

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50056769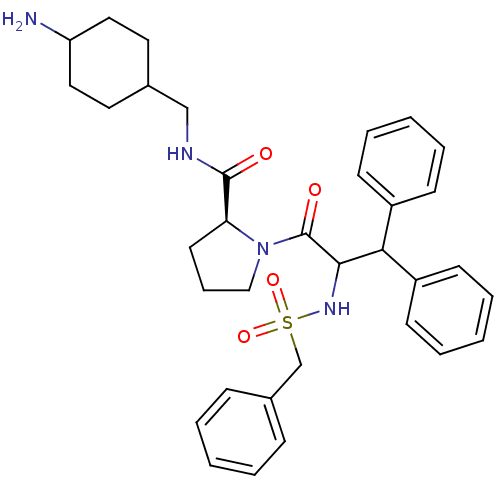 ((S)-1-(3,3-Diphenyl-2-phenylmethanesulfonylamino-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin | J Med Chem 40: 830-2 (1997) Article DOI: 10.1021/jm960762y BindingDB Entry DOI: 10.7270/Q25H7GXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458766 (CHEMBL4212386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156449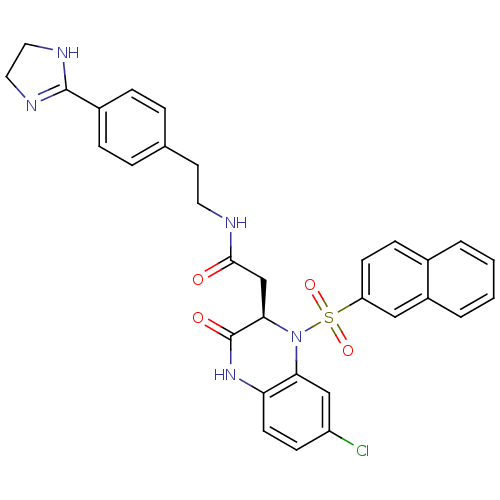 (2-[(R)-7-Chloro-1-(naphthalene-2-sulfonyl)-3-oxo-1...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM85357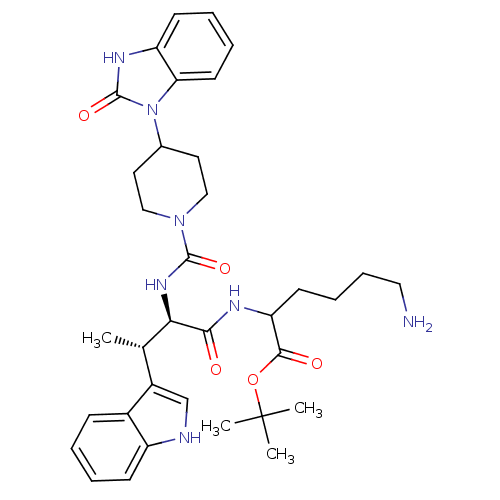 (2-[[(2R,3S)-2-[[4-[(2-Oxo-2,3-dihydro-1H-benzimida...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 95: 10836-41 (1998) Article DOI: 10.1073/pnas.95.18.10836 BindingDB Entry DOI: 10.7270/Q2XW4HCM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156451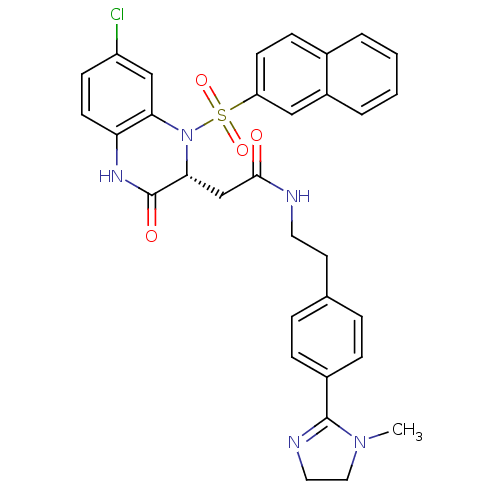 (2-[(R)-7-Chloro-1-(naphthalene-2-sulfonyl)-3-oxo-1...