 Found 141 hits with Last Name = 'maurya' and Initial = 'r'
Found 141 hits with Last Name = 'maurya' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50150105
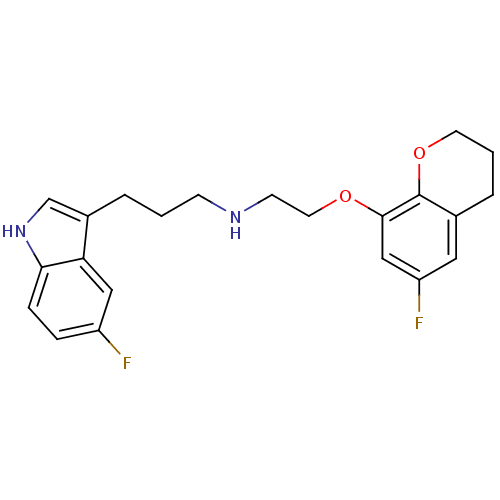
(CHEMBL124069 | [2-(6-Fluoro-chroman-8-yloxy)-ethyl...)Show SMILES Fc1cc2CCCOc2c(OCCNCCCc2c[nH]c3ccc(F)cc23)c1 Show InChI InChI=1S/C22H24F2N2O2/c23-17-5-6-20-19(12-17)16(14-26-20)3-1-7-25-8-10-27-21-13-18(24)11-15-4-2-9-28-22(15)21/h5-6,11-14,25-26H,1-4,7-10H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ISF College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of 5HT transporter (unknown origin) |
Eur J Med Chem 180: 562-612 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.019
BindingDB Entry DOI: 10.7270/Q2K93BWX |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50150093
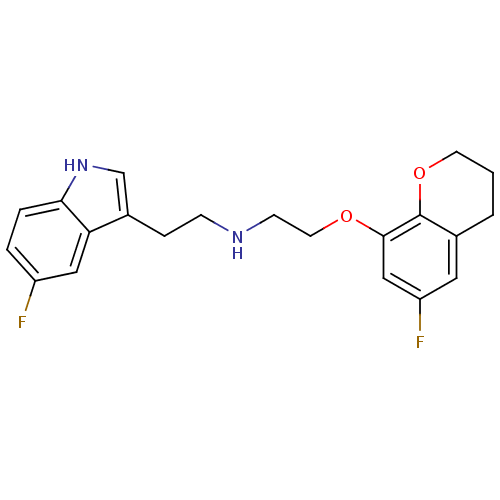
(CHEMBL340873 | [2-(6-Fluoro-chroman-8-yloxy)-ethyl...)Show InChI InChI=1S/C21H22F2N2O2/c22-16-3-4-19-18(11-16)15(13-25-19)5-6-24-7-9-26-20-12-17(23)10-14-2-1-8-27-21(14)20/h3-4,10-13,24-25H,1-2,5-9H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ISF College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of 5HT transporter (unknown origin) |
Eur J Med Chem 180: 562-612 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.019
BindingDB Entry DOI: 10.7270/Q2K93BWX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50150105

(CHEMBL124069 | [2-(6-Fluoro-chroman-8-yloxy)-ethyl...)Show SMILES Fc1cc2CCCOc2c(OCCNCCCc2c[nH]c3ccc(F)cc23)c1 Show InChI InChI=1S/C22H24F2N2O2/c23-17-5-6-20-19(12-17)16(14-26-20)3-1-7-25-8-10-27-21-13-18(24)11-15-4-2-9-28-22(15)21/h5-6,11-14,25-26H,1-4,7-10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ISF College of Pharmacy
Curated by ChEMBL
| Assay Description
Agonist activity at 5HT1A receptor (unknown origin) |
Eur J Med Chem 180: 562-612 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.019
BindingDB Entry DOI: 10.7270/Q2K93BWX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50150093

(CHEMBL340873 | [2-(6-Fluoro-chroman-8-yloxy)-ethyl...)Show InChI InChI=1S/C21H22F2N2O2/c22-16-3-4-19-18(11-16)15(13-25-19)5-6-24-7-9-26-20-12-17(23)10-14-2-1-8-27-21(14)20/h3-4,10-13,24-25H,1-2,5-9H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ISF College of Pharmacy
Curated by ChEMBL
| Assay Description
Agonist activity at 5HT1A receptor (unknown origin) |
Eur J Med Chem 180: 562-612 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.019
BindingDB Entry DOI: 10.7270/Q2K93BWX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM93118

