

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50285024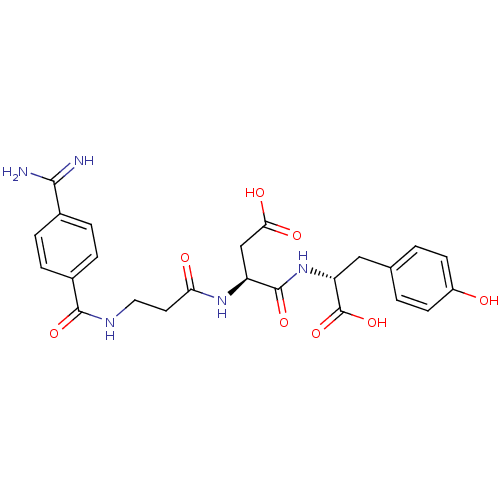 (3-[3-(4-Carbamimidoyl-benzoylamino)-propionylamino...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-SKF-107260 from alpha IIb beta3 integrin | Bioorg Med Chem Lett 5: 1941-1946 (1995) Article DOI: 10.1016/0960-894X(95)00329-R BindingDB Entry DOI: 10.7270/Q2Z60P2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50285023 ((R)-3-[3-(4-Carbamimidoyl-benzoylamino)-propionyla...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-SKF-107260 from alpha IIb beta3 integrin | Bioorg Med Chem Lett 5: 1941-1946 (1995) Article DOI: 10.1016/0960-894X(95)00329-R BindingDB Entry DOI: 10.7270/Q2Z60P2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-tetrahydrodipicolinate reductase (Escherichia coli) | BDBM59098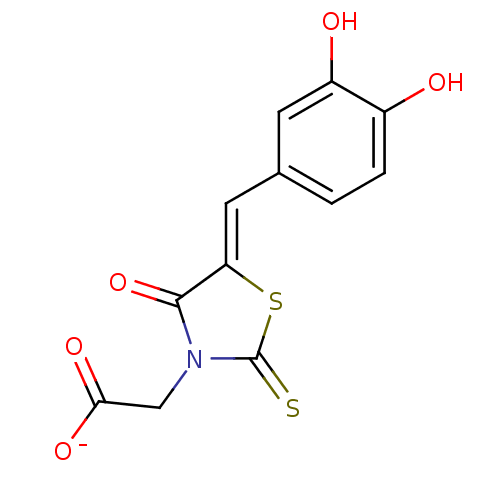 (Bi-ligand, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Quinone-dependent D-lactate dehydrogenase (Escherichia coli) | BDBM59099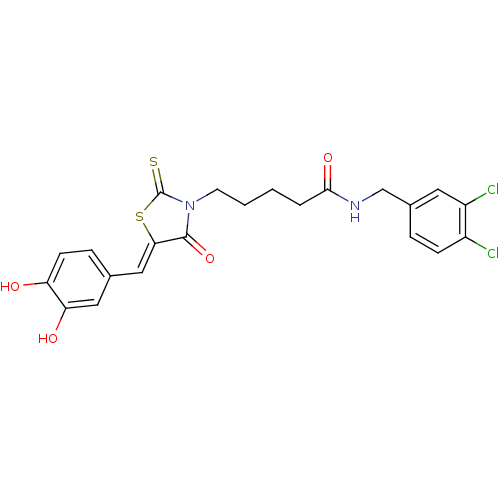 (Bi-ligand, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-tetrahydrodipicolinate reductase (Escherichia coli) | BDBM59101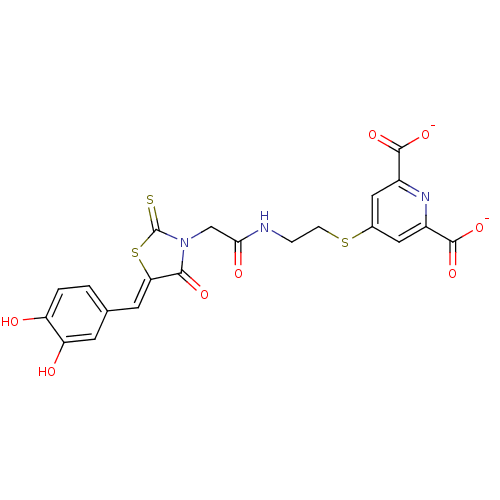 (Bi-ligand, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM59100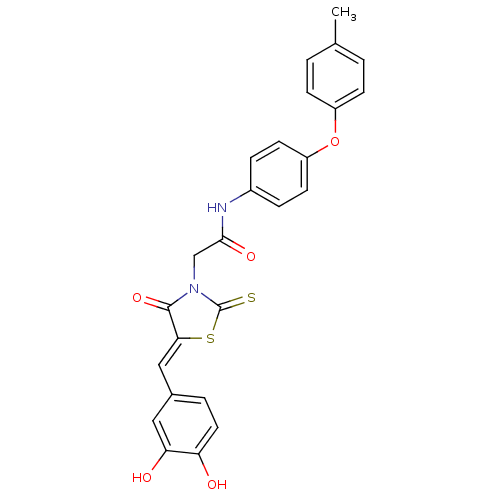 (Bi-ligand, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 202 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Quinone-dependent D-lactate dehydrogenase (Escherichia coli) | BDBM59101 (Bi-ligand, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM59101 (Bi-ligand, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM59099 (Bi-ligand, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Quinone-dependent D-lactate dehydrogenase (Escherichia coli) | BDBM59100 (Bi-ligand, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-tetrahydrodipicolinate reductase (Escherichia coli) | BDBM59100 (Bi-ligand, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-tetrahydrodipicolinate reductase (Escherichia coli) | BDBM59099 (Bi-ligand, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM59098 (Bi-ligand, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Quinone-dependent D-lactate dehydrogenase (Escherichia coli) | BDBM59098 (Bi-ligand, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM405268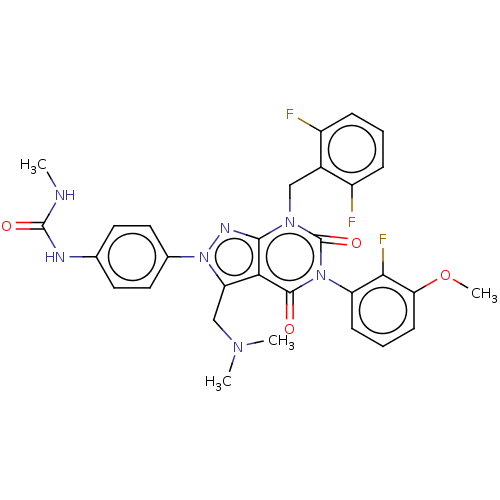 (1-(4-(7-(2,6-difluorobenzyl)-3-((dimethylamino)met...