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156446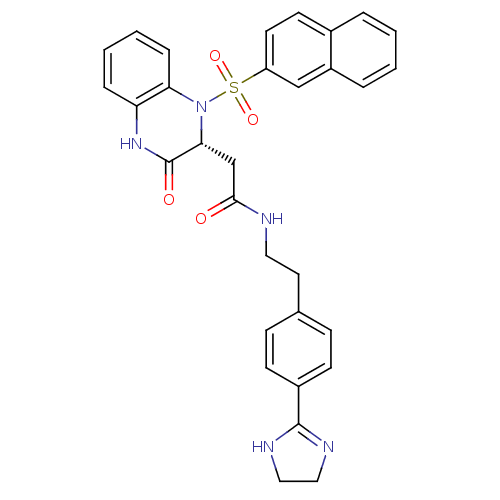 (CHEMBL359553 | N-{2-[4-(4,5-Dihydro-1H-imidazol-2-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the [35S]- radiolabelled compound to rhesus monkey Bradykinin receptor B1 | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156446 (CHEMBL359553 | N-{2-[4-(4,5-Dihydro-1H-imidazol-2-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the [35S]- radiolabelled compound to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo rec... | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156455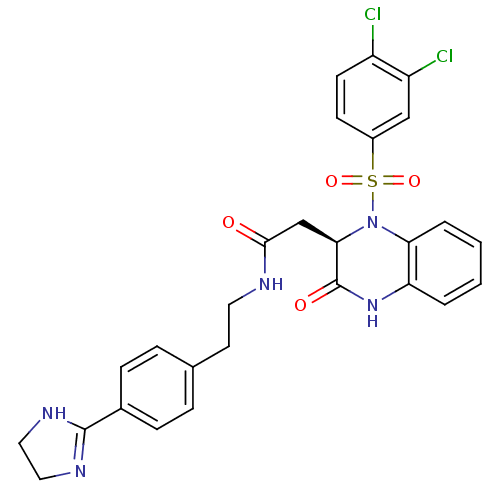 ((R)-N-(4-(4,5-dihydro-1H-imidazol-2-yl)phenethyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 1 (Homo sapiens) | BDBM50458766 (CHEMBL4212386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tolloid-like protein 2 (Homo sapiens) | BDBM50458766 (CHEMBL4212386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM81767 (15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 95: 10836-41 (1998) Article DOI: 10.1073/pnas.95.18.10836 BindingDB Entry DOI: 10.7270/Q2XW4HCM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458771 (CHEMBL4214046) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Oryctolagus