 Found 205 hits with Last Name = 'bury' and Initial = 'ps'
Found 205 hits with Last Name = 'bury' and Initial = 'ps' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Displacement of [3H]17-beta-estradiol from Estrogen receptor of rabbit uterine tissue |
Bioorg Med Chem Lett 12: 17-9 (2002)
BindingDB Entry DOI: 10.7270/Q2X350NF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory activity against Estrogen receptor by displacement of [3H]17-beta-estradiol in ER-rich cytosol from rabbit uterine tissue |
Bioorg Med Chem Lett 10: 2383-6 (2000)
BindingDB Entry DOI: 10.7270/Q28G8NWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity for estrogen receptor of rabbit uterine skeletal muscle tissue |
Bioorg Med Chem Lett 10: 399-402 (2000)
BindingDB Entry DOI: 10.7270/Q2RF5X6P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50218372
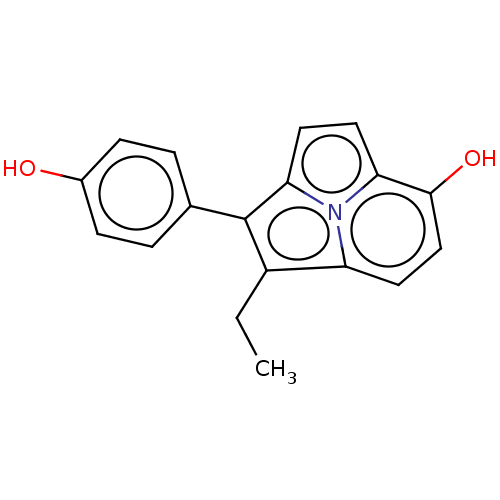
(CHEMBL80124)Show InChI InChI=1S/C18H15NO2/c1-2-13-14-9-10-17(21)15-7-8-16(19(14)15)18(13)11-3-5-12(20)6-4-11/h3-10,20-21H,2H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory activity against Estrogen receptor by displacement of [3H]17-beta-estradiol in ER-rich cytosol from rabbit uterine tissue |
Bioorg Med Chem Lett 10: 2383-6 (2000)
BindingDB Entry DOI: 10.7270/Q28G8NWG |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50218382

(CHEMBL76909)Show InChI InChI=1S/C18H15NO2/c1-2-15-17-10-14(21)9-12-5-8-16(19(12)17)18(15)11-3-6-13(20)7-4-11/h3-10,20-21H,2H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory activity against Estrogen receptor by displacement of [3H]17-beta-estradiol in ER-rich cytosol from rabbit uterine tissue |
Bioorg Med Chem Lett 10: 2383-6 (2000)
BindingDB Entry DOI: 10.7270/Q28G8NWG |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50218560
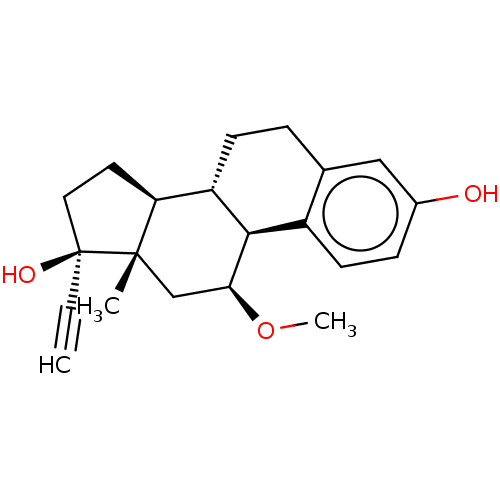
(CHEBI:34857 | Metoxiestrol | Moxestrol | R-2858)Show SMILES [H][C@@]12CC[C@@](O)(C#C)[C@@]1(C)C[C@H](OC)[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C21H26O3/c1-4-21(23)10-9-17-16-7-5-13-11-14(22)6-8-15(13)19(16)18(24-3)12-20(17,21)2/h1,6,8,11,16-19,22-23H,5,7,9-10,12H2,2-3H3/t16-,17-,18-,19+,20-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity for estrogen receptor of rabbit uterine skeletal muscle tissue |
Bioorg Med Chem Lett 10: 399-402 (2000)
BindingDB Entry DOI: 10.7270/Q2RF5X6P |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50218560

(CHEBI:34857 | Metoxiestrol | Moxestrol | R-2858)Show SMILES [H][C@@]12CC[C@@](O)(C#C)[C@@]1(C)C[C@H](OC)[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C21H26O3/c1-4-21(23)10-9-17-16-7-5-13-11-14(22)6-8-15(13)19(16)18(24-3)12-20(17,21)2/h1,6,8,11,16-19,22-23H,5,7,9-10,12H2,2-3H3/t16-,17-,18-,19+,20-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Displacement of [3H]17-beta-estradiol from Estrogen receptor of rabbit uterine tissue |
Bioorg Med Chem Lett 12: 17-9 (2002)
BindingDB Entry DOI: 10.7270/Q2X350NF |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50219402
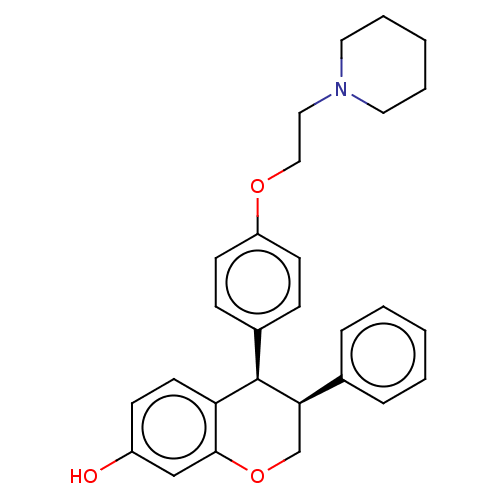
(CHEMBL299942)Show SMILES Oc1ccc2[C@H]([C@H](COc2c1)c1ccccc1)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H31NO3/c30-23-11-14-25-27(19-23)32-20-26(21-7-3-1-4-8-21)28(25)22-9-12-24(13-10-22)31-18-17-29-15-5-2-6-16-29/h1,3-4,7-14,19,26,28,30H,2,5-6,15-18,20H2/t26-,28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Displacement of [3H]17-beta-estradiol from Estrogen receptor of rabbit uterine tissue |