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., Ltd US Patent | Assay Description Human: Test Example 1. Human GnRHr (GnRH Receptor) Activity Assay of the Present Compounds.In vitro GnRHr protein activity was tested by the followin... | US Patent US10344034 (2019) BindingDB Entry DOI: 10.7270/Q29Z978Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM439612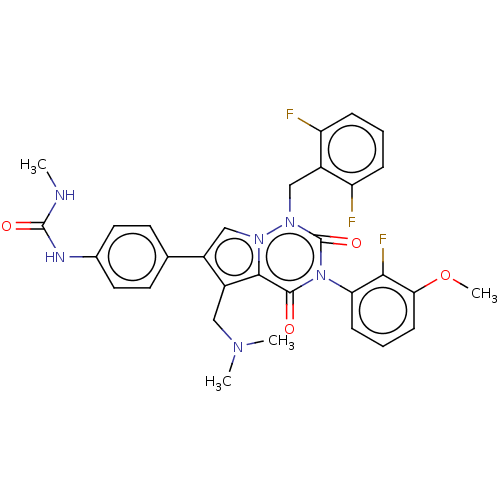 (1-(4-(1-(2,6-difluorobenzyl)-5-((dimethylamino)met...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., ltd.; Jiangsu Hangrui Medicine Co., Ltd. US Patent | Assay Description In vitro GnRHr protein activity was tested by the following methods.This assay was used to determine the inhibition effect of the present compound on... | US Patent US10633388 (2020) BindingDB Entry DOI: 10.7270/Q2F76GM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-activated protein kinase 5 Isoform 2 (Homo sapiens (Human)) | BDBM605909 (US11680056, Example 53A | US11680056, Example 53B) | MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q29G5RXB | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM260478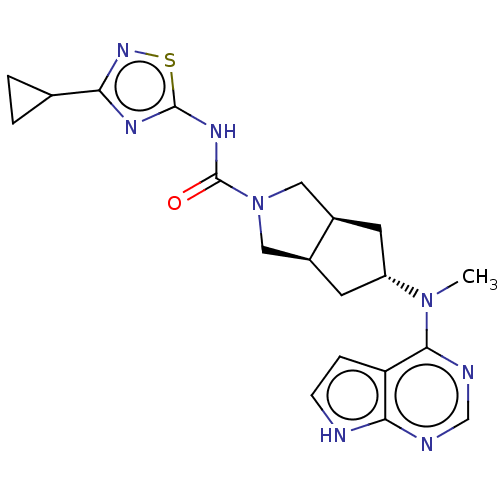 (US10428074, Example 125 | US9527851, 125 | US95278...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.3 | 27 |
Jiangsu Hengrui Medicine Co., Ltd.; Shanghai Hengrui Pharmaceutical Co., Ltd. US Patent | Assay Description In vitro kinase assays described below can be used to determine the activity of a test compound for inhibiting the activity of JAK1 kinase. The test ... | US Patent US9527851 (2016) BindingDB Entry DOI: 10.7270/Q2HD7TM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM260459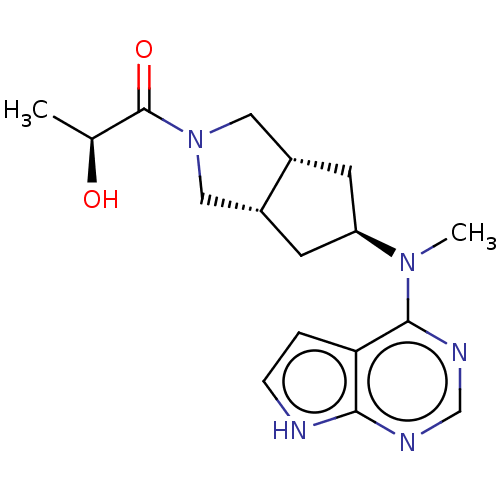 (US10428074, Example 17 | US9527851, 17 | US9527851...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.3 | 27 |
Jiangsu Hengrui Medicine Co., Ltd.; Shanghai Hengrui Pharmaceutical Co., Ltd. US Patent | Assay Description In vitro kinase assays described below can be used to determine the activity of a test compound for inhibiting the activity of JAK1 kinase. The test ... | US Patent US9527851 (2016) BindingDB Entry DOI: 10.7270/Q2HD7TM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM260478 (US10428074, Example 125 | US9527851, 125 | US95278...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.3 | 27 |