(PTP1B Inhibitor, 19)Show SMILES CC(=O)Oc1ccc(C2=NOC(C2)c2ccccc2)c(OC(C)=O)c1 |t:8| Show InChI InChI=1S/C19H17NO5/c1-12(21)23-15-8-9-16(19(10-15)24-13(2)22)17-11-18(25-20-17)14-6-4-3-5-7-14/h3-10,18H,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.50E+4 | n/a | 6.27E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266158
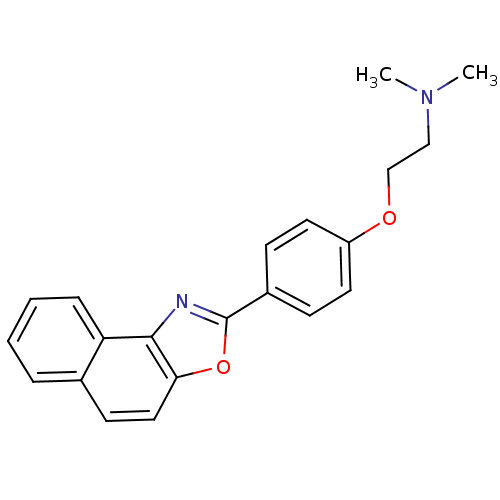
(CHEMBL510225 | Dimethyl-[2-(4-naphtho[1,2-d]oxazol...)Show InChI InChI=1S/C21H20N2O2/c1-23(2)13-14-24-17-10-7-16(8-11-17)21-22-20-18-6-4-3-5-15(18)9-12-19(20)25-21/h3-12H,13-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266161
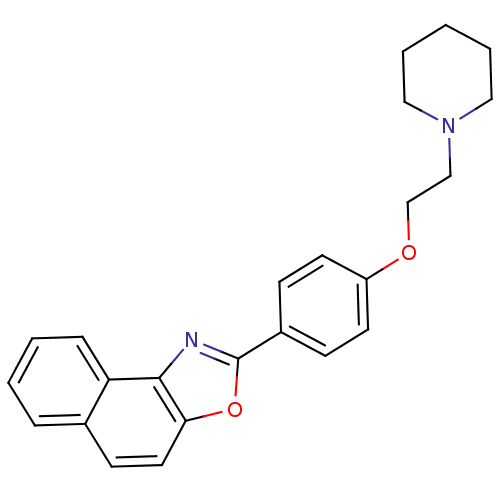
(2-[4-(2-Piperidine-1-yl-ethoxy)-phenyl]-naphtho[1,...)Show SMILES C(CN1CCCCC1)Oc1ccc(cc1)-c1nc2c(ccc3ccccc23)o1 Show InChI InChI=1S/C24H24N2O2/c1-4-14-26(15-5-1)16-17-27-20-11-8-19(9-12-20)24-25-23-21-7-3-2-6-18(21)10-13-22(23)28-24/h2-3,6-13H,1,4-5,14-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity in presence of Triton X-100 |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50182133
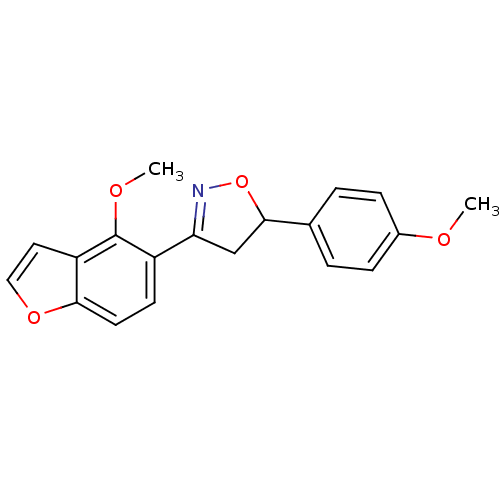
(3-(4-methoxybenzofuran-5-yl)-5-(4-methoxyphenyl)-4...)Show SMILES COc1ccc(cc1)C1CC(=NO1)c1ccc2occc2c1OC |c:11| Show InChI InChI=1S/C19H17NO4/c1-21-13-5-3-12(4-6-13)18-11-16(20-24-18)14-7-8-17-15(9-10-23-17)19(14)22-2/h3-10,18H,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 16: 2139-43 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.062
BindingDB Entry DOI: 10.7270/Q29K49ST |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266161

(2-[4-(2-Piperidine-1-yl-ethoxy)-phenyl]-naphtho[1,...)Show SMILES C(CN1CCCCC1)Oc1ccc(cc1)-c1nc2c(ccc3ccccc23)o1 Show InChI InChI=1S/C24H24N2O2/c1-4-14-26(15-5-1)16-17-27-20-11-8-19(9-12-20)24-25-23-21-7-3-2-6-18(21)10-13-22(23)28-24/h2-3,6-13H,1,4-5,14-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266158

(CHEMBL510225 | Dimethyl-[2-(4-naphtho[1,2-d]oxazol...)Show InChI InChI=1S/C21H20N2O2/c1-23(2)13-14-24-17-10-7-16(8-11-17)21-22-20-18-6-4-3-5-15(18)9-12-19(20)25-21/h3-12H,13-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity in presence of Triton X-100 |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM93109