Bioorg Med Chem Lett 12: 17-9 (2002)
BindingDB Entry DOI: 10.7270/Q2X350NF |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50218327
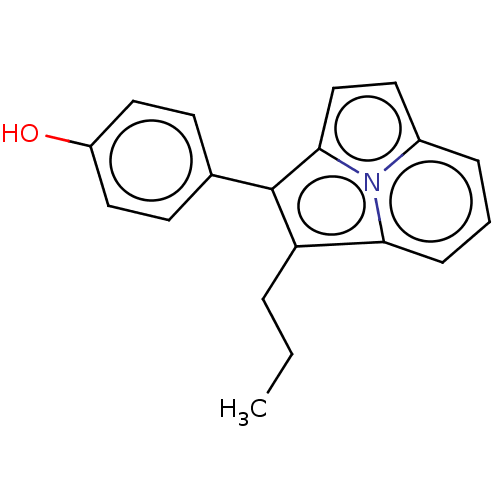
(CHEMBL312193)Show InChI InChI=1S/C19H17NO/c1-2-4-16-17-6-3-5-14-9-12-18(20(14)17)19(16)13-7-10-15(21)11-8-13/h3,5-12,21H,2,4H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory activity against Estrogen receptor by displacement of [3H]17-beta-estradiol in ER-rich cytosol from rabbit uterine tissue |
Bioorg Med Chem Lett 10: 2383-6 (2000)
BindingDB Entry DOI: 10.7270/Q28G8NWG |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50219400
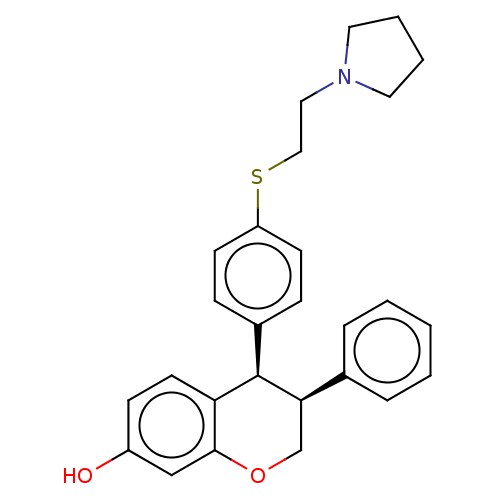
(CHEMBL554789)Show SMILES Cl.Oc1ccc2[C@H]([C@H](COc2c1)c1ccccc1)c1ccc(SCCN2CCCC2)cc1 |r| Show InChI InChI=1S/C27H29NO2S.ClH/c29-22-10-13-24-26(18-22)30-19-25(20-6-2-1-3-7-20)27(24)21-8-11-23(12-9-21)31-17-16-28-14-4-5-15-28;/h1-3,6-13,18,25,27,29H,4-5,14-17,19H2;1H/t25-,27-;/m1./s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Displacement of [3H]17-beta-estradiol from Estrogen receptor of rabbit uterine tissue |
Bioorg Med Chem Lett 12: 17-9 (2002)
BindingDB Entry DOI: 10.7270/Q2X350NF |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50219403
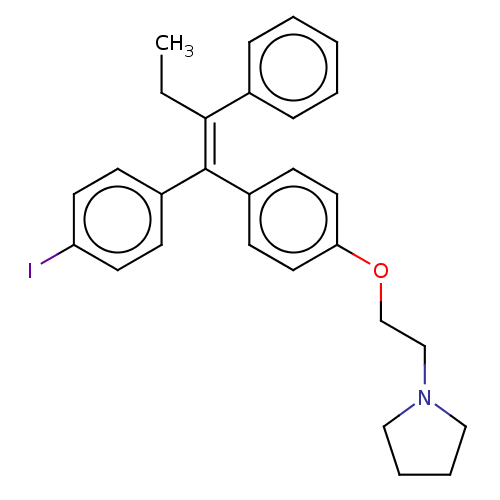
(CB-7432 | Idoxifene | SB-223030)Show SMILES CC\C(=C(/c1ccc(I)cc1)c1ccc(OCCN2CCCC2)cc1)c1ccccc1 Show InChI InChI=1S/C28H30INO/c1-2-27(22-8-4-3-5-9-22)28(23-10-14-25(29)15-11-23)24-12-16-26(17-13-24)31-21-20-30-18-6-7-19-30/h3-5,8-17H,2,6-7,18-21H2,1H3/b28-27- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Displacement of [3H]17-beta-estradiol from Estrogen receptor of rabbit uterine tissue |
Bioorg Med Chem Lett 12: 17-9 (2002)
BindingDB Entry DOI: 10.7270/Q2X350NF |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM19441

(2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...)Show SMILES Oc1ccc(cc1)-c1sc2cc(O)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H27NO4S/c30-21-8-4-20(5-9-21)28-26(24-13-10-22(31)18-25(24)34-28)27(32)19-6-11-23(12-7-19)33-17-16-29-14-2-1-3-15-29/h4-13,18,30-31H,1-3,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Displacement of [3H]17-beta-estradiol from Estrogen receptor of rabbit uterine tissue |
Bioorg Med Chem Lett 12: 17-9 (2002)
BindingDB Entry DOI: 10.7270/Q2X350NF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50218330
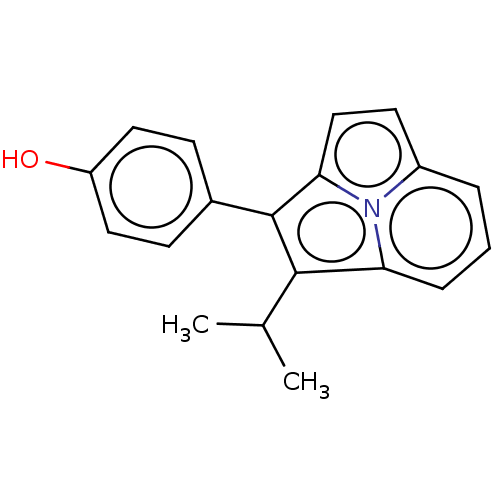
(CHEMBL312655)Show InChI InChI=1S/C19H17NO/c1-12(2)18-16-5-3-4-14-8-11-17(20(14)16)19(18)13-6-9-15(21)10-7-13/h3-12,21H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory activity against Estrogen receptor by displacement of [3H]17-beta-estradiol in ER-rich cytosol from rabbit uterine tissue |
Bioorg Med Chem Lett 10: 2383-6 (2000)
BindingDB Entry DOI: 10.7270/Q28G8NWG |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50085657
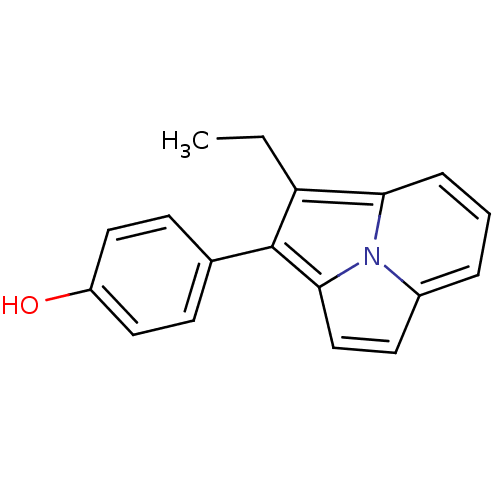
(4-(1-Ethyl-pyrrolo[2,1,5-cd]indolizin-2-yl)-phenol...)Show InChI InChI=1S/C18H15NO/c1-2-15-16-5-3-4-13-8-11-17(19(13)16)18(15)12-6-9-14(20)10-7-12/h3-11,20H,2H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory activity against Estrogen receptor by displacement of [3H]17-beta-estradiol in ER-rich cytosol from rabbit uterine tissue |
