 Found 1446 hits with Last Name = 'rampulla' and Initial = 'r'
Found 1446 hits with Last Name = 'rampulla' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50514438
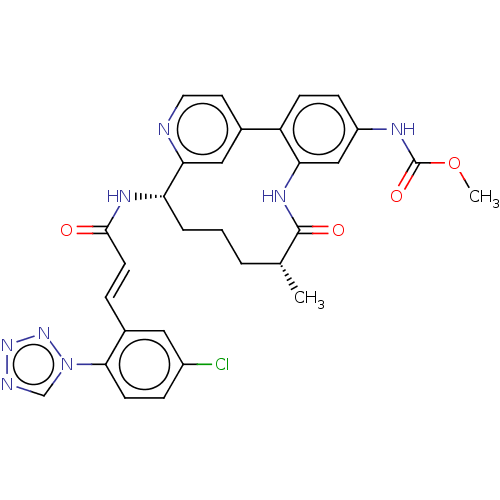
(CHEMBL4439729)Show SMILES OC(=O)C(F)(F)F.COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](NC(=O)\C=C\c3cc(Cl)ccc3-n3cnnn3)c3cc-2ccn3)c1 |r| Show InChI InChI=1S/C30H29ClN8O4.C2HF3O2/c1-18-4-3-5-24(35-28(40)11-6-20-14-21(31)7-10-27(20)39-17-33-37-38-39)26-15-19(12-13-32-26)23-9-8-22(34-30(42)43-2)16-25(23)36-29(18)41;3-2(4,5)1(6)7/h6-18,24H,3-5H2,1-2H3,(H,34,42)(H,35,40)(H,36,41);(H,6,7)/b11-6+;/t18-,24+;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
J Med Chem 63: 784-803 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01768
BindingDB Entry DOI: 10.7270/Q2J67M9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50269186
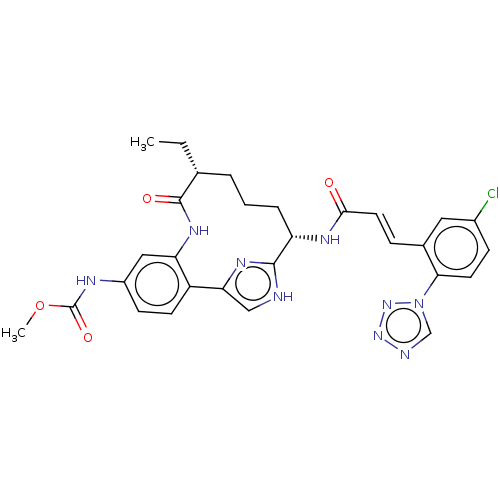
(CHEMBL4089185)Show SMILES CC[C@@H]1CCC[C@H](NC(=O)\C=C\c2cc(Cl)ccc2-n2cnnn2)c2nc(c[nH]2)-c2ccc(NC(=O)OC)cc2NC1=O |r| Show InChI InChI=1S/C29H30ClN9O4/c1-3-17-5-4-6-22(34-26(40)12-7-18-13-19(30)8-11-25(18)39-16-32-37-38-39)27-31-15-24(35-27)21-10-9-20(33-29(42)43-2)14-23(21)36-28(17)41/h7-17,22H,3-6H2,1-2H3,(H,31,35)(H,33,42)(H,34,40)(H,36,41)/b12-7+/t17-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
J Med Chem 63: 784-803 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01768
BindingDB Entry DOI: 10.7270/Q2J67M9S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50514437
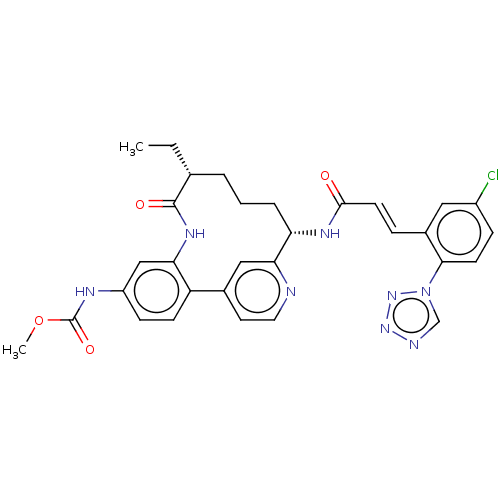
(CHEMBL4444690)Show SMILES CC[C@@H]1CCC[C@H](NC(=O)\C=C\c2cc(Cl)ccc2-n2cnnn2)c2cc(ccn2)-c2ccc(NC(=O)OC)cc2NC1=O |r| Show InChI InChI=1S/C31H31ClN8O4/c1-3-19-5-4-6-25(36-29(41)12-7-21-15-22(32)8-11-28(21)40-18-34-38-39-40)27-16-20(13-14-33-27)24-10-9-23(35-31(43)44-2)17-26(24)37-30(19)42/h7-19,25H,3-6H2,1-2H3,(H,35,43)(H,36,41)(H,37,42)/b12-7+/t19-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
J Med Chem 63: 784-803 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01768
BindingDB Entry DOI: 10.7270/Q2J67M9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50541586
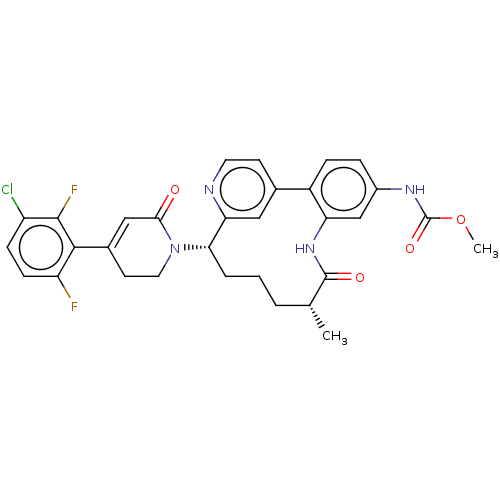
(CHEMBL4638245)Show SMILES COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](N3CCC(=CC3=O)c3c(F)ccc(Cl)c3F)c3cc-2ccn3)c1 |r,c:22| Show InChI InChI=1S/C31H29ClF2N4O4/c1-17-4-3-5-26(38-13-11-19(15-27(38)39)28-23(33)9-8-22(32)29(28)34)25-14-18(10-12-35-25)21-7-6-20(36-31(41)42-2)16-24(21)37-30(17)40/h6-10,12,14-17,26H,3-5,11,13H2,1-2H3,(H,36,41)(H,37,40)/t17-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry |
J Med Chem 63: 7226-7242 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00464
BindingDB Entry DOI: 10.7270/Q2J67MHG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50269195
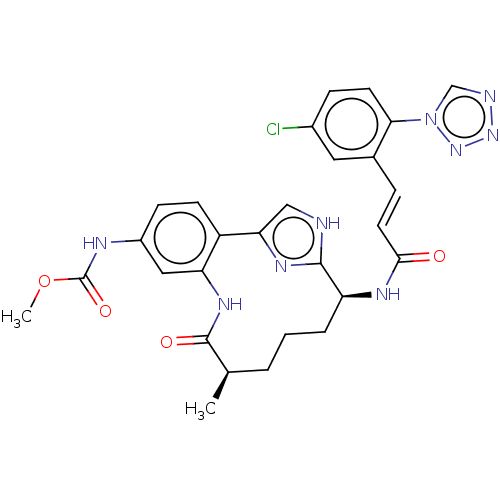
(CHEMBL4101766)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](CCC[C@@H](C)C(=O)Nc2c1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r| Show InChI InChI=1S/C28H28ClN9O4/c1-16-4-3-5-21(33-25(39)11-6-17-12-18(29)7-10-24(17)38-15-31-36-37-38)26-30-14-23(34-26)20-9-8-19(32-28(41)42-2)13-22(20)35-27(16)40/h6-16,21H,3-5H2,1-2H3,(H,30,34)(H,32,41)(H,33,39)(H,35,40)/b11-6+/t16-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
J Med Chem 63: 784-803 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01768
BindingDB Entry DOI: 10.7270/Q2J67M9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50541581