(PTP1B Inhibitor, 5)Show SMILES COc1c(ccc2OC(C)(C)C=Cc12)C1=NOC(C1)c1ccccc1 |c:11,t:16| Show InChI InChI=1S/C21H21NO3/c1-21(2)12-11-16-18(24-21)10-9-15(20(16)23-3)17-13-19(25-22-17)14-7-5-4-6-8-14/h4-12,19H,13H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 3.00E+4 | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50182134
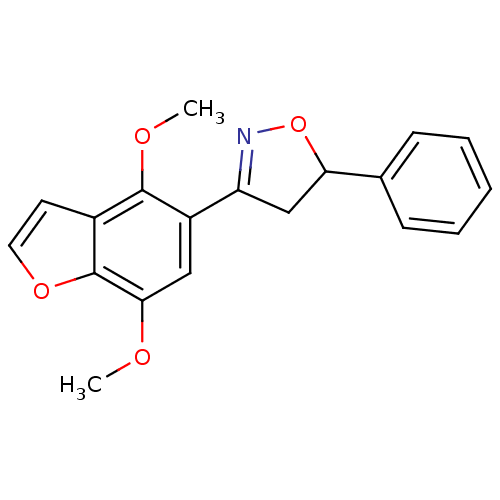
(3-(4,7-dimethoxybenzofuran-5-yl)-5-phenyl-4,5-dihy...)Show SMILES COc1cc(C2=NOC(C2)c2ccccc2)c(OC)c2ccoc12 |t:5| Show InChI InChI=1S/C19H17NO4/c1-21-17-10-14(18(22-2)13-8-9-23-19(13)17)15-11-16(24-20-15)12-6-4-3-5-7-12/h3-10,16H,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 16: 2139-43 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.062
BindingDB Entry DOI: 10.7270/Q29K49ST |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50182131
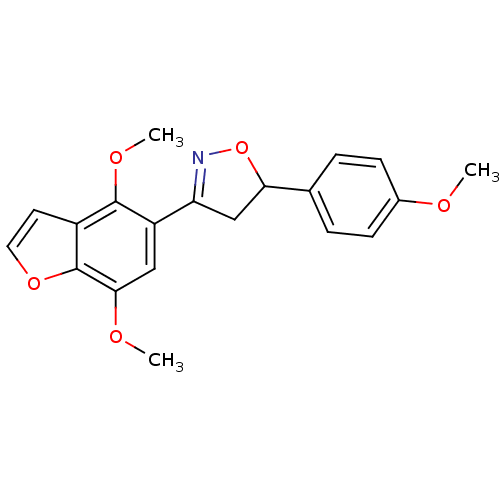
(3-(4,7-dimethoxybenzofuran-5-yl)-5-(4-methoxypheny...)Show SMILES COc1ccc(cc1)C1CC(=NO1)c1cc(OC)c2occc2c1OC |c:11| Show InChI InChI=1S/C20H19NO5/c1-22-13-6-4-12(5-7-13)17-11-16(21-26-17)15-10-18(23-2)20-14(8-9-25-20)19(15)24-3/h4-10,17H,11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 16: 2139-43 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.062
BindingDB Entry DOI: 10.7270/Q29K49ST |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM93111
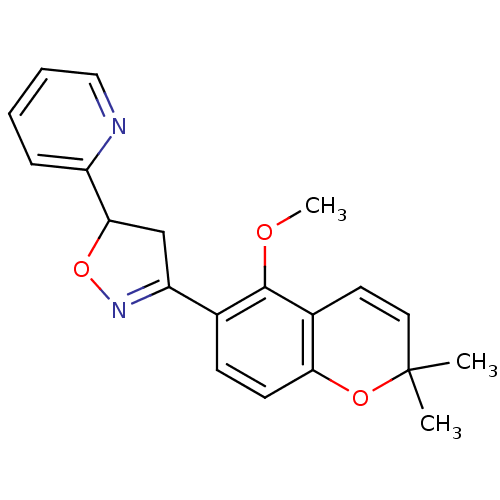
(PTP1B Inhibitor, 7)Show SMILES COc1c(ccc2OC(C)(C)C=Cc12)C1=NOC(C1)c1ccccn1 |c:11,t:16| Show InChI InChI=1S/C20H20N2O3/c1-20(2)10-9-14-17(24-20)8-7-13(19(14)23-3)16-12-18(25-22-16)15-6-4-5-11-21-15/h4-11,18H,12H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.40E+4 | n/a | 8.70E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266231

(CHEMBL510460 | Dimethyl-[3-(4-naphtho[1,2-d]oxazol...)Show InChI InChI=1S/C22H22N2O2/c1-24(2)14-5-15-25-18-11-8-17(9-12-18)22-23-21-19-7-4-3-6-16(19)10-13-20(21)26-22/h3-4,6-13H,5,14-15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266160

(2-[4-(2-Pyrrolidin-1-yl-ethoxy)-phenyl]-naphtho[1,...)Show SMILES C(CN1CCCC1)Oc1ccc(cc1)-c1nc2c(ccc3ccccc23)o1 Show InChI InChI=1S/C23H22N2O2/c1-2-6-20-17(5-1)9-12-21-22(20)24-23(27-21)18-7-10-19(11-8-18)26-16-15-25-13-3-4-14-25/h1-2,5-12H,3-4,13-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266159
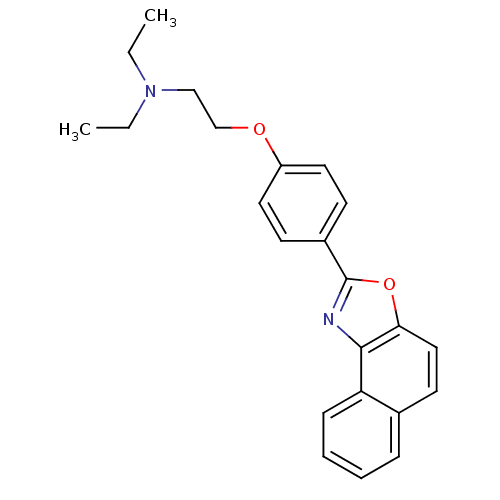
(CHEMBL506882 | Diethyl-[2-(4-naphtho[1,2-d]oxazol-...)Show InChI InChI=1S/C23H24N2O2/c1-3-25(4-2)15-16-26-19-12-9-18(10-13-19)23-24-22-20-8-6-5-7-17(20)11-14-21(22)27-23/h5-14H,3-4,15-16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266231

(CHEMBL510460 | Dimethyl-[3-(4-naphtho[1,2-d]oxazol...)Show InChI InChI=1S/C22H22N2O2/c1-24(2)14-5-15-25-18-11-8-17(9-12-18)22-23-21-19-7-4-3-6-16(19)10-13-20(21)26-22/h3-4,6-13H,5,14-15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity in presence of Triton X-100 |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266160