Bioorg Med Chem Lett 10: 2383-6 (2000)
BindingDB Entry DOI: 10.7270/Q28G8NWG |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50085657

(4-(1-Ethyl-pyrrolo[2,1,5-cd]indolizin-2-yl)-phenol...)Show InChI InChI=1S/C18H15NO/c1-2-15-16-5-3-4-13-8-11-17(19(13)16)18(15)12-6-9-14(20)10-7-12/h3-11,20H,2H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity for estrogen receptor of rabbit uterine skeletal muscle tissue |
Bioorg Med Chem Lett 10: 399-402 (2000)
BindingDB Entry DOI: 10.7270/Q2RF5X6P |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50219399
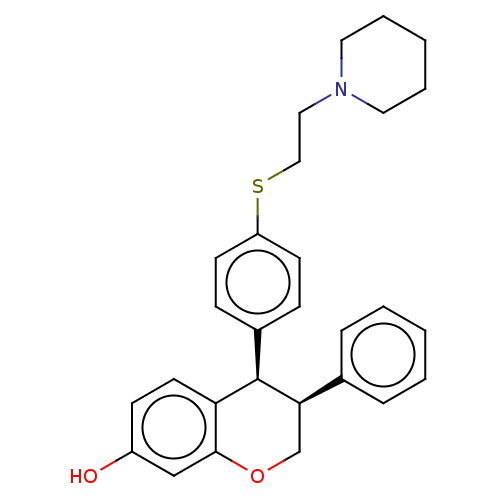
(CHEMBL52598)Show SMILES Oc1ccc2[C@H]([C@H](COc2c1)c1ccccc1)c1ccc(SCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H31NO2S/c30-23-11-14-25-27(19-23)31-20-26(21-7-3-1-4-8-21)28(25)22-9-12-24(13-10-22)32-18-17-29-15-5-2-6-16-29/h1,3-4,7-14,19,26,28,30H,2,5-6,15-18,20H2/t26-,28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Displacement of [3H]17-beta-estradiol from Estrogen receptor of rabbit uterine tissue |
Bioorg Med Chem Lett 12: 17-9 (2002)
BindingDB Entry DOI: 10.7270/Q2X350NF |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50218384
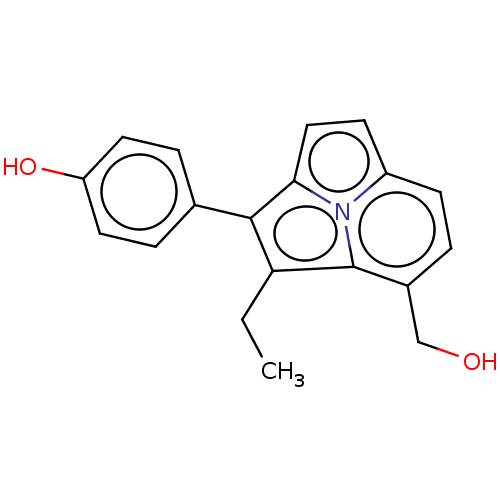
(CHEMBL407164)Show InChI InChI=1S/C19H17NO2/c1-2-16-18(12-4-8-15(22)9-5-12)17-10-7-14-6-3-13(11-21)19(16)20(14)17/h3-10,21-22H,2,11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory activity against Estrogen receptor by displacement of [3H]17-beta-estradiol in ER-rich cytosol from rabbit uterine tissue |
Bioorg Med Chem Lett 10: 2383-6 (2000)
BindingDB Entry DOI: 10.7270/Q28G8NWG |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50219401
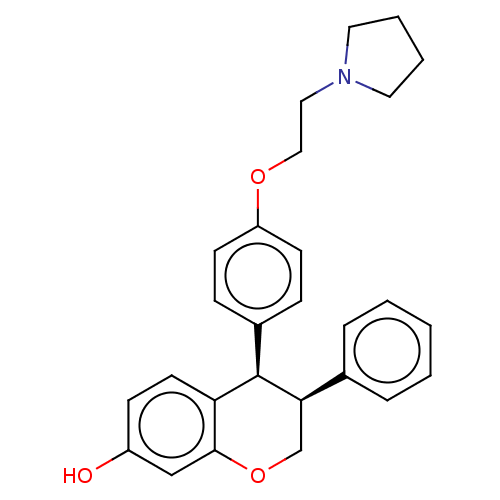
(CHEMBL417596)Show SMILES Oc1ccc2[C@H]([C@H](COc2c1)c1ccccc1)c1ccc(OCCN2CCCC2)cc1 Show InChI InChI=1S/C27H29NO3/c29-22-10-13-24-26(18-22)31-19-25(20-6-2-1-3-7-20)27(24)21-8-11-23(12-9-21)30-17-16-28-14-4-5-15-28/h1-3,6-13,18,25,27,29H,4-5,14-17,19H2/t25-,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Displacement of [3H]17-beta-estradiol from Estrogen receptor of rabbit uterine tissue |
Bioorg Med Chem Lett 12: 17-9 (2002)
BindingDB Entry DOI: 10.7270/Q2X350NF |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50218328
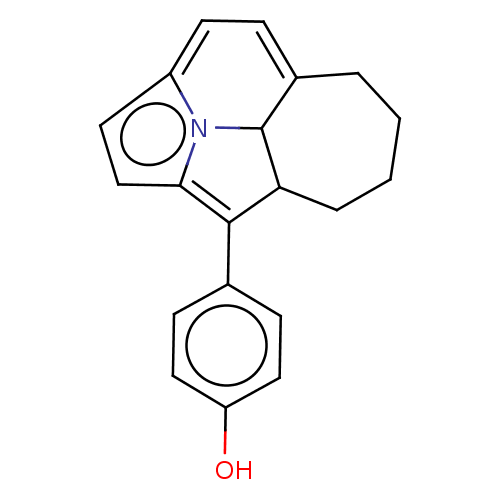
(CHEMBL80976)Show SMILES Oc1ccc(cc1)C1=c2ccc3=CC=C4CCCCC1C4n23 |c:8,t:12,14| Show InChI InChI=1S/C20H19NO/c22-16-10-6-13(7-11-16)19-17-4-2-1-3-14-5-8-15-9-12-18(19)21(15)20(14)17/h5-12,17,20,22H,1-4H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory activity against Estrogen receptor by displacement of [3H]17-beta-estradiol in ER-rich cytosol from rabbit uterine tissue |
Bioorg Med Chem Lett 10: 2383-6 (2000)
BindingDB Entry DOI: 10.7270/Q28G8NWG |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50218325
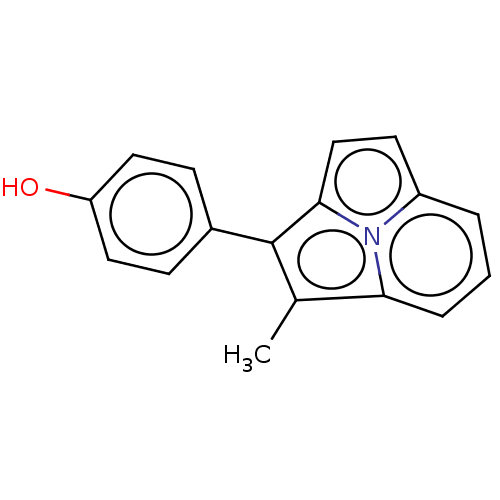
(CHEMBL308872)Show InChI InChI=1S/C17H13NO/c1-11-15-4-2-3-13-7-10-16(18(13)15)17(11)12-5-8-14(19)9-6-12/h2-10,19H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory activity against Estrogen receptor by displacement of [3H]17-beta-estradiol in ER-rich cytosol from rabbit uterine tissue |
Bioorg Med Chem Lett 10: 2383-6 (2000)
BindingDB Entry DOI: 10.7270/Q28G8NWG |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50218326