(CHEMBL4646341)Show SMILES COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](N3CCC(NC3=O)c3c(F)ccc(Cl)c3F)c3cc-2ccn3)c1 |r| Show InChI InChI=1S/C30H30ClF2N5O4/c1-16-4-3-5-25(38-13-11-22(37-29(38)40)26-21(32)9-8-20(31)27(26)33)24-14-17(10-12-34-24)19-7-6-18(35-30(41)42-2)15-23(19)36-28(16)39/h6-10,12,14-16,22,25H,3-5,11,13H2,1-2H3,(H,35,41)(H,36,39)(H,37,40)/t16-,22?,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry |
J Med Chem 63: 7226-7242 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00464
BindingDB Entry DOI: 10.7270/Q2J67MHG |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50541577
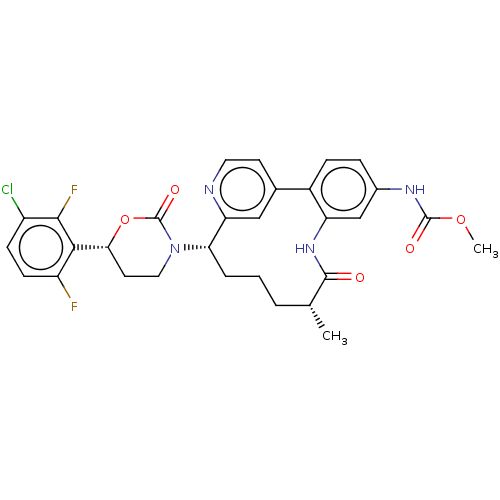
(CHEMBL4646441)Show SMILES COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](N3CC[C@@H](OC3=O)c3c(F)ccc(Cl)c3F)c3cc-2ccn3)c1 |r| Show InChI InChI=1S/C30H29ClF2N4O5/c1-16-4-3-5-24(37-13-11-25(42-30(37)40)26-21(32)9-8-20(31)27(26)33)23-14-17(10-12-34-23)19-7-6-18(35-29(39)41-2)15-22(19)36-28(16)38/h6-10,12,14-16,24-25H,3-5,11,13H2,1-2H3,(H,35,39)(H,36,38)/t16-,24+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry |
J Med Chem 63: 7226-7242 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00464
BindingDB Entry DOI: 10.7270/Q2J67MHG |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50541576
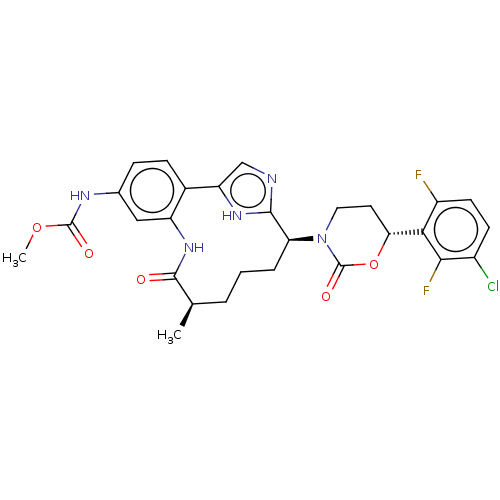
(CHEMBL4644510)Show SMILES COC(=O)Nc1ccc2-c3cnc([nH]3)[C@H](CCC[C@@H](C)C(=O)Nc2c1)N1CC[C@@H](OC1=O)c1c(F)ccc(Cl)c1F |r| Show InChI InChI=1S/C28H28ClF2N5O5/c1-14-4-3-5-21(36-11-10-22(41-28(36)39)23-18(30)9-8-17(29)24(23)31)25-32-13-20(34-25)16-7-6-15(33-27(38)40-2)12-19(16)35-26(14)37/h6-9,12-14,21-22H,3-5,10-11H2,1-2H3,(H,32,34)(H,33,38)(H,35,37)/t14-,21+,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry |
J Med Chem 63: 7226-7242 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00464
BindingDB Entry DOI: 10.7270/Q2J67MHG |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50541582
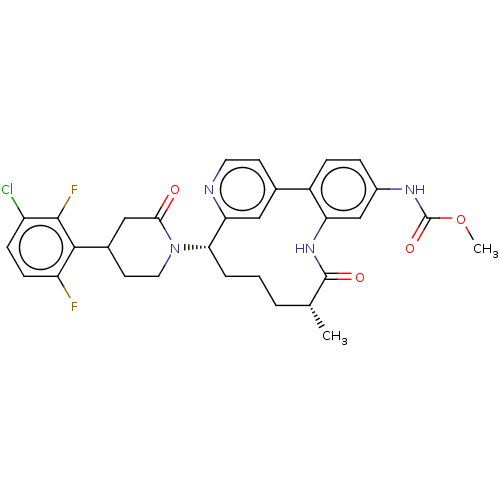
(CHEMBL4636247)Show SMILES COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](N3CCC(CC3=O)c3c(F)ccc(Cl)c3F)c3cc-2ccn3)c1 |r| Show InChI InChI=1S/C31H31ClF2N4O4/c1-17-4-3-5-26(38-13-11-19(15-27(38)39)28-23(33)9-8-22(32)29(28)34)25-14-18(10-12-35-25)21-7-6-20(36-31(41)42-2)16-24(21)37-30(17)40/h6-10,12,14,16-17,19,26H,3-5,11,13,15H2,1-2H3,(H,36,41)(H,37,40)/t17-,19?,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry |
J Med Chem 63: 7226-7242 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00464
BindingDB Entry DOI: 10.7270/Q2J67MHG |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122646