(2-[4-(2-Pyrrolidin-1-yl-ethoxy)-phenyl]-naphtho[1,...)Show SMILES C(CN1CCCC1)Oc1ccc(cc1)-c1nc2c(ccc3ccccc23)o1 Show InChI InChI=1S/C23H22N2O2/c1-2-6-20-17(5-1)9-12-21-22(20)24-23(27-21)18-7-10-19(11-8-18)26-16-15-25-13-3-4-14-25/h1-2,5-12H,3-4,13-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity in presence of Triton X-100 |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266232
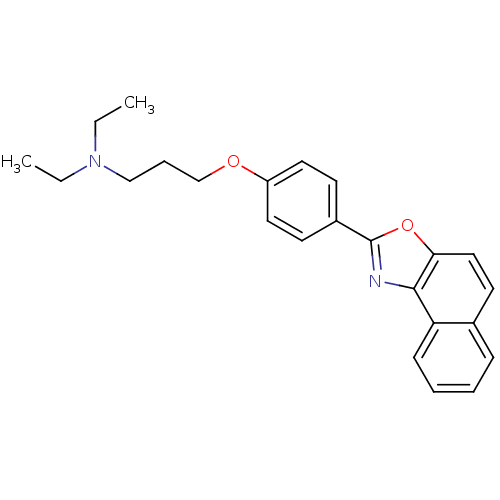
(CHEMBL458009 | Diethyl-[3-(4-naphtho[1,2-d]oxazol-...)Show SMILES CCN(CC)CCCOc1ccc(cc1)-c1nc2c(ccc3ccccc23)o1 Show InChI InChI=1S/C24H26N2O2/c1-3-26(4-2)16-7-17-27-20-13-10-19(11-14-20)24-25-23-21-9-6-5-8-18(21)12-15-22(23)28-24/h5-6,8-15H,3-4,7,16-17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266162
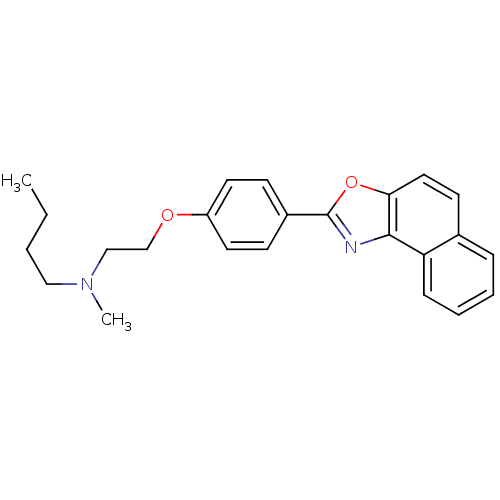
(Butyl-methyl-[2-(4-naphtho[1,2-d]oxazol-2-yl-pheno...)Show SMILES CCCCN(C)CCOc1ccc(cc1)-c1nc2c(ccc3ccccc23)o1 Show InChI InChI=1S/C24H26N2O2/c1-3-4-15-26(2)16-17-27-20-12-9-19(10-13-20)24-25-23-21-8-6-5-7-18(21)11-14-22(23)28-24/h5-14H,3-4,15-17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity in presence of Triton X-100 |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266232

(CHEMBL458009 | Diethyl-[3-(4-naphtho[1,2-d]oxazol-...)Show SMILES CCN(CC)CCCOc1ccc(cc1)-c1nc2c(ccc3ccccc23)o1 Show InChI InChI=1S/C24H26N2O2/c1-3-26(4-2)16-7-17-27-20-13-10-19(11-14-20)24-25-23-21-9-6-5-8-18(21)12-15-22(23)28-24/h5-6,8-15H,3-4,7,16-17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity in presence of Triton X-100 |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266162

(Butyl-methyl-[2-(4-naphtho[1,2-d]oxazol-2-yl-pheno...)Show SMILES CCCCN(C)CCOc1ccc(cc1)-c1nc2c(ccc3ccccc23)o1 Show InChI InChI=1S/C24H26N2O2/c1-3-4-15-26(2)16-17-27-20-12-9-19(10-13-20)24-25-23-21-8-6-5-7-18(21)11-14-22(23)28-24/h5-14H,3-4,15-17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50182135