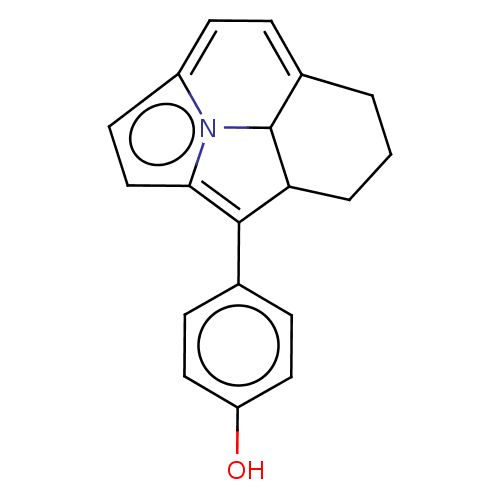
(CHEMBL80560)Show SMILES Oc1ccc(cc1)C1=c2ccc3=CC=C4CCCC1C4n23 |c:8,t:12,14| Show InChI InChI=1S/C19H17NO/c21-15-9-5-12(6-10-15)18-16-3-1-2-13-4-7-14-8-11-17(18)20(14)19(13)16/h4-11,16,19,21H,1-3H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory activity against Estrogen receptor by displacement of [3H]17-beta-estradiol in ER-rich cytosol from rabbit uterine tissue |
Bioorg Med Chem Lett 10: 2383-6 (2000)
BindingDB Entry DOI: 10.7270/Q28G8NWG |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50219398
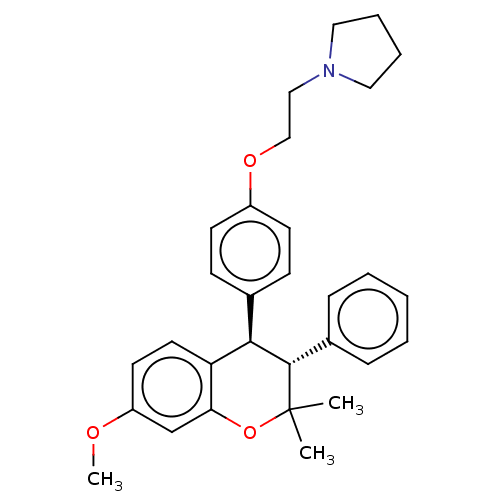
(Levormeloxifene)Show SMILES COc1ccc2[C@H]([C@H](c3ccccc3)C(C)(C)Oc2c1)c1ccc(OCCN2CCCC2)cc1 |r| Show InChI InChI=1S/C30H35NO3/c1-30(2)29(23-9-5-4-6-10-23)28(26-16-15-25(32-3)21-27(26)34-30)22-11-13-24(14-12-22)33-20-19-31-17-7-8-18-31/h4-6,9-16,21,28-29H,7-8,17-20H2,1-3H3/t28-,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Displacement of [3H]17-beta-estradiol from Estrogen receptor of rabbit uterine tissue |
Bioorg Med Chem Lett 12: 17-9 (2002)
BindingDB Entry DOI: 10.7270/Q2X350NF |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50218383
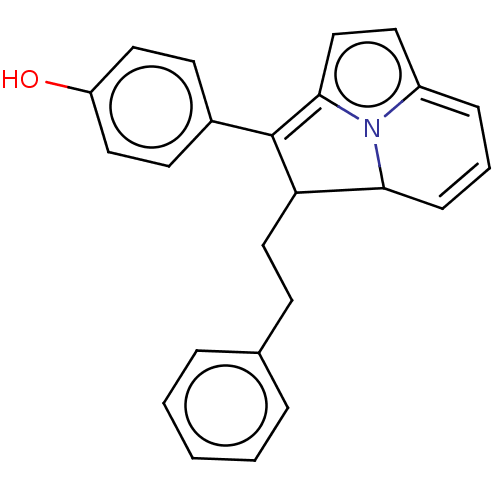
(CHEMBL309677)Show SMILES Oc1ccc(cc1)C1=c2ccc3=CC=CC(C1CCc1ccccc1)n23 |c:8,14,t:12| Show InChI InChI=1S/C24H21NO/c26-20-13-10-18(11-14-20)24-21(15-9-17-5-2-1-3-6-17)22-8-4-7-19-12-16-23(24)25(19)22/h1-8,10-14,16,21-22,26H,9,15H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory activity against Estrogen receptor by displacement of [3H]17-beta-estradiol in ER-rich cytosol from rabbit uterine tissue |
Bioorg Med Chem Lett 10: 2383-6 (2000)
BindingDB Entry DOI: 10.7270/Q28G8NWG |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50218331
(CHEMBL308754)Show InChI InChI=1S/C18H15NO/c1-2-13-14-10-11-17(20)15-8-9-16(19(14)15)18(13)12-6-4-3-5-7-12/h3-11,20H,2H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory activity against Estrogen receptor by displacement of [3H]17-beta-estradiol in ER-rich cytosol from rabbit uterine tissue |
Bioorg Med Chem Lett 10: 2383-6 (2000)
BindingDB Entry DOI: 10.7270/Q28G8NWG |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50218329
(CHEMBL78526)Show SMILES CCCCC1C2C=CC=c3ccc(=C1c1ccc(O)cc1)n23 |c:6,t:8,12| Show InChI InChI=1S/C20H21NO/c1-2-3-6-17-18-7-4-5-15-10-13-19(21(15)18)20(17)14-8-11-16(22)12-9-14/h4-5,7-13,17-18,22H,2-3,6H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory activity against Estrogen receptor by displacement of [3H]17-beta-estradiol in ER-rich cytosol from rabbit uterine tissue |
Bioorg Med Chem Lett 10: 2383-6 (2000)
BindingDB Entry DOI: 10.7270/Q28G8NWG |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50218381
(CHEMBL80470)Show InChI InChI=1S/C18H15NO/c1-2-15-16-8-4-6-13-9-10-17(19(13)16)18(15)12-5-3-7-14(20)11-12/h3-11,20H,2H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory activity against Estrogen receptor by displacement of [3H]17-beta-estradiol in ER-rich cytosol from rabbit uterine tissue |
Bioorg Med Chem Lett 10: 2383-6 (2000)
BindingDB Entry DOI: 10.7270/Q28G8NWG |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50218332
(CHEMBL80983)Show InChI InChI=1S/C16H11NO/c18-14-7-4-11(5-8-14)15-10-13-3-1-2-12-6-9-16(15)17(12)13/h1-10,18H | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory activity against Estrogen receptor by displacement of [3H]17-beta-estradiol in ER-rich cytosol from rabbit uterine tissue |