(CHEMBL3623125)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NS(=O)(=O)c1ccccc1F)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C24H19F4N3O5S/c1-22-10-17(30-37(34,35)16-6-4-3-5-15(16)25)23(2,36-22)19-18(22)20(32)31(21(19)33)13-8-7-12(11-29)14(9-13)24(26,27)28/h3-9,17-19,30H,10H2,1-2H3/t17-,18-,19+,22+,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372090
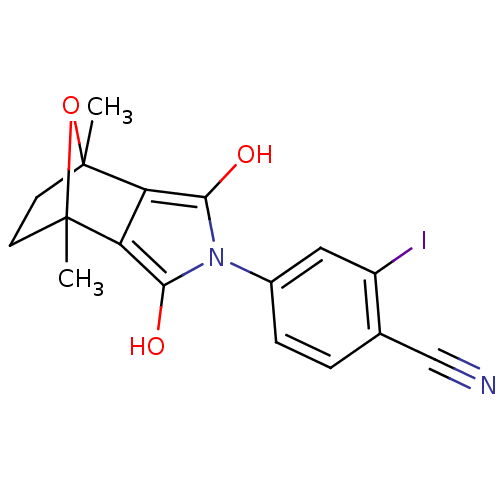
(CHEMBL403668)Show SMILES CC12CCC(C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(I)c1 |THB:11:13:6:2.3,8:7:6:2.3| Show InChI InChI=1S/C17H15IN2O3/c1-16-5-6-17(2,23-16)13-12(16)14(21)20(15(13)22)10-4-3-9(8-19)11(18)7-10/h3-4,7,21-22H,5-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50514439
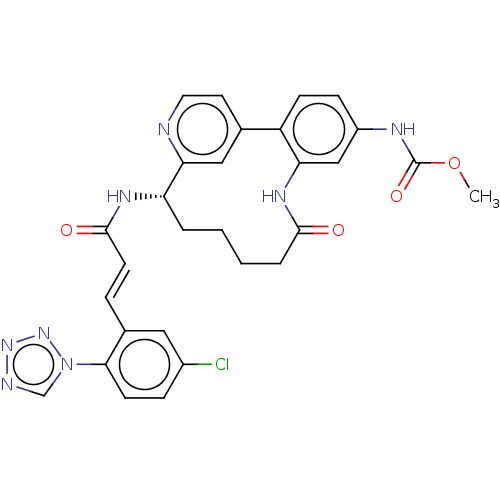
(CHEMBL4456818)Show SMILES COC(=O)Nc1ccc-2c(NC(=O)CCCC[C@H](NC(=O)\C=C\c3cc(Cl)ccc3-n3cnnn3)c3cc-2ccn3)c1 |r| Show InChI InChI=1S/C29H27ClN8O4/c1-42-29(41)33-21-8-9-22-18-12-13-31-25(15-18)23(4-2-3-5-27(39)35-24(22)16-21)34-28(40)11-6-19-14-20(30)7-10-26(19)38-17-32-36-37-38/h6-17,23H,2-5H2,1H3,(H,33,41)(H,34,40)(H,35,39)/b11-6+/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
J Med Chem 63: 784-803 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01768
BindingDB Entry DOI: 10.7270/Q2J67M9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50541579

(CHEMBL4637480)Show SMILES COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](N3CC[C@@H](OC3=O)c3c(F)ccc(Cl)c3F)c3ccnc-2c3)c1 |r| Show InChI InChI=1S/C30H29ClF2N4O5/c1-16-4-3-5-24(37-13-11-25(42-30(37)40)26-21(32)9-8-20(31)27(26)33)17-10-12-34-22(14-17)19-7-6-18(35-29(39)41-2)15-23(19)36-28(16)38/h6-10,12,14-16,24-25H,3-5,11,13H2,1-2H3,(H,35,39)(H,36,38)/t16-,24+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry |
J Med Chem 63: 7226-7242 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00464
BindingDB Entry DOI: 10.7270/Q2J67MHG |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122650
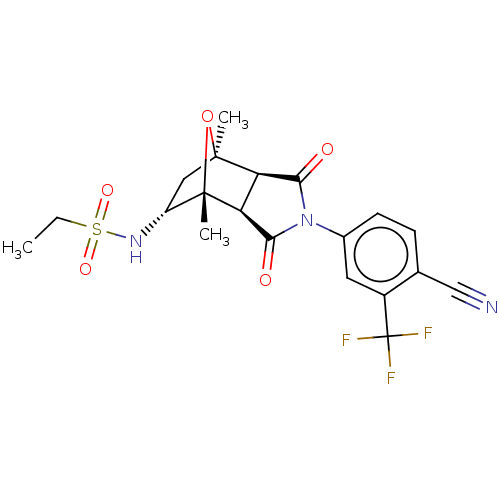
(CHEMBL3623127)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NS(=O)(=O)CC)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C20H20F3N3O5S/c1-4-32(29,30)25-13-8-18(2)14-15(19(13,3)31-18)17(28)26(16(14)27)11-6-5-10(9-24)12(7-11)20(21,22)23/h5-7,13-15,25H,4,8H2,1-3H3/t13-,14-,15+,18+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50541578
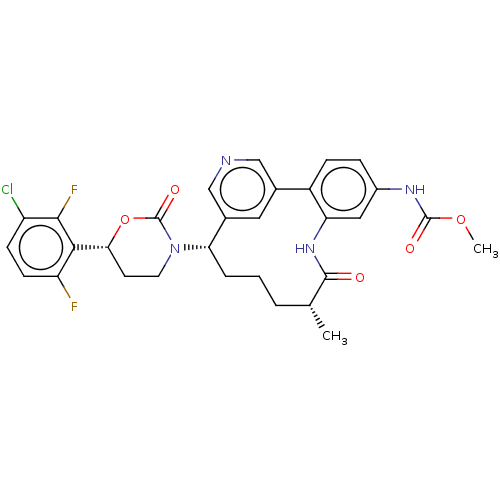
(CHEMBL4638837)Show SMILES COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](N3CC[C@@H](OC3=O)c3c(F)ccc(Cl)c3F)c3cncc-2c3)c1 |r| Show InChI InChI=1S/C30H29ClF2N4O5/c1-16-4-3-5-24(37-11-10-25(42-30(37)40)26-22(32)9-8-21(31)27(26)33)18-12-17(14-34-15-18)20-7-6-19(35-29(39)41-2)13-23(20)36-28(16)38/h6-9,12-16,24-25H,3-5,10-11H2,1-2H3,(H,35,39)(H,36,38)/t16-,24+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry |
J Med Chem 63: 7226-7242 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00464
BindingDB Entry DOI: 10.7270/Q2J67MHG |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50541586

(CHEMBL4638245)Show SMILES COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](N3CCC(=CC3=O)c3c(F)ccc(Cl)c3F)c3cc-2ccn3)c1 |r,c:22| Show InChI InChI=1S/C31H29ClF2N4O4/c1-17-4-3-5-26(38-13-11-19(15-27(38)39)28-23(33)9-8-22(32)29(28)34)25-14-18(10-12-35-25)21-7-6-20(36-31(41)42-2)16-24(21)37-30(17)40/h6-10,12,14-17,26H,3-5,11,13H2,1-2H3,(H,36,41)(H,37,40)/t17-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated protein C using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry |
J Med Chem 63: 7226-7242 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00464
BindingDB Entry DOI: 10.7270/Q2J67MHG |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122647
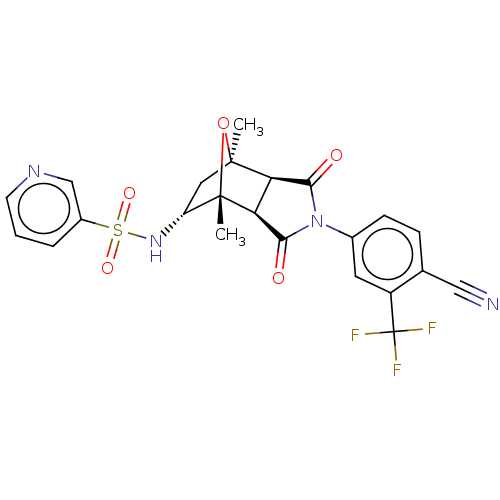
(CHEMBL3623126)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NS(=O)(=O)c1cccnc1)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C23H19F3N4O5S/c1-21-9-16(29-36(33,34)14-4-3-7-28-11-14)22(2,35-21)18-17(21)19(31)30(20(18)32)13-6-5-12(10-27)15(8-13)23(24,25)26/h3-8,11,16-18,29H,9H2,1-2H3/t16-,17-,18+,21+,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM350469

(US10208068, Example 40)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](CCC[C@@H](C)C(=O)Nc2c1)NC(=O)c1cnn(c1C)-c1cccc(Cl)c1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
J Med Chem 63: 784-803 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01768
BindingDB Entry DOI: 10.7270/Q2J67M9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM350476