Jiangsu Hengrui Medicine Co., Ltd.; Shanghai Hengrui Pharmaceutical Co., Ltd. US Patent | Assay Description In vitro kinase assays described below can be used to determine the activity of a test compound for inhibiting the activity of JAK1 kinase. The test ... | US Patent US9527851 (2016) BindingDB Entry DOI: 10.7270/Q2HD7TM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM260478 (US10428074, Example 125 | US9527851, 125 | US95278...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Hengrui Medicine Co., Ltd. US Patent | Assay Description JAK1: In vitro kinase assays described below can be used to determine the activity of a test compound for inhibiting the activity of JAK1 kinase. The... | US Patent US10428074 (2019) BindingDB Entry DOI: 10.7270/Q26D5WC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM260478 (US10428074, Example 125 | US9527851, 125 | US95278...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Hengrui Medicine Co., Ltd. US Patent | Assay Description JAK1: In vitro kinase assays described below can be used to determine the activity of a test compound for inhibiting the activity of JAK1 kinase. The... | US Patent US10428074 (2019) BindingDB Entry DOI: 10.7270/Q26D5WC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM260459 (US10428074, Example 17 | US9527851, 17 | US9527851...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Hengrui Medicine Co., Ltd. US Patent | Assay Description JAK1: In vitro kinase assays described below can be used to determine the activity of a test compound for inhibiting the activity of JAK1 kinase. The... | US Patent US10428074 (2019) BindingDB Entry DOI: 10.7270/Q26D5WC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM260478 (US10428074, Example 125 | US9527851, 125 | US95278...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Jiangsu Hengrui Medicine Co., Ltd.; Shanghai Hengrui Pharmaceutical Co., Ltd. US Patent | Assay Description TBDIn vitro kinase assays described below can be used to determine the activity of a test compound for inhibiting the activity of JAK1 kinase. The te... | US Patent US9527851 (2016) BindingDB Entry DOI: 10.7270/Q2D21VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-activated protein kinase 5 Isoform 2 (Homo sapiens (Human)) | BDBM605939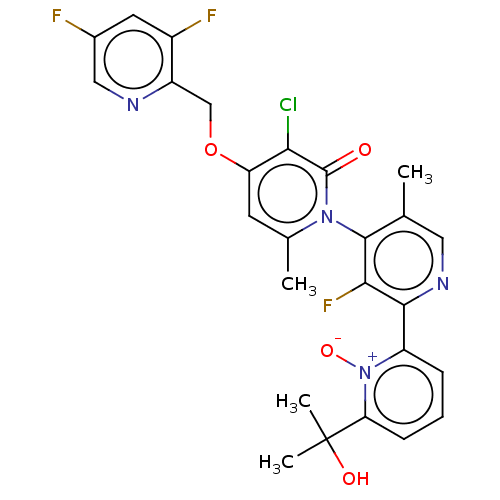 (US11680056, Example 68B) | MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q29G5RXB | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM260459 (US10428074, Example 17 | US9527851, 17 | US9527851...