Bioorg Med Chem Lett 10: 2383-6 (2000)
BindingDB Entry DOI: 10.7270/Q28G8NWG |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50218349
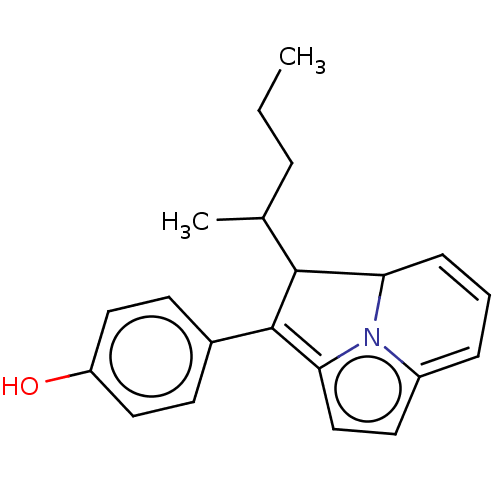
(CHEMBL77680)Show SMILES CCCC(C)C1C2C=CC=c3ccc(=C1c1ccc(O)cc1)n23 |c:7,t:9,13| Show InChI InChI=1S/C21H23NO/c1-3-5-14(2)20-18-7-4-6-16-10-13-19(22(16)18)21(20)15-8-11-17(23)12-9-15/h4,6-14,18,20,23H,3,5H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory activity against Estrogen receptor by displacement of [3H]17-beta-estradiol in ER-rich cytosol from rabbit uterine tissue |
Bioorg Med Chem Lett 10: 2383-6 (2000)
BindingDB Entry DOI: 10.7270/Q28G8NWG |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50218385
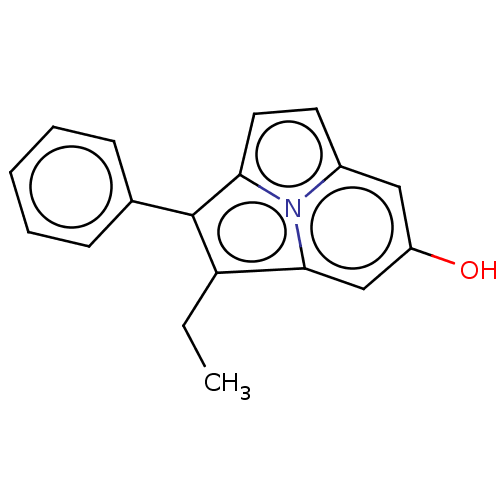
(CHEMBL309916)Show InChI InChI=1S/C18H15NO/c1-2-15-17-11-14(20)10-13-8-9-16(19(13)17)18(15)12-6-4-3-5-7-12/h3-11,20H,2H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory activity against Estrogen receptor by displacement of [3H]17-beta-estradiol in ER-rich cytosol from rabbit uterine tissue |
Bioorg Med Chem Lett 10: 2383-6 (2000)
BindingDB Entry DOI: 10.7270/Q28G8NWG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50109540
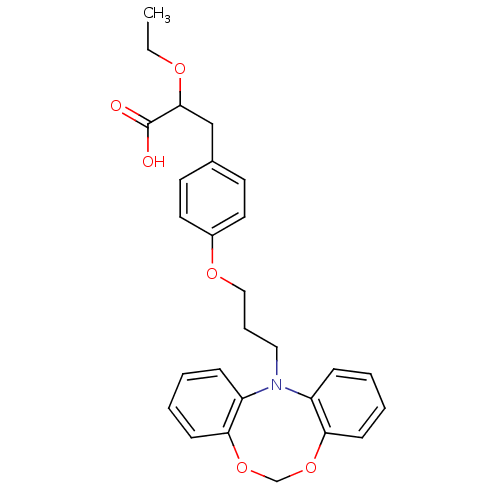
(3-{4-[3-(5,7-Dioxa-12-aza-dibenzo[a,d]cycloocten-1...)Show SMILES CCOC(Cc1ccc(OCCCN2c3ccccc3OCOc3ccccc23)cc1)C(O)=O Show InChI InChI=1S/C27H29NO6/c1-2-31-26(27(29)30)18-20-12-14-21(15-13-20)32-17-7-16-28-22-8-3-5-10-24(22)33-19-34-25-11-6-4-9-23(25)28/h3-6,8-15,26H,2,7,16-19H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transactivation using receptor transactivation assay against hPPAR gamma |
J Med Chem 45: 789-804 (2002)
BindingDB Entry DOI: 10.7270/Q2445KSG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50109541
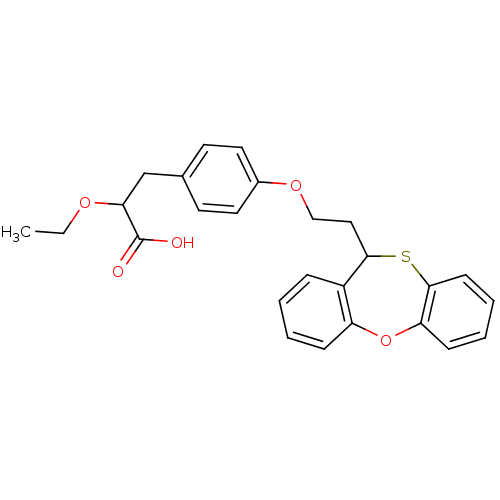
(2-Ethoxy-3-{4-[2-(11H-5-oxa-10-thia-dibenzo[a,d]cy...)Show SMILES CCOC(Cc1ccc(OCCC2Sc3ccccc3Oc3ccccc23)cc1)C(O)=O Show InChI InChI=1S/C26H26O5S/c1-2-29-23(26(27)28)17-18-11-13-19(14-12-18)30-16-15-24-20-7-3-4-8-21(20)31-22-9-5-6-10-25(22)32-24/h3-14,23-24H,2,15-17H2,1H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transactivation using receptor transactivation assay against hPPAR gamma |
J Med Chem 45: 789-804 (2002)
BindingDB Entry DOI: 10.7270/Q2445KSG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50109542

(2-Ethoxy-3-{4-[2-(9H-fluoren-9-yl)-ethoxy]-phenyl}...)Show SMILES CCOC(Cc1ccc(OCCC2c3ccccc3-c3ccccc23)cc1)C(O)=O Show InChI InChI=1S/C26H26O4/c1-2-29-25(26(27)28)17-18-11-13-19(14-12-18)30-16-15-24-22-9-5-3-7-20(22)21-8-4-6-10-23(21)24/h3-14,24-25H,2,15-17H2,1H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transactivation using receptor transactivation assay against hPPAR alpha |
J Med Chem 45: 789-804 (2002)
BindingDB Entry DOI: 10.7270/Q2445KSG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50109544

(3-[4-(2-beta-Carbolin-9-yl-ethoxy)-phenyl]-2-ethox...)Show SMILES CCO[C@@H](Cc1ccc(OCCn2c3ccccc3c3ccncc23)cc1)C(O)=O Show InChI InChI=1S/C24H24N2O4/c1-2-29-23(24(27)28)15-17-7-9-18(10-8-17)30-14-13-26-21-6-4-3-5-19(21)20-11-12-25-16-22(20)26/h3-12,16,23H,2,13-15H2,1H3,(H,27,28)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transactivation using receptor transactivation assay against hPPAR gamma |