(US10208068, Example 78)Show SMILES COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](NC(=O)c3cnn(c3C)-c3cccc(Cl)c3F)c3cc-2ccn3)c1 |r| Show InChI InChI=1S/C31H30ClFN6O4/c1-17-6-4-8-24(37-30(41)22-16-35-39(18(22)2)27-9-5-7-23(32)28(27)33)26-14-19(12-13-34-26)21-11-10-20(36-31(42)43-3)15-25(21)38-29(17)40/h5,7,9-17,24H,4,6,8H2,1-3H3,(H,36,42)(H,37,41)(H,38,40)/t17-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
J Med Chem 63: 784-803 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01768
BindingDB Entry DOI: 10.7270/Q2J67M9S |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372078
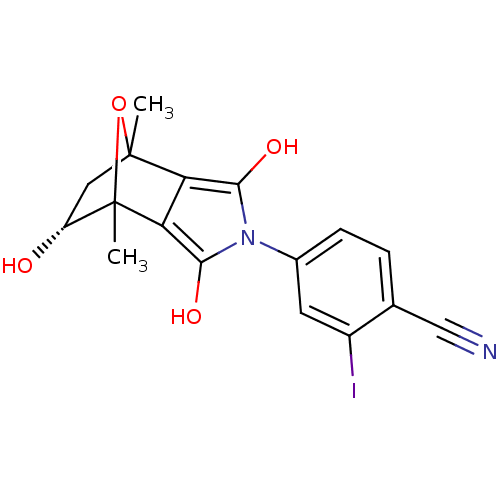
(CHEMBL272574)Show SMILES CC12C[C@@H](O)C(C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(I)c1 |TLB:4:3:7:8.14,THB:12:14:7:2.3,9:8:7:2.3| Show InChI InChI=1S/C17H15IN2O4/c1-16-6-11(21)17(2,24-16)13-12(16)14(22)20(15(13)23)9-4-3-8(7-19)10(18)5-9/h3-5,11,21-23H,6H2,1-2H3/t11-,16?,17?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50541580
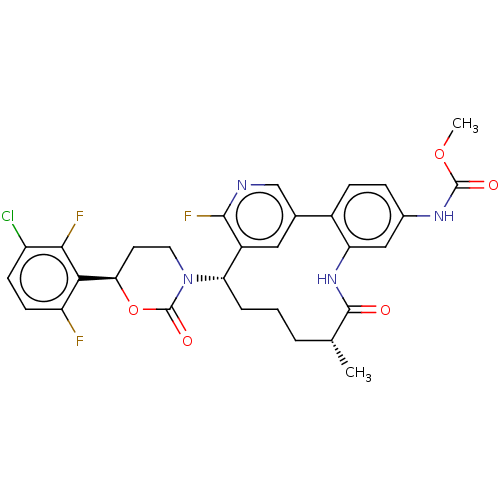
(CHEMBL4639313)Show SMILES COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](N3CC[C@@H](OC3=O)c3c(F)ccc(Cl)c3F)c3cc-2cnc3F)c1 |r| Show InChI InChI=1S/C30H28ClF3N4O5/c1-15-4-3-5-23(38-11-10-24(43-30(38)41)25-21(32)9-8-20(31)26(25)33)19-12-16(14-35-27(19)34)18-7-6-17(36-29(40)42-2)13-22(18)37-28(15)39/h6-9,12-15,23-24H,3-5,10-11H2,1-2H3,(H,36,40)(H,37,39)/t15-,23+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry |
J Med Chem 63: 7226-7242 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00464
BindingDB Entry DOI: 10.7270/Q2J67MHG |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372103

(CHEMBL402807)Show SMILES Oc1c2C3CCC(O3)c2c(O)n1-c1ccc([N+]([O-])=O)c2ccccc12 |THB:9:8:7:5.4,1:2:7:5.4,(7.88,-13.23,;8.78,-12.29,;10.06,-12.46,;11.36,-11.72,;12.65,-12.28,;13.1,-11.18,;11.68,-10.45,;11.33,-9.07,;10.63,-11.29,;9.69,-10.39,;9.71,-9.09,;8.54,-11.01,;6.99,-10.97,;6.18,-12.3,;4.63,-12.26,;3.89,-10.89,;2.34,-10.85,;1.6,-9.49,;1.53,-12.17,;4.7,-9.57,;3.96,-8.22,;4.76,-6.91,;6.31,-6.94,;7.04,-8.3,;6.24,-9.61,)| Show InChI InChI=1S/C18H14N2O5/c21-17-15-13-7-8-14(25-13)16(15)18(22)19(17)11-5-6-12(20(23)24)10-4-2-1-3-9(10)11/h1-6,13-14,21-22H,7-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372082
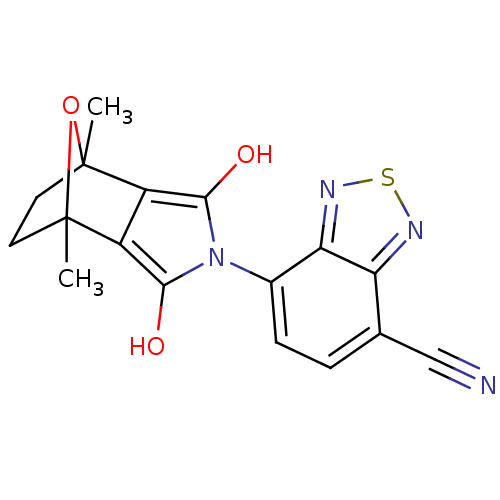
(CHEMBL446626)Show SMILES CC12CCC(C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c2nsnc12 |THB:11:13:6:2.3,8:7:6:2.3,(28.74,-48.8,;27.41,-49.58,;28.3,-50.89,;27.39,-51.64,;26.52,-50.54,;25.81,-51.91,;27.75,-48.2,;25.03,-50.57,;23.98,-49.8,;22.75,-50.2,;24.39,-48.57,;25.69,-48.58,;26.33,-47.45,;26.08,-49.81,;23.05,-47.8,;21.7,-48.58,;20.36,-47.8,;20.37,-46.25,;19.03,-45.49,;17.68,-44.73,;21.69,-45.47,;22.02,-43.95,;23.56,-43.79,;24.19,-45.21,;23.04,-46.25,)| Show InChI InChI=1S/C17H14N4O3S/c1-16-5-6-17(2,24-16)11-10(16)14(22)21(15(11)23)9-4-3-8(7-18)12-13(9)20-25-19-12/h3-4,22-23H,5-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122640

(CHEMBL3623119)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NC(=O)CC)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C21H20F3N3O4/c1-4-14(28)26-13-8-19(2)15-16(20(13,3)31-19)18(30)27(17(15)29)11-6-5-10(9-25)12(7-11)21(22,23)24/h5-7,13,15-16H,4,8H2,1-3H3,(H,26,28)/t13-,15-,16+,19+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372088
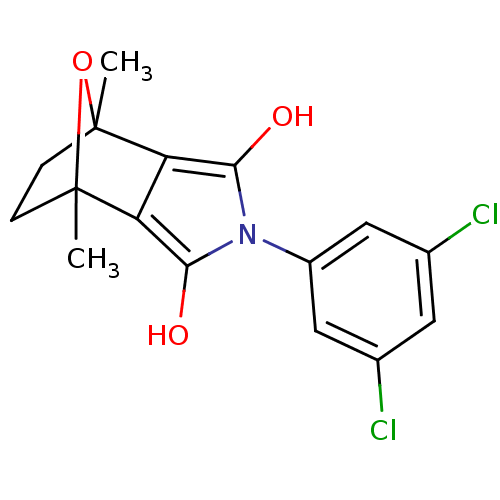
(CHEMBL257668)Show SMILES CC12CCC(C)(O1)c1c(O)n(c(O)c21)-c1cc(Cl)cc(Cl)c1 |THB:11:13:6:2.3,8:7:6:2.3| Show InChI InChI=1S/C16H15Cl2NO3/c1-15-3-4-16(2,22-15)12-11(15)13(20)19(14(12)21)10-6-8(17)5-9(18)7-10/h5-7,20-21H,3-4H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372089