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Jiangsu Hengrui Medicine Co., Ltd.; Shanghai Hengrui Pharmaceutical Co., Ltd. US Patent | Assay Description TBDIn vitro kinase assays described below can be used to determine the activity of a test compound for inhibiting the activity of JAK1 kinase. The te... | US Patent US9527851 (2016) BindingDB Entry DOI: 10.7270/Q2D21VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM260478 (US10428074, Example 125 | US9527851, 125 | US95278...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Jiangsu Hengrui Medicine Co., Ltd.; Shanghai Hengrui Pharmaceutical Co., Ltd. US Patent | Assay Description TBDIn vitro kinase assays described below can be used to determine the activity of a test compound for inhibiting the activity of JAK1 kinase. The te... | US Patent US9527851 (2016) BindingDB Entry DOI: 10.7270/Q2D21VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G_PROTEIN_RECEP_F1_2 domain-containing protein (Oryctolagus cuniculus (Rabbit)) | BDBM405272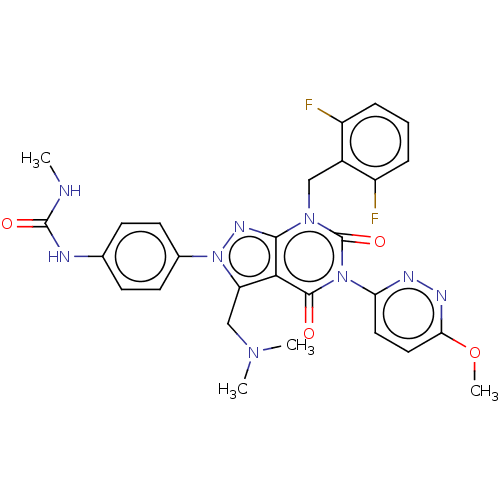 (1-(4-(7-(2,6-difluorobenzyl)-3-((dimethylamino)met...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., Ltd US Patent | Assay Description Rabbit: This assay was used to determine the inhibition effect of the present compounds on the activity of rabbit GnRHr protein expressed by rabbit G... | US Patent US10344034 (2019) BindingDB Entry DOI: 10.7270/Q29Z978Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone II receptor (Green monkey) | BDBM405272 (1-(4-(7-(2,6-difluorobenzyl)-3-((dimethylamino)met...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., ltd.; Jiangsu Hangrui Medicine Co., Ltd. US Patent | Assay Description In vitro GnRHr protein activity was tested by the following methods.This assay was used to determine the inhibition effect of the present compound on... | US Patent US10633388 (2020) BindingDB Entry DOI: 10.7270/Q2F76GM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rabbit) | BDBM405272 (1-(4-(7-(2,6-difluorobenzyl)-3-((dimethylamino)met...