J Med Chem 45: 789-804 (2002)
BindingDB Entry DOI: 10.7270/Q2445KSG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50109543

(3-{4-[2-(10,11-Dihydro-dibenzo[b,f]azepin-5-yl)-et...)Show SMILES CCOC(Cc1ccc(OCCN2c3ccccc3CCc3ccccc23)cc1)C(O)=O Show InChI InChI=1S/C27H29NO4/c1-2-31-26(27(29)30)19-20-11-15-23(16-12-20)32-18-17-28-24-9-5-3-7-21(24)13-14-22-8-4-6-10-25(22)28/h3-12,15-16,26H,2,13-14,17-19H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 750 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transactivation using receptor transactivation assay against hPPAR gamma |
J Med Chem 45: 789-804 (2002)
BindingDB Entry DOI: 10.7270/Q2445KSG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50109545
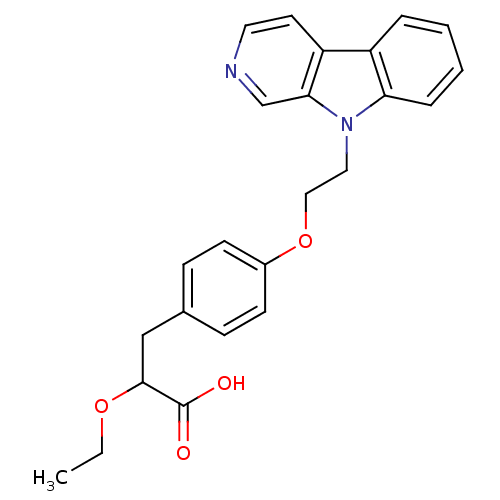
(3-[4-(2-beta-Carbolin-9-yl-ethoxy)-phenyl]-2-ethox...)Show SMILES CCOC(Cc1ccc(OCCn2c3ccccc3c3ccncc23)cc1)C(O)=O Show InChI InChI=1S/C24H24N2O4/c1-2-29-23(24(27)28)15-17-7-9-18(10-8-17)30-14-13-26-21-6-4-3-5-19(21)20-11-12-25-16-22(20)26/h3-12,16,23H,2,13-15H2,1H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 670 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transactivation using receptor transactivation assay against hPPAR alpha |
J Med Chem 45: 789-804 (2002)
BindingDB Entry DOI: 10.7270/Q2445KSG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50109546
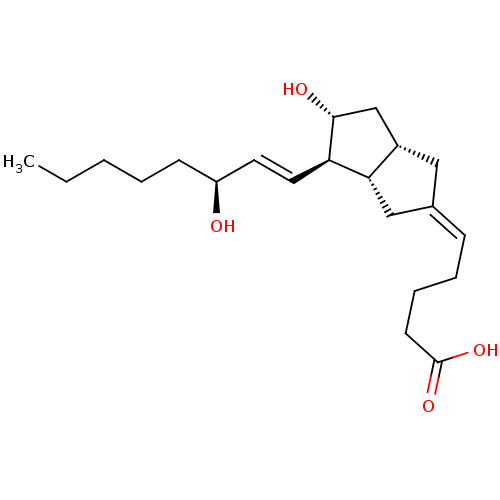
(5-[(3aS,4R,5R,6aS)-5-Hydroxy-4-((S)-3-hydroxy-oct-...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)C[C@@H]2C\C(C[C@H]12)=C\CCCC(O)=O Show InChI InChI=1S/C21H34O4/c1-2-3-4-8-17(22)10-11-18-19-13-15(7-5-6-9-21(24)25)12-16(19)14-20(18)23/h7,10-11,16-20,22-23H,2-6,8-9,12-14H2,1H3,(H,24,25)/b11-10+,15-7-/t16-,17-,18+,19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.41E+3 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transcriptional activation of hPPAR delta |
J Med Chem 45: 789-804 (2002)
BindingDB Entry DOI: 10.7270/Q2445KSG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50109547
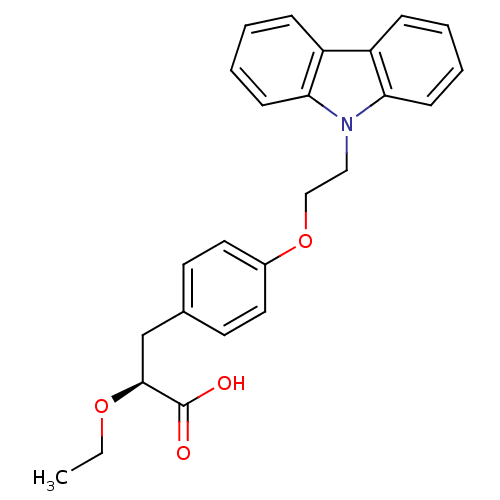
((S)-3-(4-(2-CARBAZOL-9-YL-ETHOXY)-PHENYL)-2-ETHOXY...)Show SMILES CCO[C@@H](Cc1ccc(OCCn2c3ccccc3c3ccccc23)cc1)C(O)=O Show InChI InChI=1S/C25H25NO4/c1-2-29-24(25(27)28)17-18-11-13-19(14-12-18)30-16-15-26-22-9-5-3-7-20(22)21-8-4-6-10-23(21)26/h3-14,24H,2,15-17H2,1H3,(H,27,28)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 360 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transactivation using receptor transactivation assay against hPPAR alpha |
J Med Chem 45: 789-804 (2002)
BindingDB Entry DOI: 10.7270/Q2445KSG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50109548
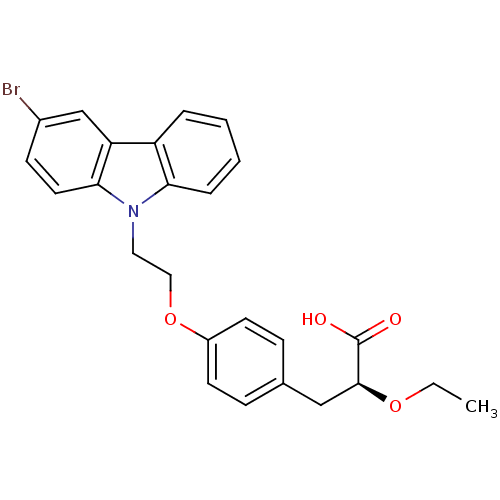
(3-{4-[2-(3-Bromo-carbazol-9-yl)-ethoxy]-phenyl}-2-...)Show SMILES CCO[C@@H](Cc1ccc(OCCn2c3ccccc3c3cc(Br)ccc23)cc1)C(O)=O Show InChI InChI=1S/C25H24BrNO4/c1-2-30-24(25(28)29)15-17-7-10-19(11-8-17)31-14-13-27-22-6-4-3-5-20(22)21-16-18(26)9-12-23(21)27/h3-12,16,24H,2,13-15H2,1H3,(H,28,29)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transactivation using receptor transactivation assay against hPPAR gamma |
J Med Chem 45: 789-804 (2002)
BindingDB Entry DOI: 10.7270/Q2445KSG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50109549