(CHEMBL272509)Show SMILES CSc1cc(ccc1C#N)-n1c(O)c2c(c1O)C1(C)CCC2(C)O1 |THB:11:13:23:20.19,15:14:23:20.19| Show InChI InChI=1S/C18H18N2O3S/c1-17-6-7-18(2,23-17)14-13(17)15(21)20(16(14)22)11-5-4-10(9-19)12(8-11)24-3/h4-5,8,21-22H,6-7H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50514435

(CHEMBL4437236)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](NC(=O)c3cnn(c3N)-c3cccc(Cl)c3F)c3cc-2ccn3)c1 |r| Show InChI InChI=1S/C30H29ClFN7O4.2C2HF3O2/c1-16-5-3-7-22(37-29(41)20-15-35-39(27(20)33)25-8-4-6-21(31)26(25)32)24-13-17(11-12-34-24)19-10-9-18(36-30(42)43-2)14-23(19)38-28(16)40;2*3-2(4,5)1(6)7/h4,6,8-16,22H,3,5,7,33H2,1-2H3,(H,36,42)(H,37,41)(H,38,40);2*(H,6,7)/t16-,22+;;/m1../s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
J Med Chem 63: 784-803 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01768
BindingDB Entry DOI: 10.7270/Q2J67M9S |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372086
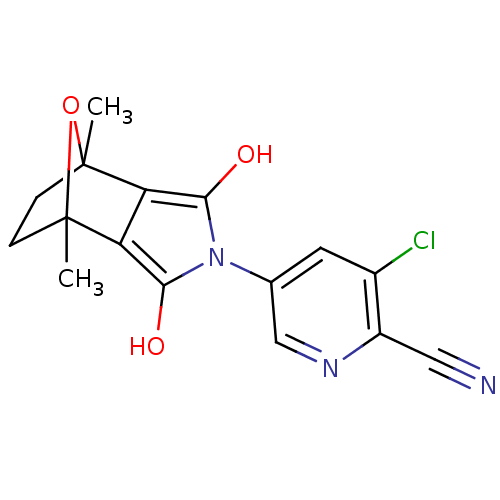
(CHEMBL403213)Show SMILES CC12CCC(C)(O1)c1c(O)n(c(O)c21)-c1cnc(C#N)c(Cl)c1 |THB:11:13:6:2.3,8:7:6:2.3| Show InChI InChI=1S/C16H14ClN3O3/c1-15-3-4-16(2,23-15)12-11(15)13(21)20(14(12)22)8-5-9(17)10(6-18)19-7-8/h5,7,21-22H,3-4H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50514432
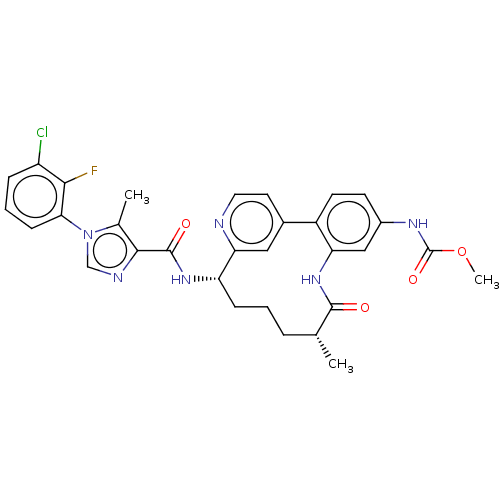
(CHEMBL4438385)Show SMILES Cl.Cl.COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](NC(=O)c3ncn(c3C)-c3cccc(Cl)c3F)c3cc-2ccn3)c1 |r| Show InChI InChI=1S/C31H30ClFN6O4.2ClH/c1-17-6-4-8-23(37-30(41)28-18(2)39(16-35-28)26-9-5-7-22(32)27(26)33)25-14-19(12-13-34-25)21-11-10-20(36-31(42)43-3)15-24(21)38-29(17)40;;/h5,7,9-17,23H,4,6,8H2,1-3H3,(H,36,42)(H,37,41)(H,38,40);2*1H/t17-,23+;;/m1../s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
J Med Chem 63: 784-803 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01768
BindingDB Entry DOI: 10.7270/Q2J67M9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50541583
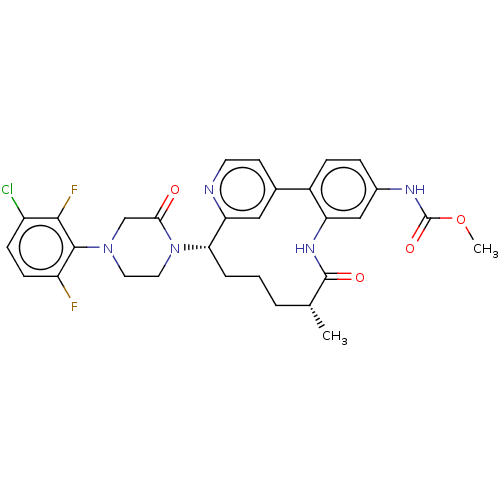
(CHEMBL4633639)Show SMILES COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](N3CCN(CC3=O)c3c(F)ccc(Cl)c3F)c3cc-2ccn3)c1 |r| Show InChI InChI=1S/C30H30ClF2N5O4/c1-17-4-3-5-25(38-13-12-37(16-26(38)39)28-22(32)9-8-21(31)27(28)33)24-14-18(10-11-34-24)20-7-6-19(35-30(41)42-2)15-23(20)36-29(17)40/h6-11,14-15,17,25H,3-5,12-13,16H2,1-2H3,(H,35,41)(H,36,40)/t17-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry |
J Med Chem 63: 7226-7242 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00464
BindingDB Entry DOI: 10.7270/Q2J67MHG |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50541585
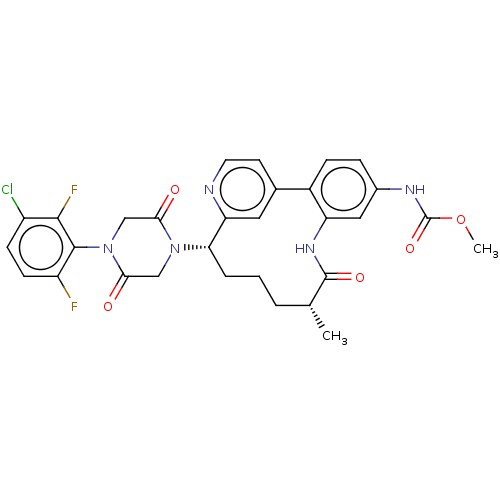
(CHEMBL4635155)Show SMILES COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](N3CC(=O)N(CC3=O)c3c(F)ccc(Cl)c3F)c3cc-2ccn3)c1 |r| Show InChI InChI=1S/C30H28ClF2N5O5/c1-16-4-3-5-24(37-14-26(40)38(15-25(37)39)28-21(32)9-8-20(31)27(28)33)23-12-17(10-11-34-23)19-7-6-18(35-30(42)43-2)13-22(19)36-29(16)41/h6-13,16,24H,3-5,14-15H2,1-2H3,(H,35,42)(H,36,41)/t16-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry |
J Med Chem 63: 7226-7242 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00464
BindingDB Entry DOI: 10.7270/Q2J67MHG |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50514441
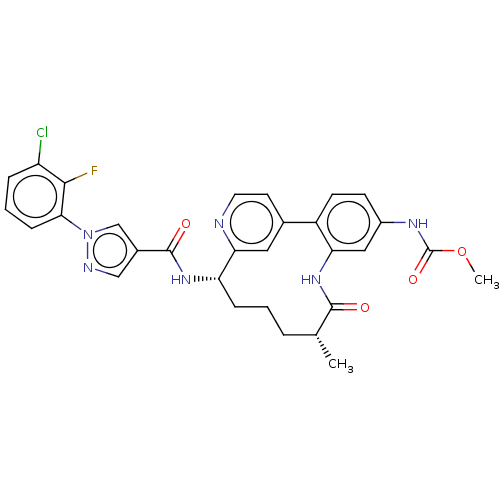
(CHEMBL4476061)Show SMILES OC(=O)C(F)(F)F.COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](NC(=O)c3cnn(c3)-c3cccc(Cl)c3F)c3cc-2ccn3)c1 |r| Show InChI InChI=1S/C30H28ClFN6O4.C2HF3O2/c1-17-5-3-7-23(36-29(40)19-15-34-38(16-19)26-8-4-6-22(31)27(26)32)25-13-18(11-12-33-25)21-10-9-20(35-30(41)42-2)14-24(21)37-28(17)39;3-2(4,5)1(6)7/h4,6,8-17,23H,3,5,7H2,1-2H3,(H,35,41)(H,36,40)(H,37,39);(H,6,7)/t17-,23+;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
J Med Chem 63: 784-803 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01768
BindingDB Entry DOI: 10.7270/Q2J67M9S |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50514432