(3-(4-methoxybenzofuran-5-yl)-5-phenyl-4,5-dihydroi...)Show InChI InChI=1S/C18H15NO3/c1-20-18-13(7-8-16-14(18)9-10-21-16)15-11-17(22-19-15)12-5-3-2-4-6-12/h2-10,17H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 16: 2139-43 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.062
BindingDB Entry DOI: 10.7270/Q29K49ST |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM93113
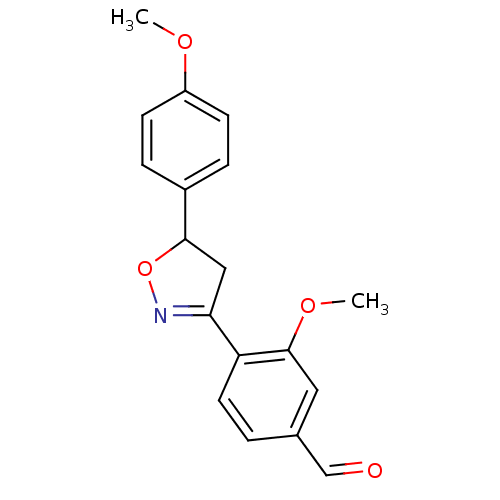
(PTP1B Inhibitor, 11)Show SMILES COc1ccc(cc1)C1CC(=NO1)c1ccc(C=O)cc1OC |c:11| Show InChI InChI=1S/C18H17NO4/c1-21-14-6-4-13(5-7-14)17-10-16(19-23-17)15-8-3-12(11-20)9-18(15)22-2/h3-9,11,17H,10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 4.80E+4 | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM93112
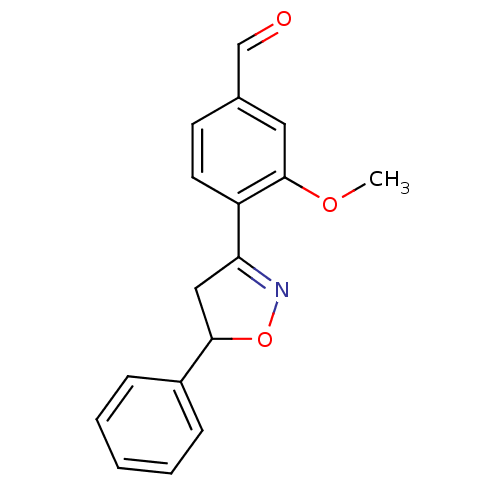
(PTP1B Inhibitor, 10)Show InChI InChI=1S/C17H15NO3/c1-20-17-9-12(11-19)7-8-14(17)15-10-16(21-18-15)13-5-3-2-4-6-13/h2-9,11,16H,10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 4.80E+4 | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50487368
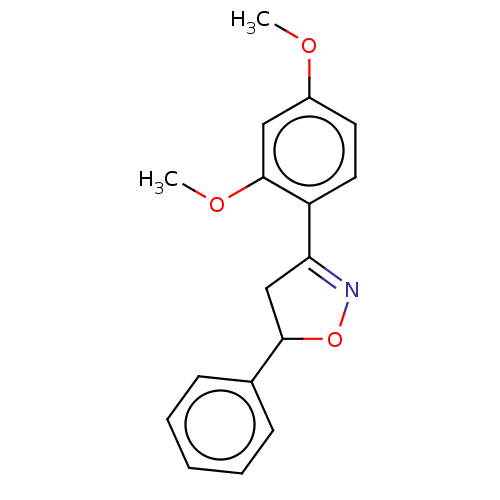
(CHEMBL2259740)Show InChI InChI=1S/C17H17NO3/c1-19-13-8-9-14(17(10-13)20-2)15-11-16(21-18-15)12-6-4-3-5-7-12/h3-10,16H,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50487364
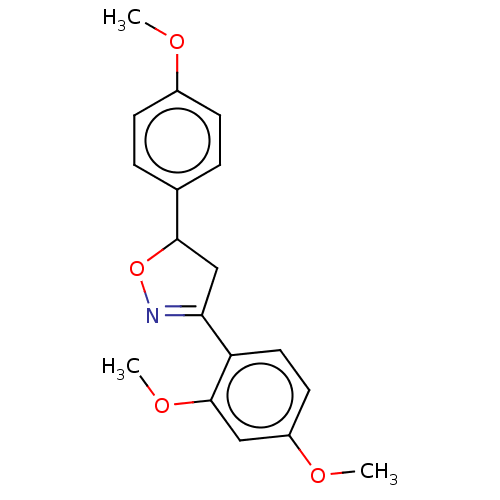
(CHEMBL2259741)Show SMILES COc1ccc(cc1)C1CC(=NO1)c1ccc(OC)cc1OC |c:11| Show InChI InChI=1S/C18H19NO4/c1-20-13-6-4-12(5-7-13)17-11-16(19-23-17)15-9-8-14(21-2)10-18(15)22-3/h4-10,17H,11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM93110
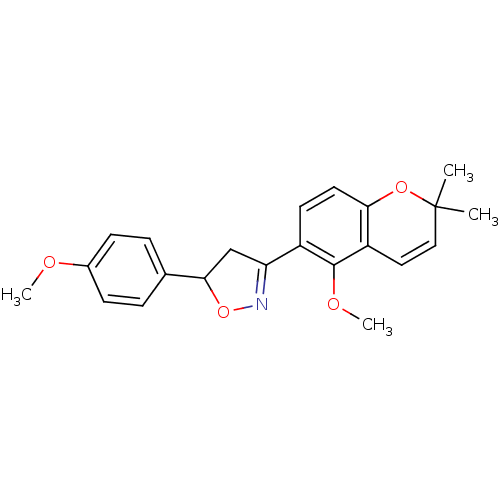
(PTP1B Inhibitor, 6)Show SMILES COc1ccc(cc1)C1CC(=NO1)c1ccc2OC(C)(C)C=Cc2c1OC |c:11,23| Show InChI InChI=1S/C22H23NO4/c1-22(2)12-11-17-19(26-22)10-9-16(21(17)25-4)18-13-20(27-23-18)14-5-7-15(24-3)8-6-14/h5-12,20H,13H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 5.60E+4 | n/a | 9.10E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM93116