(2-Ethoxy-3-{4-[2-(9H-xanthen-9-yl)-ethoxy]-phenyl}...)Show SMILES CCOC(Cc1ccc(OCCC2c3ccccc3Oc3ccccc23)cc1)C(O)=O Show InChI InChI=1S/C26H26O5/c1-2-29-25(26(27)28)17-18-11-13-19(14-12-18)30-16-15-20-21-7-3-5-9-23(21)31-24-10-6-4-8-22(20)24/h3-14,20,25H,2,15-17H2,1H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | n/a | 460 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transactivation using receptor transactivation assay against hPPAR gamma |
J Med Chem 45: 789-804 (2002)
BindingDB Entry DOI: 10.7270/Q2445KSG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50109550

(3-[4-(2-Dibenzo[b,f]azepin-5-yl-ethoxy)-phenyl]-2-...)Show SMILES CCOC(Cc1ccc(OCCN2c3ccccc3C=Cc3ccccc23)cc1)C(O)=O |c:20| Show InChI InChI=1S/C27H27NO4/c1-2-31-26(27(29)30)19-20-11-15-23(16-12-20)32-18-17-28-24-9-5-3-7-21(24)13-14-22-8-4-6-10-25(22)28/h3-16,26H,2,17-19H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | n/a | 760 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transactivation using receptor transactivation assay against hPPAR gamma |
J Med Chem 45: 789-804 (2002)
BindingDB Entry DOI: 10.7270/Q2445KSG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50109552

(2-Ethoxy-3-[4-(2-fluoren-9-ylidene-ethoxy)-phenyl]...)Show SMILES [#6]-[#6]-[#8]-[#6](-[#6]-c1ccc(-[#8]-[#6]\[#6]=[#6]-2/c3ccccc3-c3ccccc-23)cc1)-[#6](-[#8])=O Show InChI InChI=1S/C26H24O4/c1-2-29-25(26(27)28)17-18-11-13-19(14-12-18)30-16-15-24-22-9-5-3-7-20(22)21-8-4-6-10-23(21)24/h3-15,25H,2,16-17H2,1H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transactivation using receptor transactivation assay against hPPAR gamma |
J Med Chem 45: 789-804 (2002)
BindingDB Entry DOI: 10.7270/Q2445KSG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50109551
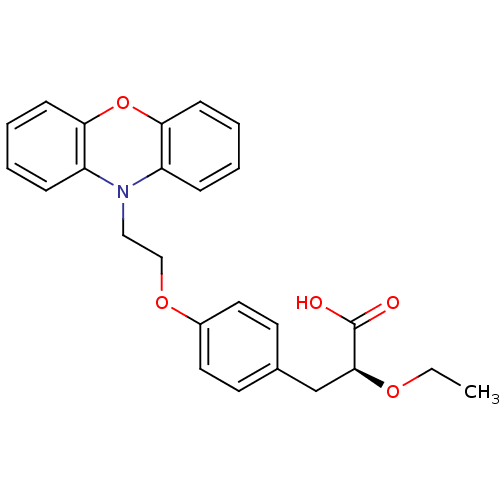
((2S)-2-ETHOXY-3-{4-[2-(10H-PHENOXAZIN-10-YL)ETHOXY...)Show SMILES CCO[C@@H](Cc1ccc(OCCN2c3ccccc3Oc3ccccc23)cc1)C(O)=O Show InChI InChI=1S/C25H25NO5/c1-2-29-24(25(27)28)17-18-11-13-19(14-12-18)30-16-15-26-20-7-3-5-9-22(20)31-23-10-6-4-8-21(23)26/h3-14,24H,2,15-17H2,1H3,(H,27,28)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 3.21E+3 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transactivation using receptor transactivation assay against hPPAR alpha |
J Med Chem 45: 789-804 (2002)
BindingDB Entry DOI: 10.7270/Q2445KSG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50109553
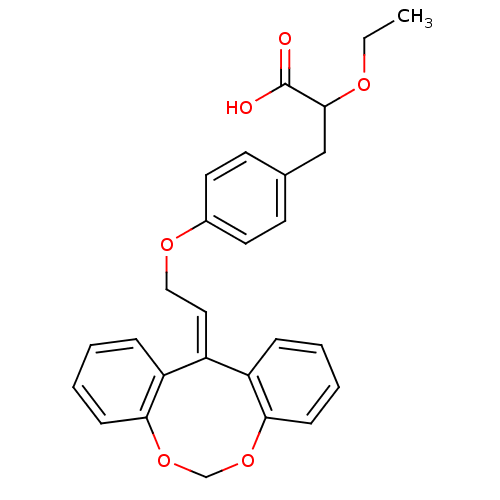
(3-{4-[2-(5,7-Dioxa-dibenzo[a,d]cycloocten-12-ylide...)Show SMILES [#6]-[#6]-[#8]-[#6](-[#6]-c1ccc(-[#8]-[#6]\[#6]=[#6]-2\c3ccccc3-[#8]-[#6]-[#8]-c3ccccc-23)cc1)-[#6](-[#8])=O Show InChI InChI=1S/C27H26O6/c1-2-30-26(27(28)29)17-19-11-13-20(14-12-19)31-16-15-21-22-7-3-5-9-24(22)32-18-33-25-10-6-4-8-23(21)25/h3-15,26H,2,16-18H2,1H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transactivation using receptor transactivation assay against hPPAR gamma |
J Med Chem 45: 789-804 (2002)
BindingDB Entry DOI: 10.7270/Q2445KSG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50109547

((S)-3-(4-(2-CARBAZOL-9-YL-ETHOXY)-PHENYL)-2-ETHOXY...)Show SMILES CCO[C@@H](Cc1ccc(OCCn2c3ccccc3c3ccccc23)cc1)C(O)=O Show InChI InChI=1S/C25H25NO4/c1-2-29-24(25(27)28)17-18-11-13-19(14-12-18)30-16-15-26-22-9-5-3-7-20(22)21-8-4-6-10-23(21)26/h3-14,24H,2,15-17H2,1H3,(H,27,28)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transactivation using receptor transactivation assay against hPPAR gamma |