(CHEMBL4438385)Show SMILES Cl.Cl.COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](NC(=O)c3ncn(c3C)-c3cccc(Cl)c3F)c3cc-2ccn3)c1 |r| Show InChI InChI=1S/C31H30ClFN6O4.2ClH/c1-17-6-4-8-23(37-30(41)28-18(2)39(16-35-28)26-9-5-7-22(32)27(26)33)25-14-19(12-13-34-25)21-11-10-20(36-31(42)43-3)15-24(21)38-29(17)40;;/h5,7,9-17,23H,4,6,8H2,1-3H3,(H,36,42)(H,37,41)(H,38,40);2*1H/t17-,23+;;/m1../s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human kallikrein using H-(D)-Pro-Phe-Arg-pNA as substrate by spectrophotometry |
J Med Chem 63: 784-803 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01768
BindingDB Entry DOI: 10.7270/Q2J67M9S |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122643

(CHEMBL3623122)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NC(=O)ON1CCN(C)CC1)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C24H26F3N5O5/c1-22-11-16(29-21(35)36-31-8-6-30(3)7-9-31)23(2,37-22)18-17(22)19(33)32(20(18)34)14-5-4-13(12-28)15(10-14)24(25,26)27/h4-5,10,16-18H,6-9,11H2,1-3H3,(H,29,35)/t16-,17-,18+,22+,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50514442
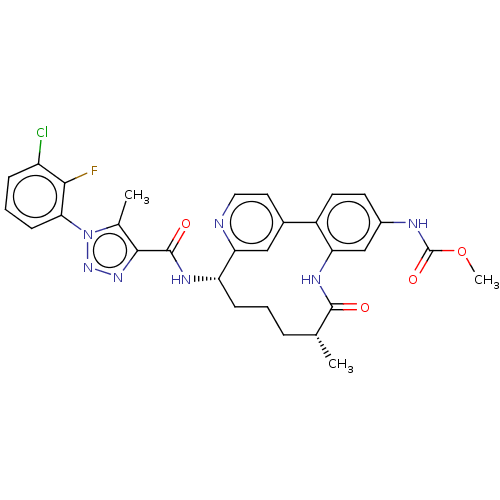
(CHEMBL4470992)Show SMILES Cl.COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](NC(=O)c3nnn(c3C)-c3cccc(Cl)c3F)c3cc-2ccn3)c1 |r| Show InChI InChI=1S/C30H29ClFN7O4.ClH/c1-16-6-4-8-22(35-29(41)27-17(2)39(38-37-27)25-9-5-7-21(31)26(25)32)24-14-18(12-13-33-24)20-11-10-19(34-30(42)43-3)15-23(20)36-28(16)40;/h5,7,9-16,22H,4,6,8H2,1-3H3,(H,34,42)(H,35,41)(H,36,40);1H/t16-,22+;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
J Med Chem 63: 784-803 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01768
BindingDB Entry DOI: 10.7270/Q2J67M9S |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372079

(CHEMBL255331)Show SMILES CC12C[C@H](O)C(C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(I)c1 |TLB:4:3:7:14.8,THB:9:8:7:3.2,12:14:7:3.2| Show InChI InChI=1S/C17H15IN2O4/c1-16-6-11(21)17(2,24-16)13-12(16)14(22)20(15(13)23)9-4-3-8(7-19)10(18)5-9/h3-5,11,21-23H,6H2,1-2H3/t11-,16?,17?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122635
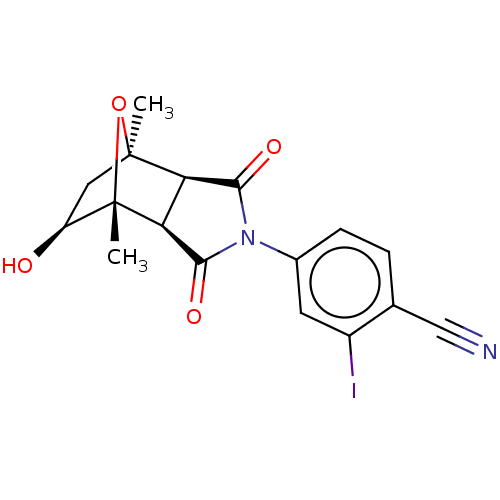
(CHEMBL3623114)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@@H]1O)c1ccc(C#N)c(I)c1 |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C17H15IN2O4/c1-16-6-11(21)17(2,24-16)13-12(16)14(22)20(15(13)23)9-4-3-8(7-19)10(18)5-9/h3-5,11-13,21H,6H2,1-2H3/t11-,12+,13-,16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50559797

