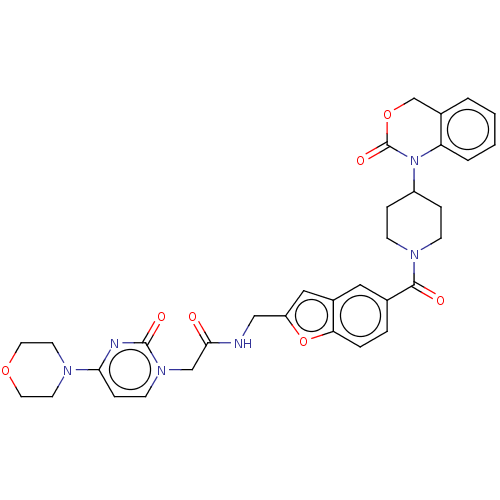
(CHEMBL286895)Show SMILES O=C(Cn1ccc(nc1=O)N1CCOCC1)NCc1cc2cc(ccc2o1)C(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C33H34N6O7/c40-30(20-38-12-9-29(35-32(38)42)36-13-15-44-16-14-36)34-19-26-18-24-17-22(5-6-28(24)46-26)31(41)37-10-7-25(8-11-37)39-27-4-2-1-3-23(27)21-45-33(39)43/h1-6,9,12,17-18,25H,7-8,10-11,13-16,19-21H2,(H,34,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50150107
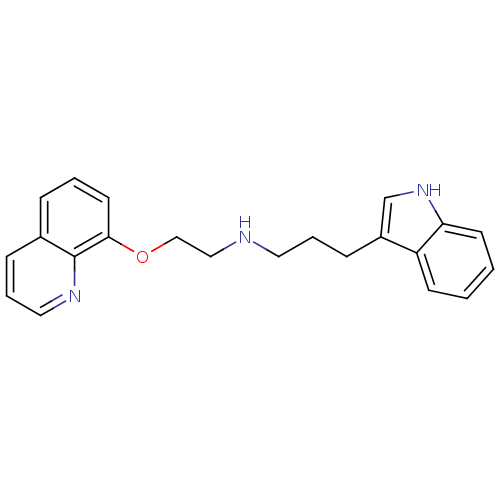
(CHEMBL340705 | [3-(1H-Indol-3-yl)-propyl]-[2-(quin...)Show InChI InChI=1S/C22H23N3O/c1-2-10-20-19(9-1)18(16-25-20)8-4-12-23-14-15-26-21-11-3-6-17-7-5-13-24-22(17)21/h1-3,5-7,9-11,13,16,23,25H,4,8,12,14-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity against human 5-hydroxytryptamine 1A receptor in CHO cells labeled with [3H]-8-OH-DPAT radioligand |
J Med Chem 47: 3823-42 (2004)
Article DOI: 10.1021/jm0304010
BindingDB Entry DOI: 10.7270/Q2S46RDW |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM92267

(CS257)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#CC#Cc1ccccc1)C(=O)NO Show InChI InChI=1S/C21H18N2O4/c1-15(24)19(21(26)23-27)22-20(25)18-13-11-17(12-14-18)10-6-5-9-16-7-3-2-4-8-16/h2-4,7-8,11-15,19,24,27H,1H3,(H,22,25)(H,23,26)/t15-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem 19: 852-60 (2011)
Article DOI: 10.1016/j.bmc.2010.12.017
BindingDB Entry DOI: 10.7270/Q2DV1NQM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM92267