cuniculus) | BDBM50156446 (CHEMBL359553 | N-{2-[4-(4,5-Dihydro-1H-imidazol-2-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the [35S]- radiolabelled compound to rabbit Bradykinin receptor B1 | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM81766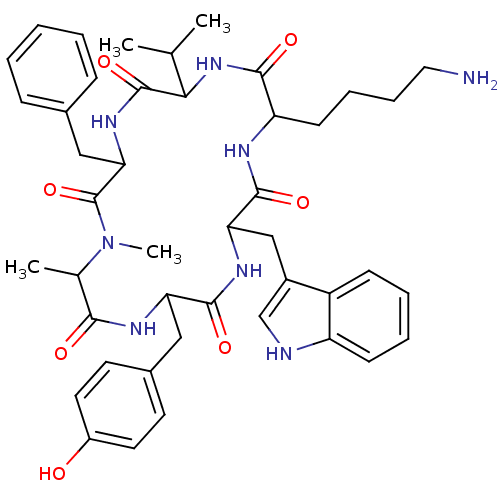 (CAS_3086456 | MK 678 | NSC_3086456) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 95: 10836-41 (1998) Article DOI: 10.1073/pnas.95.18.10836 BindingDB Entry DOI: 10.7270/Q2XW4HCM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090035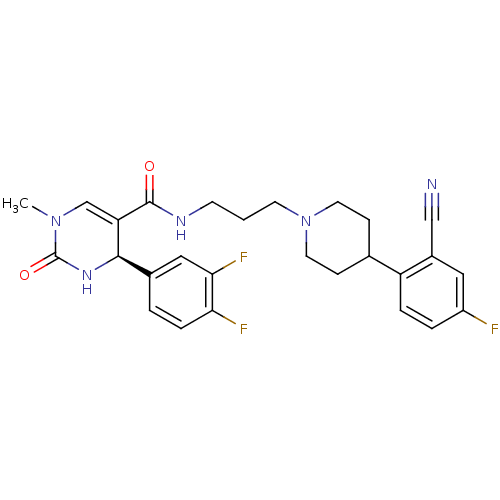 (4-(3,4-Difluoro-phenyl)-1-methyl-2-oxo-1,2,3,4-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090023 (4-(3,4-Difluoro-phenyl)-2-oxo-1,2,3,4-tetrahydro-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156450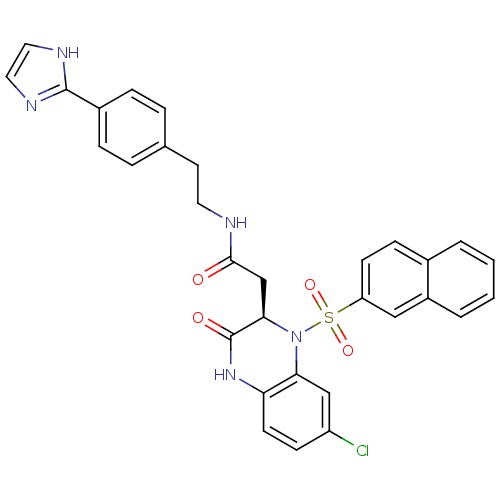 (2-[(R)-7-Chloro-1-(naphthalene-2-sulfonyl)-3-oxo-1...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156448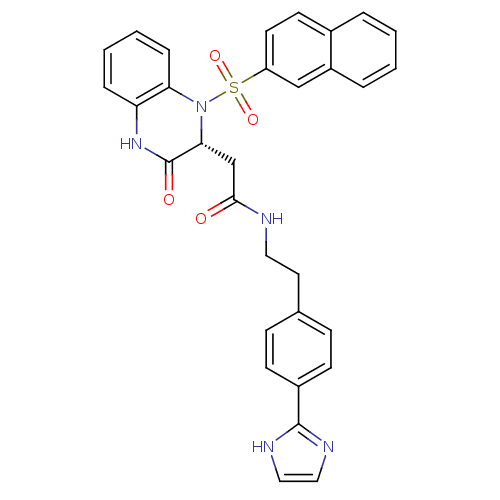 (CHEMBL185811 | N-{2-[4-(1H-Imidazol-2-yl)-phenyl]-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50454822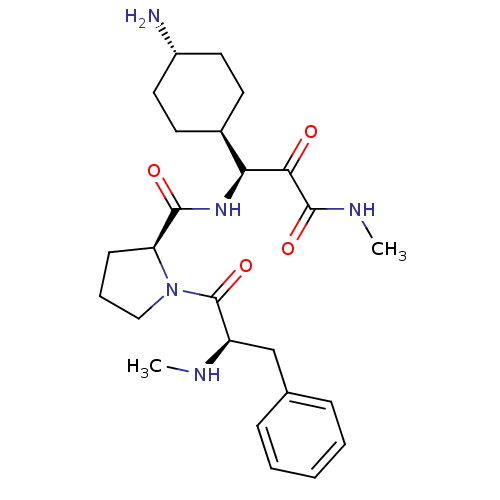 (CHEMBL2062141 | L-370518) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin | J Med Chem 40: 830-2 (1997) Article DOI: 10.1021/jm960762y BindingDB Entry DOI: 10.7270/Q25H7GXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50056772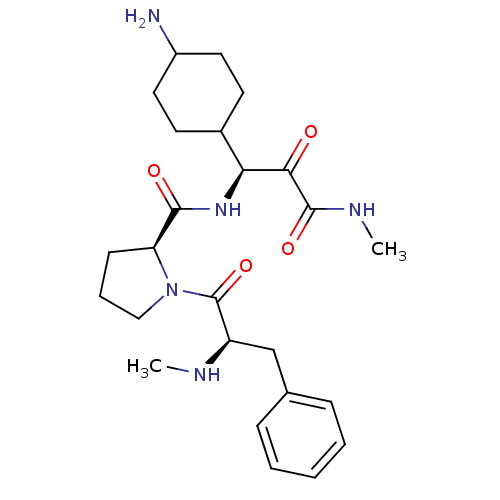 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of the compound against human thrombin was determined | Bioorg Med Chem Lett 7: 67-72 (1997) Article DOI: 10.1016/S0960-894X(96)00583-5 BindingDB Entry DOI: 10.7270/Q2639PQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50056773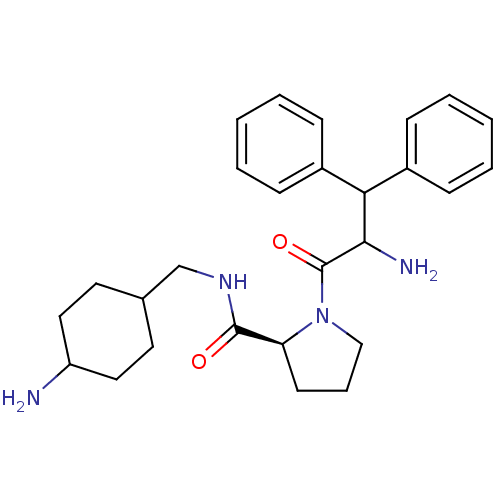 ((S)-1-(2-Amino-3,3-diphenyl-propionyl)-pyrrolidine...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin | J Med Chem 40: 830-2 (1997) Article DOI: 10.1021/jm960762y BindingDB Entry DOI: 10.7270/Q25H7GXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090010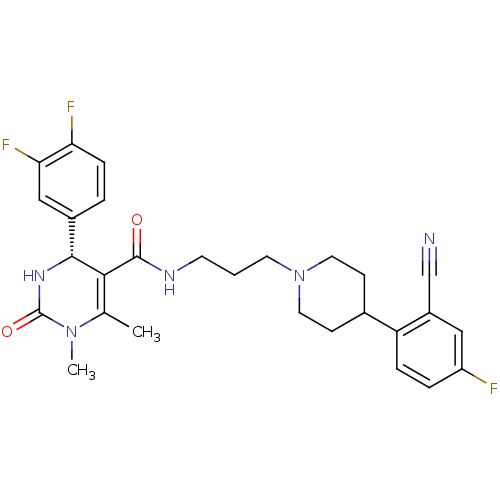 (4-(3,4-Difluoro-phenyl)-1,6-dimethyl-2-oxo-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090032 ((R)-4-(3,4-Difluoro-phenyl)-1-methyl-2-oxo-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090018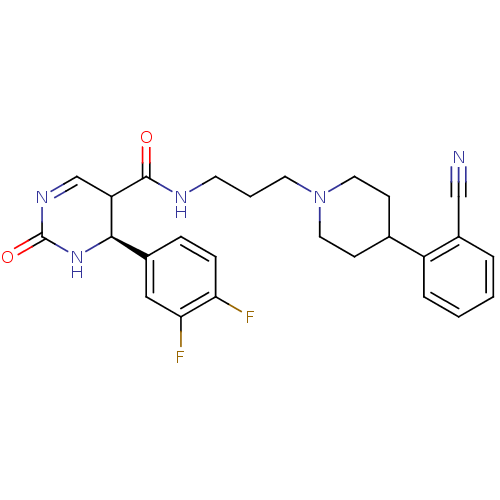 (4-(3,4-Difluoro-phenyl)-2-oxo-1,2,3,4-tetrahydro-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090019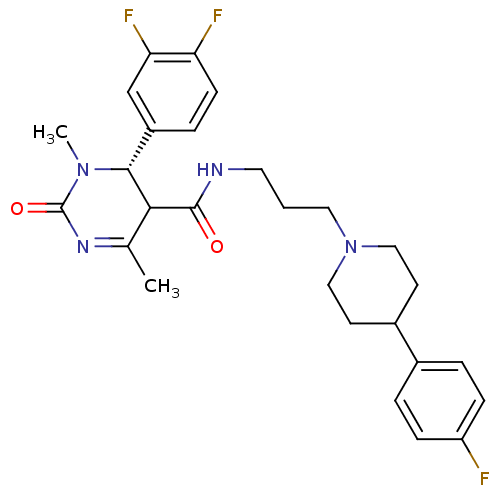 (4-(3,4-Difluoro-phenyl)-3,6-dimethyl-2-oxo-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090042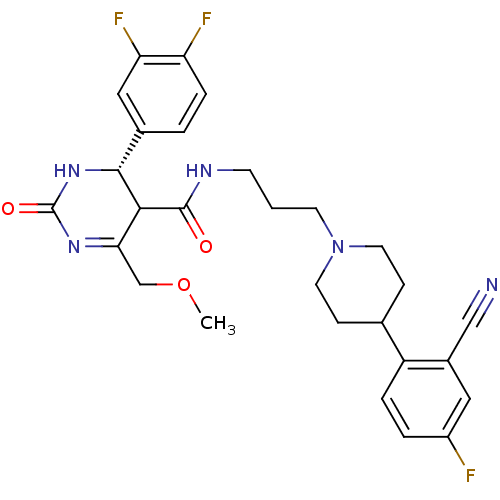 (4-(3,4-Difluoro-phenyl)-6-methoxymethyl-2-oxo-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090036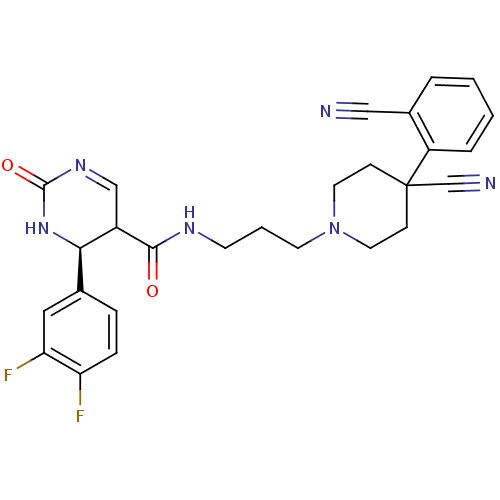 (4-(3,4-Difluoro-phenyl)-2-oxo-1,2,3,4-tetrahydro-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090647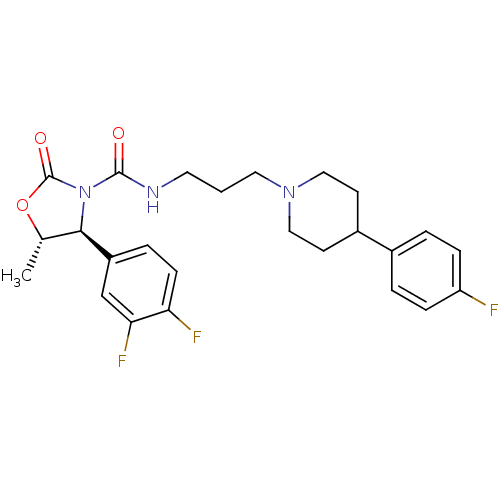 ((4S,5S)-4-(3,4-Difluoro-phenyl)-5-methyl-2-oxo-oxa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity of the compound towards Alpha1A human adrenergic receptors, using [125I]-HEAT as radioligand. | J Med Chem 43: 2775-8 (2000) BindingDB Entry DOI: 10.7270/Q2PZ582K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090025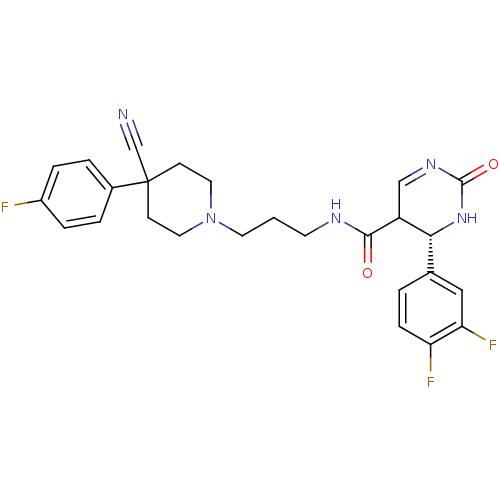 (4-(3,4-Difluoro-phenyl)-2-oxo-1,2,3,4-tetrahydro-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090015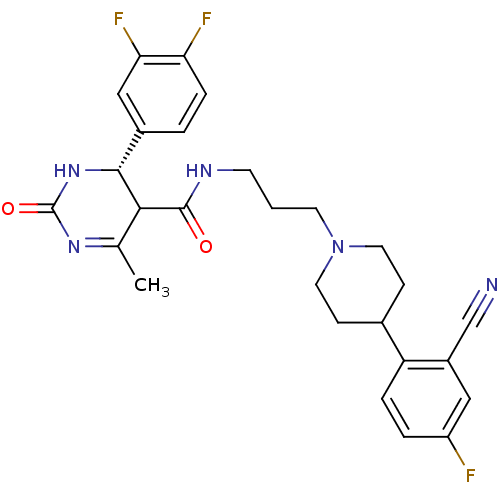 (4-(3,4-Difluoro-phenyl)-6-methyl-2-oxo-1,2,3,4-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090031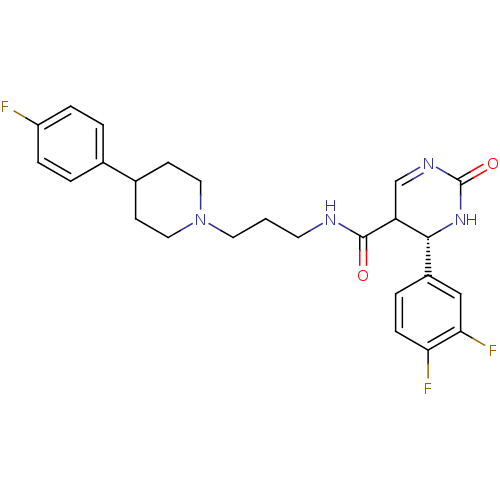 (4-(3,4-Difluoro-phenyl)-2-oxo-1,2,3,4-tetrahydro-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090027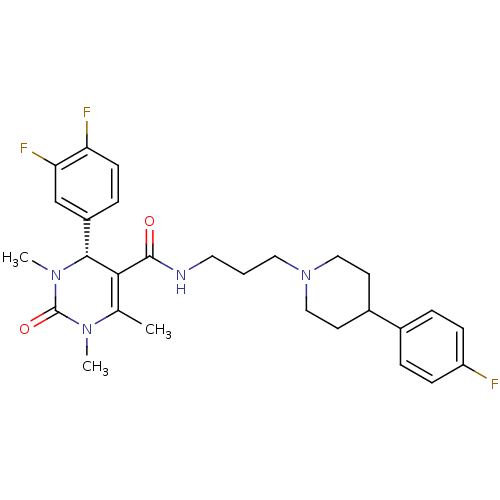 ((R)-4-(3,4-Difluoro-phenyl)-1,3,6-trimethyl-2-oxo-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090017 ((R)-4-(3,4-Difluoro-phenyl)-1,6-dimethyl-2-oxo-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090016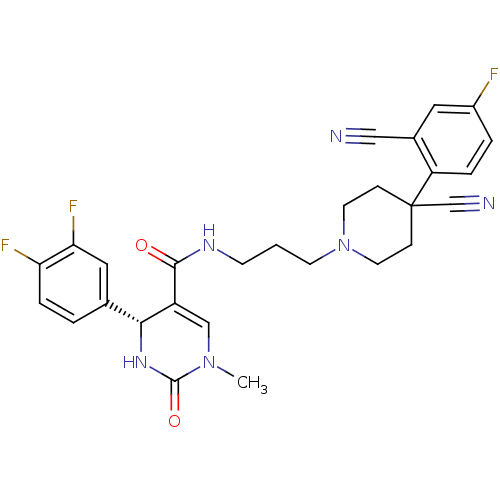 (4-(3,4-Difluoro-phenyl)-1-methyl-2-oxo-1,2,3,4-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM84745 (CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by PDSP Ki Database | Neuropsychopharmacology 8: 23-33 (1993) Article DOI: 10.1038/npp.1993.4 BindingDB Entry DOI: 10.7270/Q2XS5SXF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090339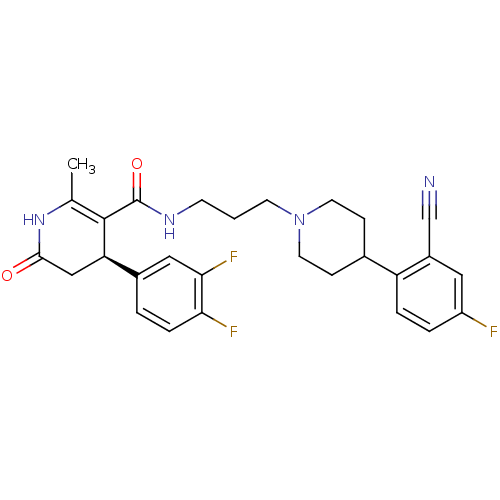 ((R)-4-(3,4-Difluoro-phenyl)-2-methyl-6-oxo-1,4,5,6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description