(CHEMBL4786078)Show SMILES CN(C)C(=O)C(=O)NC12CCC(CC1)Cn1c2nc(C(=O)NCc2ccc(F)c(C)c2)c(O)c1=O |(11.1,-26.94,;12.4,-26.12,;12.34,-24.58,;13.76,-26.83,;13.83,-28.37,;15.07,-26.01,;15,-24.47,;16.43,-26.72,;17.73,-25.9,;18.79,-27.04,;20.33,-26.91,;21.21,-25.64,;19.57,-26.13,;18.81,-24.8,;20.74,-24.16,;19.3,-23.6,;17.96,-24.37,;16.64,-23.61,;16.64,-22.07,;15.3,-21.31,;15.29,-19.77,;13.97,-22.08,;12.63,-21.31,;11.3,-22.09,;11.31,-23.62,;9.98,-24.39,;8.64,-23.63,;7.31,-24.4,;8.64,-22.09,;7.3,-21.32,;9.97,-21.31,;17.96,-21.29,;17.96,-19.75,;19.29,-22.07,;20.63,-21.31,)| | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to wild type HIV1 integrase G140S/Q148H mutant |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115541
BindingDB Entry DOI: 10.7270/Q23R0XKX |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122645
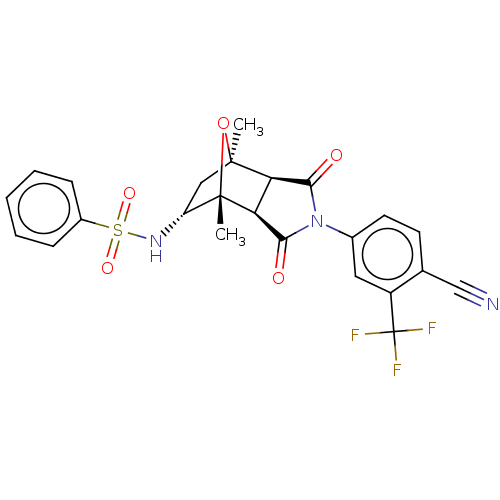
(CHEMBL3623124)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NS(=O)(=O)c1ccccc1)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C24H20F3N3O5S/c1-22-11-17(29-36(33,34)15-6-4-3-5-7-15)23(2,35-22)19-18(22)20(31)30(21(19)32)14-9-8-13(12-28)16(10-14)24(25,26)27/h3-10,17-19,29H,11H2,1-2H3/t17-,18-,19+,22+,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267028

(CHEMBL4073525)Show SMILES Cc1cc(OCc2ccccc2)ccc1Oc1ccc(cc1)N1C[C@H](C[C@H]1CC(O)=O)C(F)(F)F |r| Show InChI InChI=1S/C27H26F3NO4/c1-18-13-24(34-17-19-5-3-2-4-6-19)11-12-25(18)35-23-9-7-21(8-10-23)31-16-20(27(28,29)30)14-22(31)15-26(32)33/h2-13,20,22H,14-17H2,1H3,(H,32,33)/t20-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50514436
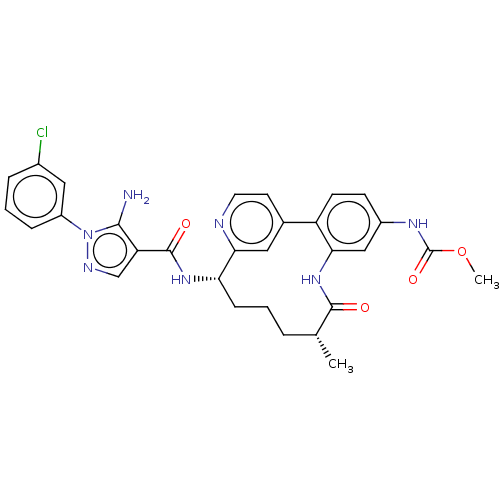
(CHEMBL4464434)Show SMILES COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](NC(=O)c3cnn(c3N)-c3cccc(Cl)c3)c3cc-2ccn3)c1 |r| Show InChI InChI=1S/C30H30ClN7O4/c1-17-5-3-8-24(36-29(40)23-16-34-38(27(23)32)21-7-4-6-19(31)14-21)26-13-18(11-12-33-26)22-10-9-20(35-30(41)42-2)15-25(22)37-28(17)39/h4,6-7,9-17,24H,3,5,8,32H2,1-2H3,(H,35,41)(H,36,40)(H,37,39)/t17-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
J Med Chem 63: 784-803 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01768
BindingDB Entry DOI: 10.7270/Q2J67M9S |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122644
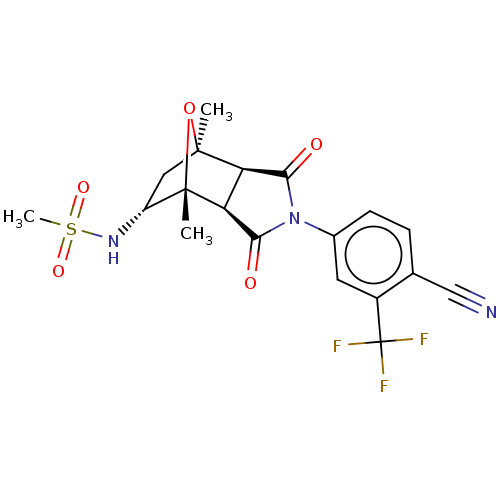
(CHEMBL3623123)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NS(C)(=O)=O)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C19H18F3N3O5S/c1-17-7-12(24-31(3,28)29)18(2,30-17)14-13(17)15(26)25(16(14)27)10-5-4-9(8-23)11(6-10)19(20,21)22/h4-6,12-14,24H,7H2,1-3H3/t12-,13-,14+,17+,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372085
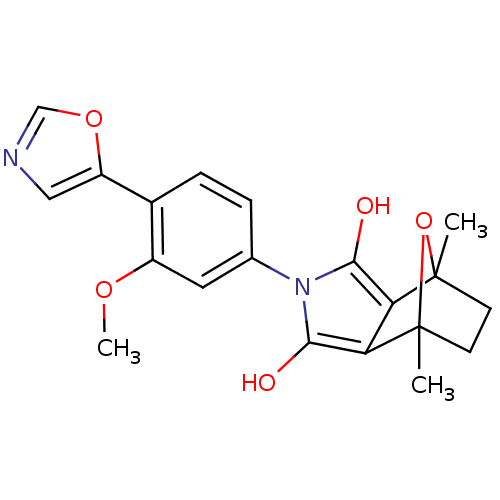
(CHEMBL403061)Show SMILES COc1cc(ccc1-c1cnco1)-n1c(O)c2c(c1O)C1(C)CCC2(C)O1 |THB:14:16:26:23.22,18:17:26:23.22| Show InChI InChI=1S/C20H20N2O5/c1-19-6-7-20(2,27-19)16-15(19)17(23)22(18(16)24)11-4-5-12(13(8-11)25-3)14-9-21-10-26-14/h4-5,8-10,23-24H,6-7H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267031