(CS257)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#CC#Cc1ccccc1)C(=O)NO Show InChI InChI=1S/C21H18N2O4/c1-15(24)19(21(26)23-27)22-20(25)18-13-11-17(12-14-18)10-6-5-9-16-7-3-2-4-8-16/h2-4,7-8,11-15,19,24,27H,1H3,(H,22,25)(H,23,26)/t15-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem 19: 852-60 (2011)
Article DOI: 10.1016/j.bmc.2010.12.017
BindingDB Entry DOI: 10.7270/Q2DV1NQM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339718
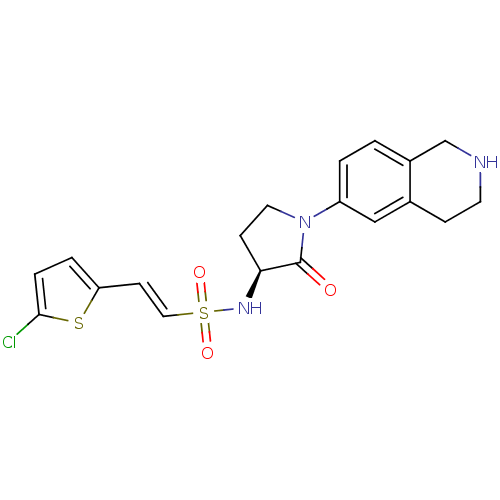
((S)-2-(5-chlorothiophen-2-yl)-N-(2-oxo-1-(1,2,3,4-...)Show SMILES Clc1ccc(\C=C\S(=O)(=O)N[C@H]2CCN(C2=O)c2ccc3CNCCc3c2)s1 |r| Show InChI InChI=1S/C19H20ClN3O3S2/c20-18-4-3-16(27-18)7-10-28(25,26)22-17-6-9-23(19(17)24)15-2-1-14-12-21-8-5-13(14)11-15/h1-4,7,10-11,17,21-22H,5-6,8-9,12H2/b10-7+/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374263
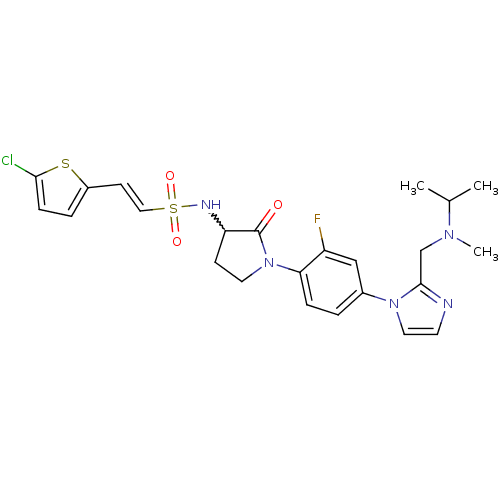
(CHEMBL437097)Show SMILES CC(C)N(C)Cc1nccn1-c1ccc(N2CCC(NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 |w:18.19| Show InChI InChI=1S/C24H27ClFN5O3S2/c1-16(2)29(3)15-23-27-10-12-30(23)17-4-6-21(19(26)14-17)31-11-8-20(24(31)32)28-36(33,34)13-9-18-5-7-22(25)35-18/h4-7,9-10,12-14,16,20,28H,8,11,15H2,1-3H3/b13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 18: 28-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.019
BindingDB Entry DOI: 10.7270/Q29024PS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328712
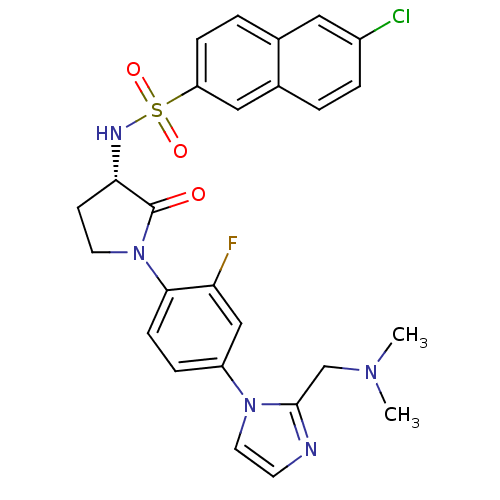
((S)-6-chloro-N-(1-(4-(2-((dimethylamino)methyl)-1H...)Show SMILES CN(C)Cc1nccn1-c1ccc(N2CC[C@H](NS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C2=O)c(F)c1 |r| Show InChI InChI=1S/C26H25ClFN5O3S/c1-31(2)16-25-29-10-12-32(25)20-6-8-24(22(28)15-20)33-11-9-23(26(33)34)30-37(35,36)21-7-4-17-13-19(27)5-3-18(17)14-21/h3-8,10,12-15,23,30H,9,11,16H2,1-2H3/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 18: 28-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.019
BindingDB Entry DOI: 10.7270/Q29024PS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339708

((S)-2-(5-chlorothiophen-2-yl)-N-(1-(5-fluoro-1,2,3...)Show SMILES Fc1c2CCNCc2ccc1N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |r| Show InChI InChI=1S/C19H19ClFN3O3S2/c20-17-4-2-13(28-17)7-10-29(26,27)23-15-6-9-24(19(15)25)16-3-1-12-11-22-8-5-14(12)18(16)21/h1-4,7,10,15,22-23H,5-6,8-9,11H2/b10-7+/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50102023