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., ltd.; Jiangsu Hangrui Medicine Co., Ltd. US Patent | Assay Description In vitro GnRHr protein activity was tested by the following methods.This assay was used to determine the inhibition effect of the present compound on... | US Patent US10633388 (2020) BindingDB Entry DOI: 10.7270/Q2F76GM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rabbit) | BDBM405271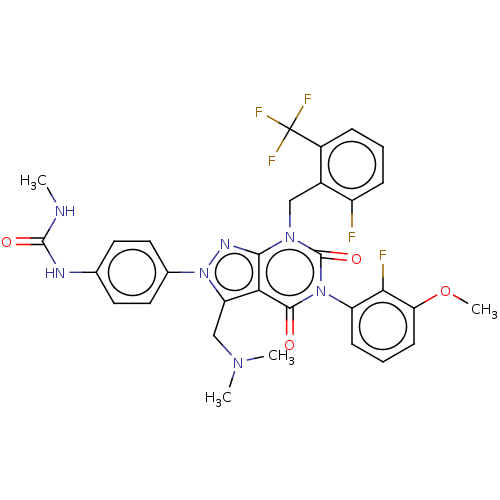 (1-(4-(3-((dimethylamino)methyl)-5-(2-fluoro-3-meth...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., ltd.; Jiangsu Hangrui Medicine Co., Ltd. US Patent | Assay Description In vitro GnRHr protein activity was tested by the following methods.This assay was used to determine the inhibition effect of the present compound on... | US Patent US10633388 (2020) BindingDB Entry DOI: 10.7270/Q2F76GM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G_PROTEIN_RECEP_F1_2 domain-containing protein (Oryctolagus cuniculus (Rabbit)) | BDBM405271 (1-(4-(3-((dimethylamino)methyl)-5-(2-fluoro-3-meth...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., Ltd US Patent | Assay Description Rabbit: This assay was used to determine the inhibition effect of the present compounds on the activity of rabbit GnRHr protein expressed by rabbit G... | US Patent US10344034 (2019) BindingDB Entry DOI: 10.7270/Q29Z978Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM260459 (US10428074, Example 17 | US9527851, 17 | US9527851...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Jiangsu Hengrui Medicine Co., Ltd.; Shanghai Hengrui Pharmaceutical Co., Ltd. US Patent | Assay Description TBDIn vitro kinase assays described below can be used to determine the activity of a test compound for inhibiting the activity of JAK3 kinase. The te... | US Patent US9527851 (2016) BindingDB Entry DOI: 10.7270/Q2D21VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM260459 (US10428074, Example 17 | US9527851, 17 | US9527851...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Hengrui Medicine Co., Ltd. US Patent | Assay Description JAK3: In vitro kinase assays described below can be used to determine the activity of a test compound for inhibiting the activity of JAK3 kinase. The... | US Patent US10428074 (2019) BindingDB Entry DOI: 10.7270/Q26D5WC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM415495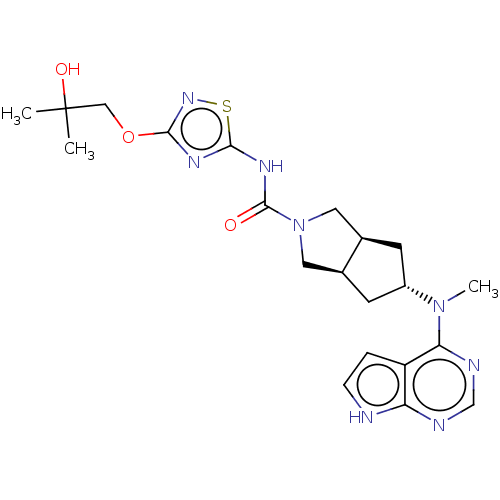 (US10428074, Example 99) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Hengrui Medicine Co., Ltd. US Patent | Assay Description JAK1: In vitro kinase assays described below can be used to determine the activity of a test compound for inhibiting the activity of JAK1 kinase. The... | US Patent US10428074 (2019) BindingDB Entry DOI: 10.7270/Q26D5WC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM260459 (US10428074, Example 17 | US9527851, 17 | US9527851...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.3 | 27 |
Jiangsu Hengrui Medicine Co., Ltd.; Shanghai Hengrui Pharmaceutical Co., Ltd. US Patent | Assay Description In vitro kinase assays described below can be used to determine the activity of a test compound for inhibiting the activity of JAK3 kinase. The test ... | US Patent US9527851 (2016) BindingDB Entry DOI: 10.7270/Q2HD7TM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM202489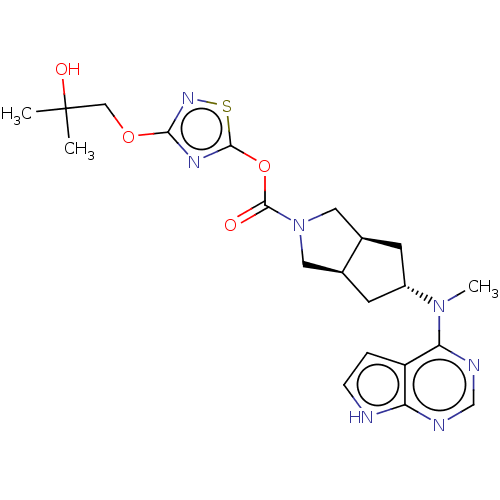 (US9527851, example 99) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Jiangsu Hengrui Medicine Co., Ltd.; Shanghai Hengrui Pharmaceutical Co., Ltd. US Patent | Assay Description TBDIn vitro kinase assays described below can be used to determine the activity of a test compound for inhibiting the activity of JAK1 kinase. The te... | US Patent US9527851 (2016) BindingDB Entry DOI: 10.7270/Q2D21VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM260502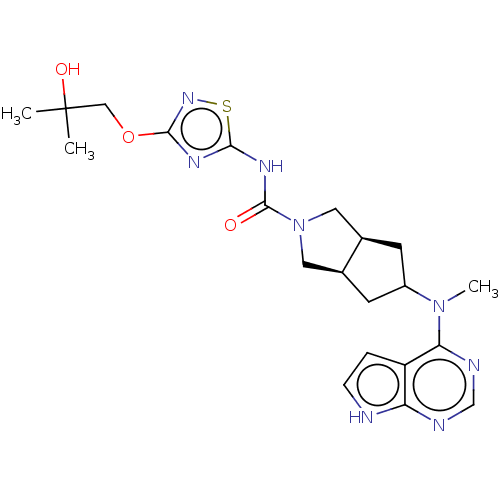 (US9527851, 99) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.3 | 27 |
Jiangsu Hengrui Medicine Co., Ltd.; Shanghai Hengrui Pharmaceutical Co., Ltd. US Patent | Assay Description In vitro kinase assays described below can be used to determine the activity of a test compound for inhibiting the activity of JAK1 kinase. The test ... | US Patent US9527851 (2016) BindingDB Entry DOI: 10.7270/Q2HD7TM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-activated protein kinase 5 Isoform 2 (Homo sapiens (Human)) | BDBM605929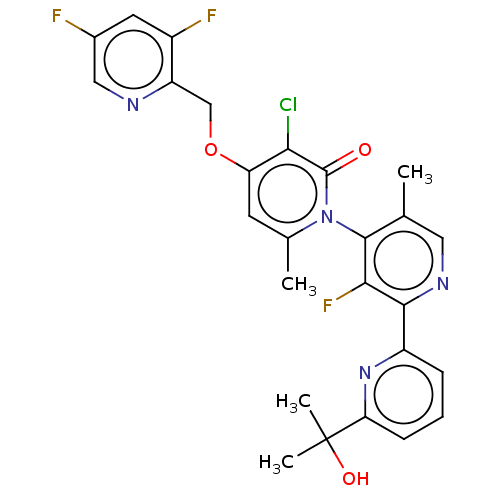 (US11680056, Example 63B) | MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q29G5RXB | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM405272 (1-(4-(7-(2,6-difluorobenzyl)-3-((dimethylamino)met...