(PTP1B Inhibitor, 14)Show InChI InChI=1S/C14H13N3O3/c1-19-13-6-10(8-18)2-3-11(13)12-7-14(20-16-12)17-5-4-15-9-17/h2-6,8-9,14H,7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 6.30E+4 | n/a | 8.80E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50487366
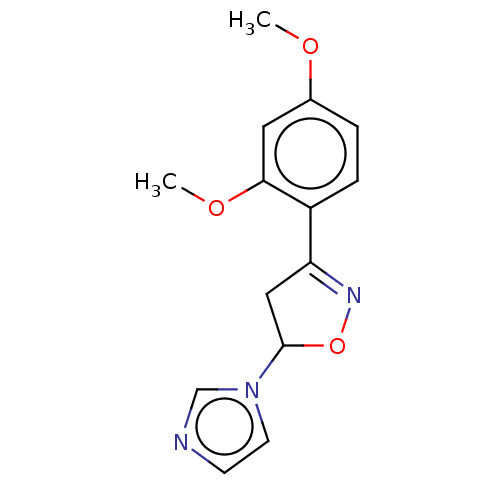
(CHEMBL2259730)Show InChI InChI=1S/C14H15N3O3/c1-18-10-3-4-11(13(7-10)19-2)12-8-14(20-16-12)17-6-5-15-9-17/h3-7,9,14H,8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50182132
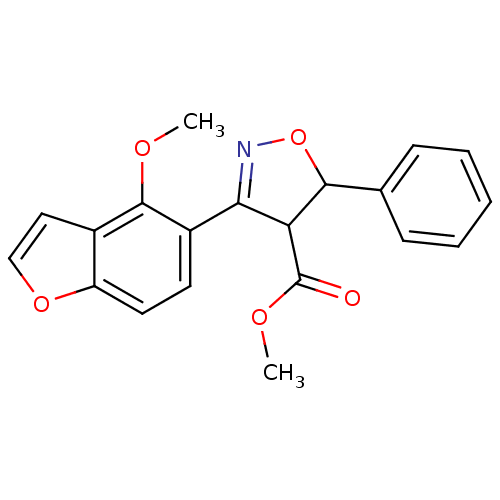
(CHEMBL205730 | methyl 3-(4-methoxybenzofuran-5-yl)...)Show SMILES COC(=O)C1C(ON=C1c1ccc2occc2c1OC)c1ccccc1 |c:7| Show InChI InChI=1S/C20H17NO5/c1-23-19-13-10-11-25-15(13)9-8-14(19)17-16(20(22)24-2)18(26-21-17)12-6-4-3-5-7-12/h3-11,16,18H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 16: 2139-43 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.062
BindingDB Entry DOI: 10.7270/Q29K49ST |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM93117
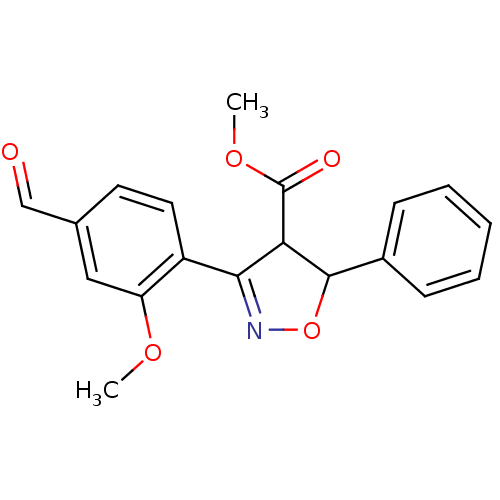
(PTP1B Inhibitor, 15)Show SMILES COC(=O)C1C(ON=C1c1ccc(C=O)cc1OC)c1ccccc1 |c:7| Show InChI InChI=1S/C19H17NO5/c1-23-15-10-12(11-21)8-9-14(15)17-16(19(22)24-2)18(25-20-17)13-6-4-3-5-7-13/h3-11,16,18H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.35E+5 | n/a | 1.65E+5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50487365
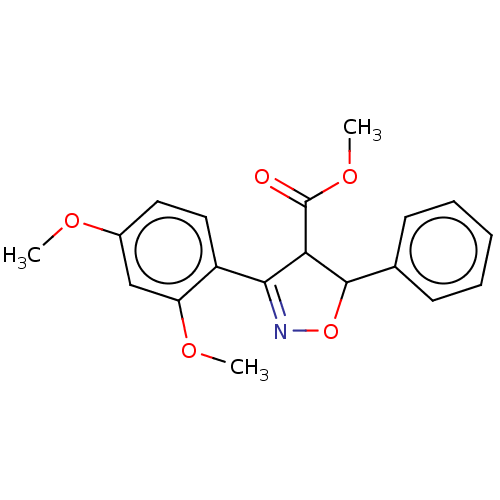
(CHEMBL2259731)Show SMILES COC(=O)C1C(ON=C1c1ccc(OC)cc1OC)c1ccccc1 |c:7| Show InChI InChI=1S/C19H19NO5/c1-22-13-9-10-14(15(11-13)23-2)17-16(19(21)24-3)18(25-20-17)12-7-5-4-6-8-12/h4-11,16,18H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM93115
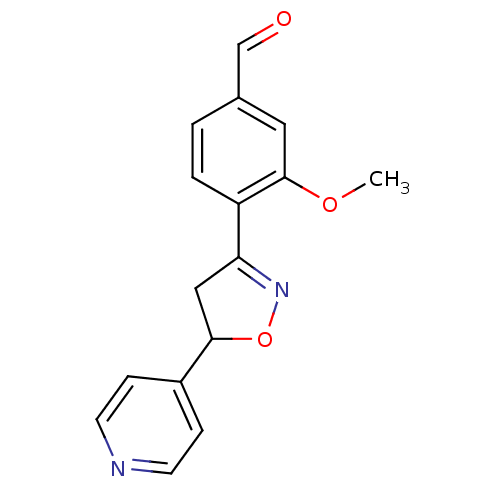
(PTP1B Inhibitor, 13)Show InChI InChI=1S/C16H14N2O3/c1-20-16-8-11(10-19)2-3-13(16)14-9-15(21-18-14)12-4-6-17-7-5-12/h2-8,10,15H,9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 1.50E+5 | n/a | 2.75E+5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50487367
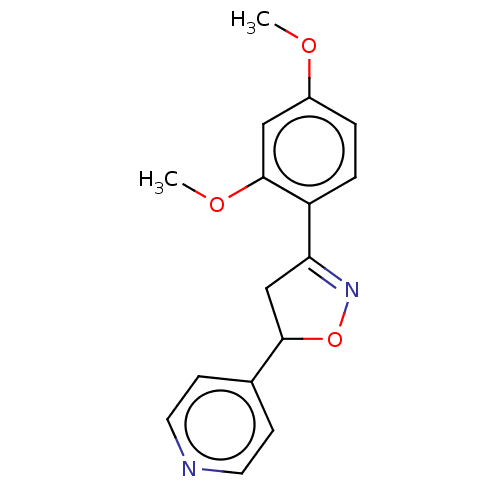
(CHEMBL2259743)Show InChI InChI=1S/C16H16N2O3/c1-19-12-3-4-13(16(9-12)20-2)14-10-15(21-18-14)11-5-7-17-8-6-11/h3-9,15H,10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM93114