(3-[4-(5-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-c...)Show SMILES COc1cccc2CN(CCc12)[C@H]1CC[C@H](CC1)c1c[nH]c2ccc(cc12)C#N |wU:12.13,15.20,(4.13,-4.89,;3.36,-6.22,;4.13,-7.55,;3.35,-8.88,;4.13,-10.22,;5.66,-10.22,;6.42,-8.89,;7.97,-8.9,;8.74,-7.57,;7.97,-6.22,;6.43,-6.22,;5.66,-7.55,;10.27,-7.57,;11.06,-6.22,;12.59,-6.24,;13.36,-7.57,;12.59,-8.9,;11.04,-8.9,;14.9,-7.57,;15.37,-9.04,;16.92,-9.03,;17.39,-7.57,;18.77,-6.94,;18.95,-5.4,;17.69,-4.5,;16.3,-5.13,;16.14,-6.67,;17.84,-2.96,;17.99,-1.43,)| Show InChI InChI=1S/C25H27N3O/c1-29-25-4-2-3-19-16-28(12-11-21(19)25)20-8-6-18(7-9-20)23-15-27-24-10-5-17(14-26)13-22(23)24/h2-5,10,13,15,18,20,27H,6-9,11-12,16H2,1H3/t18-,20+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro for its binding affinity for serotonin transporter RB5-HT-T in rat cortical membranes |
Bioorg Med Chem Lett 11: 1885-8 (2001)
BindingDB Entry DOI: 10.7270/Q2GM86K9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50369890
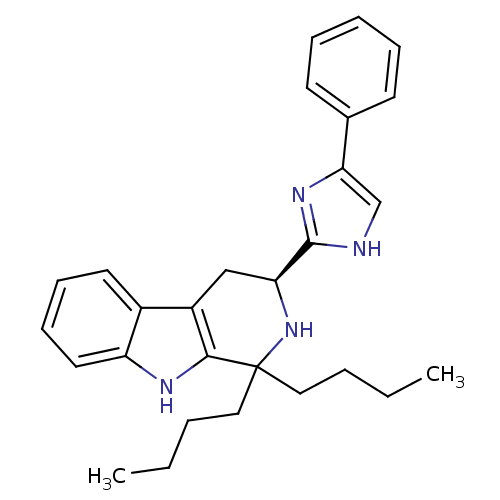
(CHEMBL1237140 | CHEMBL1788167)Show SMILES CCCCC1(CCCC)N[C@@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C28H34N4/c1-3-5-16-28(17-6-4-2)26-22(21-14-10-11-15-23(21)30-26)18-24(32-28)27-29-19-25(31-27)20-12-8-7-9-13-20/h7-15,19,24,30,32H,3-6,16-18H2,1-2H3,(H,29,31)/t24-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50389603

(CHEMBL2069499)Show SMILES CCCCC1(CCCC)N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C28H34N4/c1-3-5-16-28(17-6-4-2)26-22(21-14-10-11-15-23(21)30-26)18-24(32-28)27-29-19-25(31-27)20-12-8-7-9-13-20/h7-15,19,24,30,32H,3-6,16-18H2,1-2H3,(H,29,31)/t24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human SST3 receptor expressed in CHO cells after 60 mins by scintillation counting |
ACS Med Chem Lett 3: 289-293 (2012)
Article DOI: 10.1021/ml200272z
BindingDB Entry DOI: 10.7270/Q2K938MH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50150096

(CHEMBL330827 | [2-(1H-Benzoimidazol-4-yloxy)-ethyl...)Show InChI InChI=1S/C20H21FN4O/c21-15-6-7-17-16(11-15)14(12-23-17)3-2-8-22-9-10-26-19-5-1-4-18-20(19)25-13-24-18/h1,4-7,11-13,22-23H,2-3,8-10H2,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity against human 5-hydroxytryptamine 1A receptor in CHO cells labeled with [3H]-8-OH-DPAT radioligand |
J Med Chem 47: 3823-42 (2004)
Article DOI: 10.1021/jm0304010
BindingDB Entry DOI: 10.7270/Q2S46RDW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50150101