J Med Chem 45: 789-804 (2002)
BindingDB Entry DOI: 10.7270/Q2445KSG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50109554

(3-{4-[2-(3,6-Dibromo-carbazol-9-yl)-ethoxy]-phenyl...)Show SMILES CCO[C@@H](Cc1ccc(OCCn2c3ccc(Br)cc3c3cc(Br)ccc23)cc1)C(O)=O Show InChI InChI=1S/C25H23Br2NO4/c1-2-31-24(25(29)30)13-16-3-7-19(8-4-16)32-12-11-28-22-9-5-17(26)14-20(22)21-15-18(27)6-10-23(21)28/h3-10,14-15,24H,2,11-13H2,1H3,(H,29,30)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transactivation using receptor transactivation assay against hPPAR alpha |
J Med Chem 45: 789-804 (2002)
BindingDB Entry DOI: 10.7270/Q2445KSG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM28701
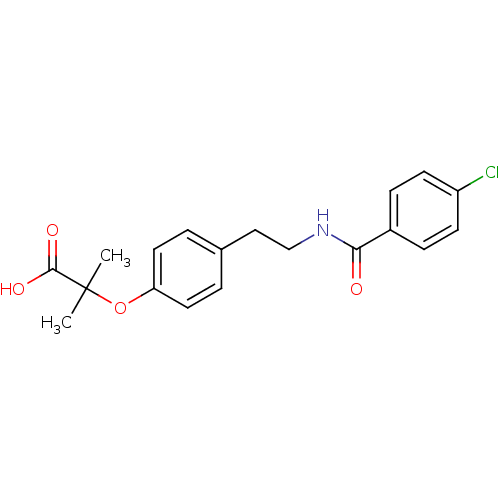
(2-(4-{2-[(4-chlorophenyl)formamido]ethyl}phenoxy)-...)Show SMILES CC(C)(Oc1ccc(CCNC(=O)c2ccc(Cl)cc2)cc1)C(O)=O Show InChI InChI=1S/C19H20ClNO4/c1-19(2,18(23)24)25-16-9-3-13(4-10-16)11-12-21-17(22)14-5-7-15(20)8-6-14/h3-10H,11-12H2,1-2H3,(H,21,22)(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
PubMed
| n/a | n/a | n/a | n/a | 1.02E+5 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transcriptional activation of hPPAR delta |
J Med Chem 45: 789-804 (2002)
BindingDB Entry DOI: 10.7270/Q2445KSG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50109544

(3-[4-(2-beta-Carbolin-9-yl-ethoxy)-phenyl]-2-ethox...)Show SMILES CCO[C@@H](Cc1ccc(OCCn2c3ccccc3c3ccncc23)cc1)C(O)=O Show InChI InChI=1S/C24H24N2O4/c1-2-29-23(24(27)28)15-17-7-9-18(10-8-17)30-14-13-26-21-6-4-3-5-19(21)20-11-12-25-16-22(20)26/h3-12,16,23H,2,13-15H2,1H3,(H,27,28)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transactivation using receptor transactivation assay against hPPAR alpha |
J Med Chem 45: 789-804 (2002)
BindingDB Entry DOI: 10.7270/Q2445KSG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28701

(2-(4-{2-[(4-chlorophenyl)formamido]ethyl}phenoxy)-...)Show SMILES CC(C)(Oc1ccc(CCNC(=O)c2ccc(Cl)cc2)cc1)C(O)=O Show InChI InChI=1S/C19H20ClNO4/c1-19(2,18(23)24)25-16-9-3-13(4-10-16)11-12-21-17(22)14-5-7-15(20)8-6-14/h3-10H,11-12H2,1-2H3,(H,21,22)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
PDB
PubMed
| n/a | n/a | n/a | n/a | 7.35E+4 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transactivation using receptor transactivation assay against hPPAR gamma |
J Med Chem 45: 789-804 (2002)
BindingDB Entry DOI: 10.7270/Q2445KSG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transactivation using receptor transactivation assay against hPPAR gamma |
J Med Chem 45: 789-804 (2002)
BindingDB Entry DOI: 10.7270/Q2445KSG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50109556
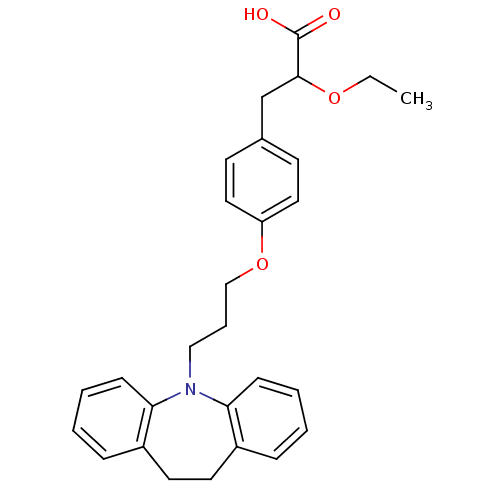
(3-{4-[3-(10,11-Dihydro-dibenzo[b,f]azepin-5-yl)-pr...)Show SMILES CCOC(Cc1ccc(OCCCN2c3ccccc3CCc3ccccc23)cc1)C(O)=O Show InChI InChI=1S/C28H31NO4/c1-2-32-27(28(30)31)20-21-12-16-24(17-13-21)33-19-7-18-29-25-10-5-3-8-22(25)14-15-23-9-4-6-11-26(23)29/h3-6,8-13,16-17,27H,2,7,14-15,18-20H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 760 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transactivation using receptor transactivation assay against hPPAR gamma |
J Med Chem 45: 789-804 (2002)
BindingDB Entry DOI: 10.7270/Q2445KSG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data