(PTP1B Inhibitor, 12)Show InChI InChI=1S/C16H14N2O3/c1-20-15-8-11(10-19)5-6-12(15)14-9-16(21-18-14)13-4-2-3-7-17-13/h2-8,10,16H,9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.70E+5 | n/a | 4.85E+5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50487369
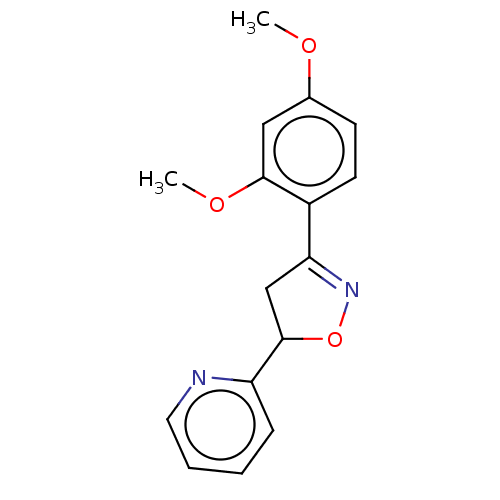
(CHEMBL2259742)Show InChI InChI=1S/C16H16N2O3/c1-19-11-6-7-12(15(9-11)20-2)14-10-16(21-18-14)13-5-3-4-8-17-13/h3-9,16H,10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266159

(CHEMBL506882 | Diethyl-[2-(4-naphtho[1,2-d]oxazol-...)Show InChI InChI=1S/C23H24N2O2/c1-3-25(4-2)15-16-26-19-12-9-18(10-13-19)23-24-22-20-8-6-5-7-17(20)11-14-21(22)27-23/h5-14H,3-4,15-16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.55E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity in presence of Triton X-100 |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50346150

(5-(2-(2-(1H-pyrrol-1-yl)ethoxy)-4-methoxyphenyl)-3...)Show SMILES COc1ccc(-c2cc(no2)-c2ccccc2)c(OCCN2CCCC2)c1 Show InChI InChI=1S/C22H24N2O3/c1-25-18-9-10-19(21(15-18)26-14-13-24-11-5-6-12-24)22-16-20(23-27-22)17-7-3-2-4-8-17/h2-4,7-10,15-16H,5-6,11-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of protein tyrosine phosphatase-1B at 10 uM incubated for 10 mins using pNPP substrate in absence of Triton X-100 by modified Goldstein me... |
Bioorg Med Chem 17: 5285-92 (2009)
Article DOI: 10.1016/j.bmc.2009.05.033
BindingDB Entry DOI: 10.7270/Q2J966QV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50346150

(5-(2-(2-(1H-pyrrol-1-yl)ethoxy)-4-methoxyphenyl)-3...)Show SMILES COc1ccc(-c2cc(no2)-c2ccccc2)c(OCCN2CCCC2)c1 Show InChI InChI=1S/C22H24N2O3/c1-25-18-9-10-19(21(15-18)26-14-13-24-11-5-6-12-24)22-16-20(23-27-22)17-7-3-2-4-8-17/h2-4,7-10,15-16H,5-6,11-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of protein tyrosine phosphatase-1B at 10 uM incubated for 10 mins using pNPP substrate in presence of 0.01% Triton X-100 by modified Golds... |
Bioorg Med Chem 17: 5285-92 (2009)
Article DOI: 10.1016/j.bmc.2009.05.033
BindingDB Entry DOI: 10.7270/Q2J966QV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266161

(2-[4-(2-Piperidine-1-yl-ethoxy)-phenyl]-naphtho[1,...)Show SMILES C(CN1CCCCC1)Oc1ccc(cc1)-c1nc2c(ccc3ccccc23)o1 Show InChI InChI=1S/C24H24N2O2/c1-4-14-26(15-5-1)16-17-27-20-11-8-19(9-12-20)24-25-23-21-7-3-2-6-18(21)10-13-22(23)28-24/h2-3,6-13H,1,4-5,14-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity in presence of Triton X-100 |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266161

(2-[4-(2-Piperidine-1-yl-ethoxy)-phenyl]-naphtho[1,...)Show SMILES C(CN1CCCCC1)Oc1ccc(cc1)-c1nc2c(ccc3ccccc23)o1 Show InChI InChI=1S/C24H24N2O2/c1-4-14-26(15-5-1)16-17-27-20-11-8-19(9-12-20)24-25-23-21-7-3-2-6-18(21)10-13-22(23)28-24/h2-3,6-13H,1,4-5,14-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50516934