(CHEMBL4079930)Show SMILES Cc1c(Cl)cccc1Oc1ccc(cc1)N1C[C@H](C[C@H]1CC(O)=O)C(F)(F)F |r| Show InChI InChI=1S/C20H19ClF3NO3/c1-12-17(21)3-2-4-18(12)28-16-7-5-14(6-8-16)25-11-13(20(22,23)24)9-15(25)10-19(26)27/h2-8,13,15H,9-11H2,1H3,(H,26,27)/t13-,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50541584
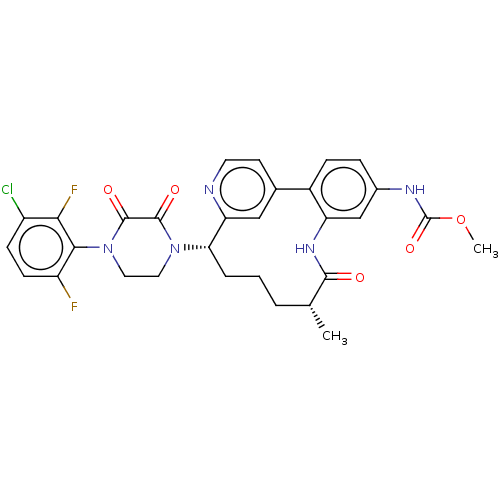
(CHEMBL4639498)Show SMILES COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](N3CCN(C(=O)C3=O)c3c(F)ccc(Cl)c3F)c3cc-2ccn3)c1 |r| Show InChI InChI=1S/C30H28ClF2N5O5/c1-16-4-3-5-24(37-12-13-38(29(41)28(37)40)26-21(32)9-8-20(31)25(26)33)23-14-17(10-11-34-23)19-7-6-18(35-30(42)43-2)15-22(19)36-27(16)39/h6-11,14-16,24H,3-5,12-13H2,1-2H3,(H,35,42)(H,36,39)/t16-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry |
J Med Chem 63: 7226-7242 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00464
BindingDB Entry DOI: 10.7270/Q2J67MHG |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50559796
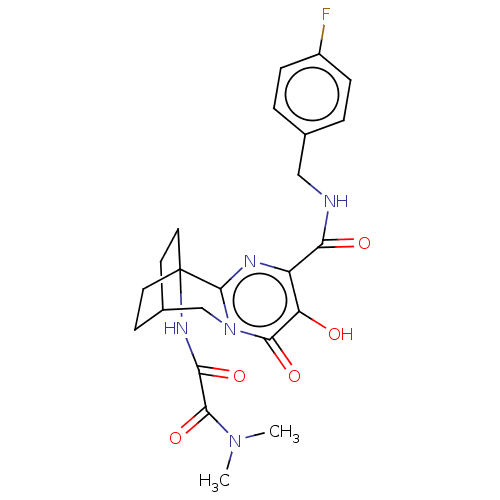
(CHEMBL4779601)Show SMILES CN(C)C(=O)C(=O)NC12CCC(CC1)Cn1c2nc(C(=O)NCc2ccc(F)cc2)c(O)c1=O |(8.88,-35.92,;8.94,-37.46,;7.64,-38.28,;10.3,-38.18,;10.37,-39.71,;11.61,-37.35,;11.54,-35.81,;12.97,-38.07,;14.27,-37.24,;15.33,-38.38,;16.87,-38.26,;17.75,-36.98,;16.12,-37.47,;15.67,-36.2,;17.28,-35.51,;15.84,-34.94,;14.5,-35.71,;13.18,-34.95,;13.18,-33.41,;11.84,-32.65,;11.83,-31.11,;10.51,-33.42,;9.17,-32.65,;7.84,-33.43,;6.51,-32.66,;5.18,-33.43,;5.18,-34.97,;3.85,-35.75,;6.52,-35.74,;7.85,-34.96,;14.5,-32.64,;14.5,-31.1,;15.83,-33.41,;17.17,-32.65,)| | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to wild type HIV1 integrase G140S/Q148H mutant |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115541
BindingDB Entry DOI: 10.7270/Q23R0XKX |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372080

(CHEMBL403039)Show SMILES CC12CCC(C)(O1)c1c(O)n(c(O)c21)-c1ccc([N+]([O-])=O)c2c(Br)cccc12 |THB:11:13:6:2.3,8:7:6:2.3,(31.1,-48.31,;29.77,-49.09,;30.66,-50.4,;29.75,-51.15,;28.88,-50.05,;28.17,-51.42,;30.11,-47.72,;27.39,-50.08,;26.35,-49.32,;25.12,-49.71,;26.75,-48.09,;28.05,-48.09,;28.69,-46.97,;28.44,-49.33,;25.41,-47.32,;25.4,-45.77,;24.06,-44.99,;22.74,-45.77,;21.39,-45.01,;21.39,-43.46,;20.06,-45.78,;22.74,-47.32,;21.41,-48.09,;20.07,-47.32,;21.41,-49.64,;22.75,-50.4,;24.08,-49.63,;24.07,-48.09,)| Show InChI InChI=1S/C20H17BrN2O5/c1-19-8-9-20(2,28-19)16-15(19)17(24)22(18(16)25)12-6-7-13(23(26)27)14-10(12)4-3-5-11(14)21/h3-7,24-25H,8-9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372087
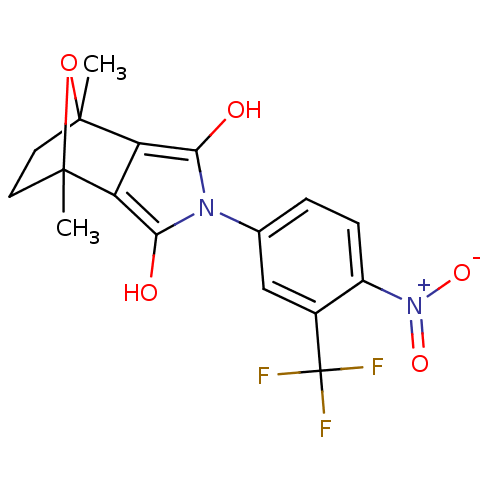
(CHEMBL403214)Show SMILES CC12CCC(C)(O1)c1c(O)n(c(O)c21)-c1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |THB:11:13:6:2.3,8:7:6:2.3| Show InChI InChI=1S/C17H15F3N2O5/c1-15-5-6-16(2,27-15)12-11(15)13(23)21(14(12)24)8-3-4-10(22(25)26)9(7-8)17(18,19)20/h3-4,7,23-24H,5-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372084

(CHEMBL271262)Show SMILES CC12CCC(C)(O1)c1c(O)n(c(O)c21)-c1c(N)ccc2ncsc12 |THB:11:13:6:2.3,8:7:6:2.3,(43.68,-48.16,;42.36,-48.94,;43.25,-50.24,;42.34,-50.99,;41.47,-49.89,;40.77,-51.26,;42.7,-47.56,;39.99,-49.92,;38.94,-49.16,;38.06,-49.55,;39.35,-47.93,;40.64,-47.94,;41.28,-46.81,;41.04,-49.17,;38.01,-47.17,;36.67,-47.94,;36.67,-49.48,;35.34,-47.17,;35.34,-45.62,;36.66,-44.84,;36.98,-43.33,;38.52,-43.17,;39.15,-44.59,;38,-45.62,)| Show InChI InChI=1S/C17H17N3O3S/c1-16-5-6-17(2,23-16)11-10(16)14(21)20(15(11)22)12-8(18)3-4-9-13(12)24-7-19-9/h3-4,7,21-22H,5-6,18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372091
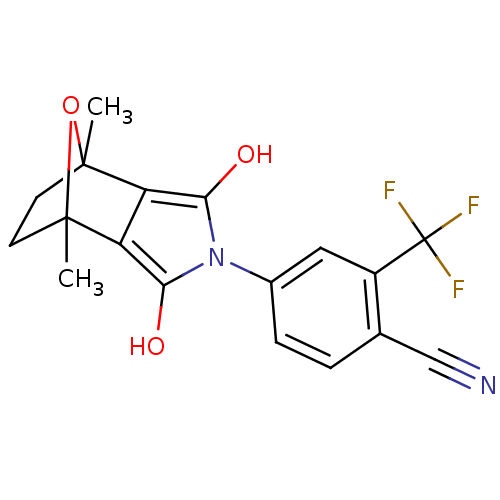
(CHEMBL403669)Show SMILES CC12CCC(C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F |THB:11:13:6:2.3,8:7:6:2.3| Show InChI InChI=1S/C18H15F3N2O3/c1-16-5-6-17(2,26-16)13-12(16)14(24)23(15(13)25)10-4-3-9(8-22)11(7-10)18(19,20)21/h3-4,7,24-25H,5-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data