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., ltd.; Jiangsu Hangrui Medicine Co., Ltd. US Patent | Assay Description In vitro GnRHr protein activity was tested by the following methods.This assay was used to determine the inhibition effect of the present compound on... | US Patent US10633388 (2020) BindingDB Entry DOI: 10.7270/Q2F76GM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM405272 (1-(4-(7-(2,6-difluorobenzyl)-3-((dimethylamino)met...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., Ltd US Patent | Assay Description Human: Test Example 1. Human GnRHr (GnRH Receptor) Activity Assay of the Present Compounds.In vitro GnRHr protein activity was tested by the followin... | US Patent US10344034 (2019) BindingDB Entry DOI: 10.7270/Q29Z978Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM405278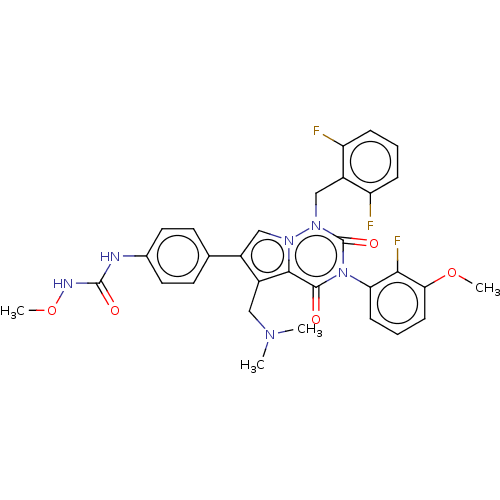 (1-(4-(1-(2,6-difluorobenzyl)-5-((dimethylamino)met...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., Ltd US Patent | Assay Description Human: Test Example 1. Human GnRHr (GnRH Receptor) Activity Assay of the Present Compounds.In vitro GnRHr protein activity was tested by the followin... | US Patent US10344034 (2019) BindingDB Entry DOI: 10.7270/Q29Z978Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM405278 (1-(4-(1-(2,6-difluorobenzyl)-5-((dimethylamino)met...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., ltd.; Jiangsu Hangrui Medicine Co., Ltd. US Patent | Assay Description In vitro GnRHr protein activity was tested by the following methods.This assay was used to determine the inhibition effect of the present compound on... | US Patent US10633388 (2020) BindingDB Entry DOI: 10.7270/Q2F76GM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM405270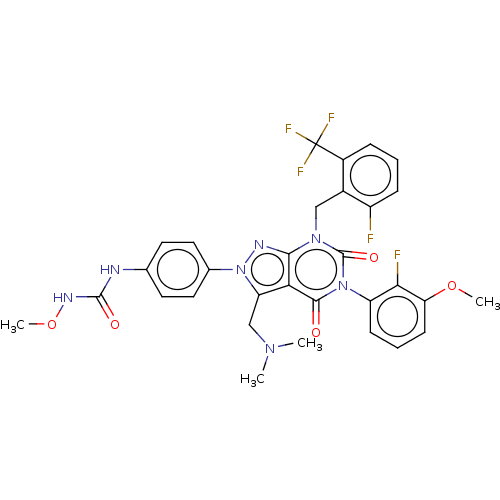 (1-(4-(3-((dimethylamino)methyl)-5-(2-fluoro-3-meth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., ltd.; Jiangsu Hangrui Medicine Co., Ltd. US Patent | Assay Description In vitro GnRHr protein activity was tested by the following methods.This assay was used to determine the inhibition effect of the present compound on... | US Patent US10633388 (2020) BindingDB Entry DOI: 10.7270/Q2F76GM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM405270 (1-(4-(3-((dimethylamino)methyl)-5-(2-fluoro-3-meth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., Ltd US Patent | Assay Description Human: Test Example 1. Human GnRHr (GnRH Receptor) Activity Assay of the Present