(CHEMBL4468611)Show SMILES CCCCC(O)(c1c(NC(=O)OCC)[nH]c2ccc(Br)cc12)C(F)(F)F Show InChI InChI=1S/C17H20BrF3N2O3/c1-3-5-8-16(25,17(19,20)21)13-11-9-10(18)6-7-12(11)22-14(13)23-15(24)26-4-2/h6-7,9,22,25H,3-5,8H2,1-2H3,(H,23,24) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ISF College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 3B Y181C mutant infected in human MT2 cells assessed as reduction in virus-induced cytopathogenicity after 3 days by BrightGlo luc... |
Eur J Med Chem 180: 562-612 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.019
BindingDB Entry DOI: 10.7270/Q2K93BWX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50346151

(4-(2-(5-methoxy-2-(3-phenylisoxazol-5-yl)phenoxy)e...)Show SMILES COc1ccc(-c2cc(no2)-c2ccccc2)c(OCCN2CCOCC2)c1 Show InChI InChI=1S/C22H24N2O4/c1-25-18-7-8-19(21(15-18)27-14-11-24-9-12-26-13-10-24)22-16-20(23-28-22)17-5-3-2-4-6-17/h2-8,15-16H,9-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of protein tyrosine phosphatase-1B at 10 uM incubated for 10 mins using pNPP substrate in absence of Triton X-100 by modified Goldstein me... |
Bioorg Med Chem 17: 5285-92 (2009)
Article DOI: 10.1016/j.bmc.2009.05.033
BindingDB Entry DOI: 10.7270/Q2J966QV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266162

(Butyl-methyl-[2-(4-naphtho[1,2-d]oxazol-2-yl-pheno...)Show SMILES CCCCN(C)CCOc1ccc(cc1)-c1nc2c(ccc3ccccc23)o1 Show InChI InChI=1S/C24H26N2O2/c1-3-4-15-26(2)16-17-27-20-12-9-19(10-13-20)24-25-23-21-8-6-5-7-18(21)11-14-22(23)28-24/h5-14H,3-4,15-17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266162

(Butyl-methyl-[2-(4-naphtho[1,2-d]oxazol-2-yl-pheno...)Show SMILES CCCCN(C)CCOc1ccc(cc1)-c1nc2c(ccc3ccccc23)o1 Show InChI InChI=1S/C24H26N2O2/c1-3-4-15-26(2)16-17-27-20-12-9-19(10-13-20)24-25-23-21-8-6-5-7-18(21)11-14-22(23)28-24/h5-14H,3-4,15-17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity in presence of Triton X-100 |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50346151

(4-(2-(5-methoxy-2-(3-phenylisoxazol-5-yl)phenoxy)e...)Show SMILES COc1ccc(-c2cc(no2)-c2ccccc2)c(OCCN2CCOCC2)c1 Show InChI InChI=1S/C22H24N2O4/c1-25-18-7-8-19(21(15-18)27-14-11-24-9-12-26-13-10-24)22-16-20(23-28-22)17-5-3-2-4-6-17/h2-8,15-16H,9-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of protein tyrosine phosphatase-1B at 10 uM incubated for 10 mins using pNPP substrate in presence of 0.01% Triton X-100 by modified Golds... |
Bioorg Med Chem 17: 5285-92 (2009)
Article DOI: 10.1016/j.bmc.2009.05.033
BindingDB Entry DOI: 10.7270/Q2J966QV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50346148
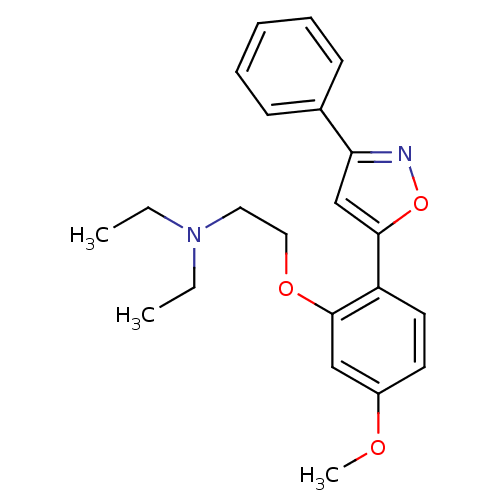
(CHEMBL1783773 | N,N-diethyl-2-(5-methoxy-2-(3-phen...)Show InChI InChI=1S/C22H26N2O3/c1-4-24(5-2)13-14-26-21-15-18(25-3)11-12-19(21)22-16-20(23-27-22)17-9-7-6-8-10-17/h6-12,15-16H,4-5,13-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of protein tyrosine phosphatase-1B at 10 uM incubated for 10 mins using pNPP substrate in presence of 0.01% Triton X-100 by modified Golds... |
Bioorg Med Chem 17: 5285-92 (2009)
Article DOI: 10.1016/j.bmc.2009.05.033
BindingDB Entry DOI: 10.7270/Q2J966QV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50320877

(4-(4-Fluorophenyl)-7,7-dimethyl-2-phenyl-4,6,7,8-t...)Show SMILES CC1(C)CC(=O)C2C(C=C(N=C2C1)c1ccccc1)c1ccc(F)cc1 |c:8,10| Show InChI InChI=1S/C23H22FNO/c1-23(2)13-20-22(21(26)14-23)18(15-8-10-17(24)11-9-15)12-19(25-20)16-6-4-3-5-7-16/h3-12,18,22H,13-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as p-nitrophenolate ion production pretreated for 10 mins measured after 30 mins |
Bioorg Med Chem 18: 4138-48 (2010)
Article DOI: 10.1016/j.bmc.2009.11.061
BindingDB Entry DOI: 10.7270/Q2DJ5FSF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data