(CHEMBL419240 | [2-(2,3-Dihydro-benzo[1,4]dioxin-5-...)Show InChI InChI=1S/C21H23FN2O3/c22-16-6-7-18-17(13-16)15(14-24-18)3-2-8-23-9-10-25-19-4-1-5-20-21(19)27-12-11-26-20/h1,4-7,13-14,23-24H,2-3,8-12H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity against human 5-hydroxytryptamine 1A receptor in CHO cells labeled with [3H]-8-OH-DPAT radioligand |
J Med Chem 47: 3823-42 (2004)
Article DOI: 10.1021/jm0304010
BindingDB Entry DOI: 10.7270/Q2S46RDW |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Rhizobium leguminosarum bv. trifolii (strain WSM13...) | BDBM50478376

(BB-78485 | CHEMBL261713)Show SMILES ONC(=O)[C@@H](Cc1ccc2ccccc2c1)NS(=O)(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C23H20N2O4S/c26-23(24-27)22(14-16-9-10-17-5-1-3-7-19(17)13-16)25-30(28,29)21-12-11-18-6-2-4-8-20(18)15-21/h1-13,15,22,25,27H,14H2,(H,24,26)/t22-/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of Rhizobium leguminosarum LpxC W206Q/S214G mutant |
Proc Natl Acad Sci U S A 104: 18433-8 (2007)
Article DOI: 10.1073/pnas.0709412104
BindingDB Entry DOI: 10.7270/Q2SB48HR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50306142
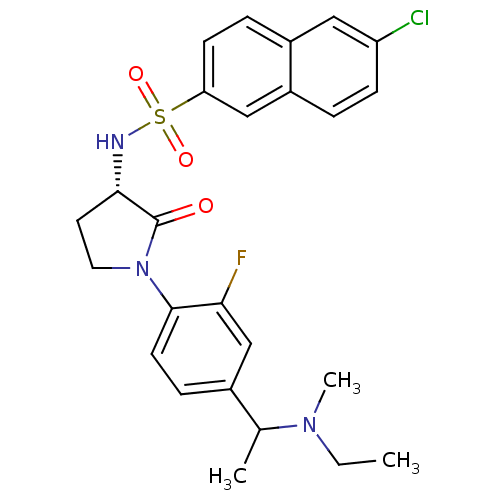
(6-chloro-N-((3S)-1-(4-(1-(ethyl(methyl)amino)ethyl...)Show SMILES CCN(C)C(C)c1ccc(N2CC[C@H](NS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C2=O)c(F)c1 |r| Show InChI InChI=1S/C25H27ClFN3O3S/c1-4-29(3)16(2)17-7-10-24(22(27)15-17)30-12-11-23(25(30)31)28-34(32,33)21-9-6-18-13-20(26)8-5-19(18)14-21/h5-10,13-16,23,28H,4,11-12H2,1-3H3/t16?,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by fluorescence assay |
Bioorg Med Chem Lett 20: 618-22 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.077
BindingDB Entry DOI: 10.7270/Q24M94NN |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50306138
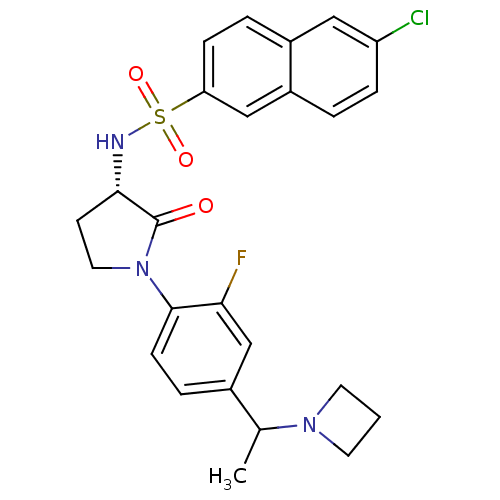
(CHEMBL604887 | N-((3S)-1-(4-(1-(azetidin-1-yl)ethy...)Show SMILES CC(N1CCC1)c1ccc(N2CC[C@H](NS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C2=O)c(F)c1 |r| Show InChI InChI=1S/C25H25ClFN3O3S/c1-16(29-10-2-11-29)17-5-8-24(22(27)15-17)30-12-9-23(25(30)31)28-34(32,33)21-7-4-18-13-20(26)6-3-19(18)14-21/h3-8,13-16,23,28H,2,9-12H2,1H3/t16?,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by fluorescence assay |
Bioorg Med Chem Lett 20: 618-22 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.077
BindingDB Entry DOI: 10.7270/Q24M94NN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data