Compounds.In vitro GnRHr protein activity was tested by the followin... | US Patent US10344034 (2019) BindingDB Entry DOI: 10.7270/Q29Z978Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM202338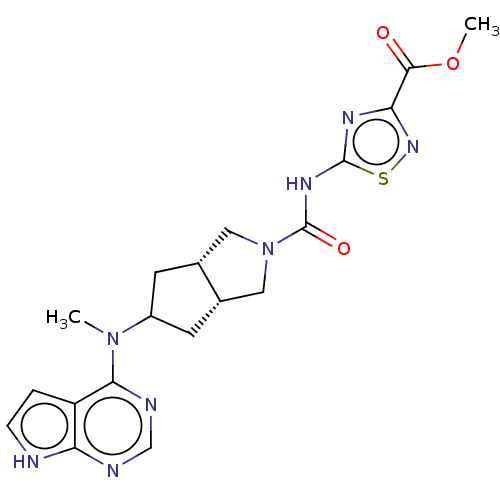 ((3ar,5s,6as)-n-(3-(hydroxymethyl)-1,2,4-thiadiazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Jiangsu Hengrui Medicine Co., Ltd.; Shanghai Hengrui Pharmaceutical Co., Ltd. US Patent | Assay Description TBDIn vitro kinase assays described below can be used to determine the activity of a test compound for inhibiting the activity of JAK1 kinase. The te... | US Patent US9527851 (2016) BindingDB Entry DOI: 10.7270/Q2D21VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50204291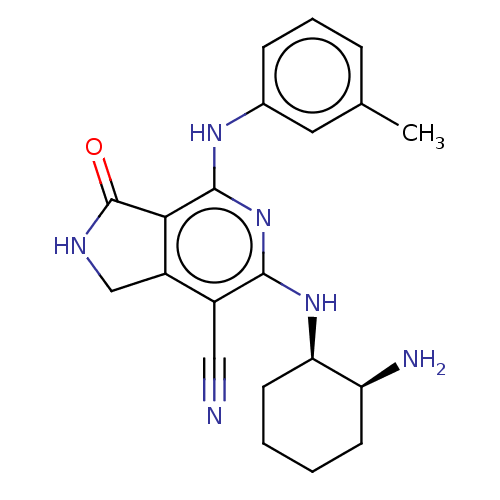 (CHEMBL3953104 | US20230295171, Example 76) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, Inc. Curated by ChEMBL | Assay Description Inhibition of human C-terminal 6His-tagged SYK (356 to 635 residues) expressed in Sf9 insect cells using 5-carboxyfluorescein(FAM)-EEPLYWSFPAKKK-NH2 ... | Bioorg Med Chem Lett 26: 5947-5950 (2016) Article DOI: 10.1016/j.bmcl.2016.10.087 BindingDB Entry DOI: 10.7270/Q29888Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM260479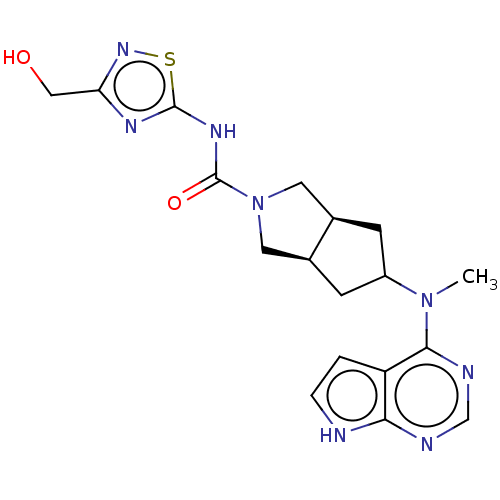 (US9527851, 50) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.3 | 27 |
Jiangsu Hengrui Medicine Co., Ltd.; Shanghai Hengrui Pharmaceutical Co., Ltd. US Patent | Assay Description In vitro kinase assays described below can be used to determine the activity of a test compound for inhibiting the activity of JAK1 kinase. The test ... | US Patent US9527851 (2016) BindingDB Entry DOI: 10.7270/Q2HD7TM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM415473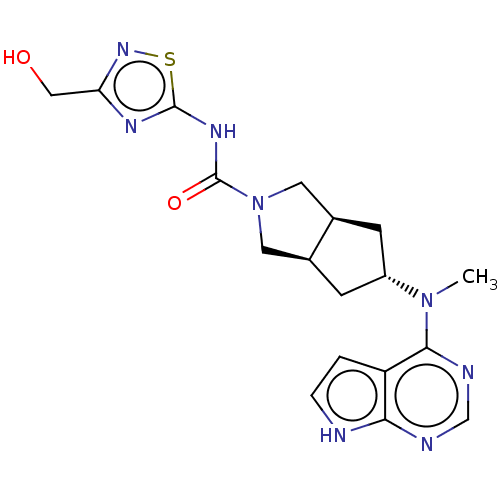 ((3aR,5S,6aS)?N-(3-(Hydroxymethyl)-1,2,4-thiadiazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Hengrui Medicine Co., Ltd. US Patent | Assay Description JAK1: In vitro kinase assays described below can be used to determine the activity of a test compound for inhibiting the activity of JAK1 kinase. The... | US Patent US10428074 (2019) BindingDB Entry DOI: 10.7270/Q26D5WC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50204291 (CHEMBL3953104 | US20230295171, Example 76) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2358 total ) | Next | Last >> |