In vitro antagonism at Alpha-1A adrenergic receptor | Bioorg Med Chem Lett 10: 1625-8 (2000) BindingDB Entry DOI: 10.7270/Q2445KQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090647 ((4S,5S)-4-(3,4-Difluoro-phenyl)-5-methyl-2-oxo-oxa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity towards Alpha1A dog adrenergic receptors, using [125I]HEAT as radioligand. | J Med Chem 43: 2775-8 (2000) BindingDB Entry DOI: 10.7270/Q2PZ582K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090043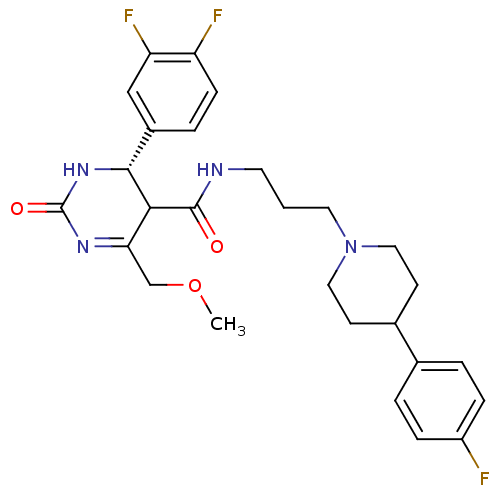 (4-(3,4-Difluoro-phenyl)-6-methoxymethyl-2-oxo-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50091723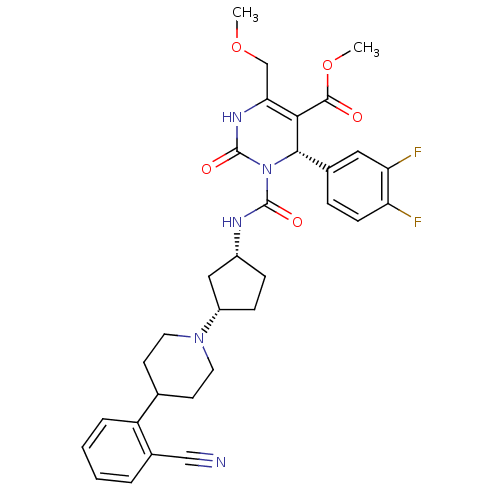 ((S)-3-{(1R,3S)-3-[4-(2-Cyano-phenyl)-piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]HEAT from human Alpha-1A adrenergic receptor stably expressed in CHO cells | Bioorg Med Chem Lett 10: 1917-20 (2001) BindingDB Entry DOI: 10.7270/Q2KH0NWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 1 (Homo sapiens) | BDBM50458771 (CHEMBL4214046) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50243173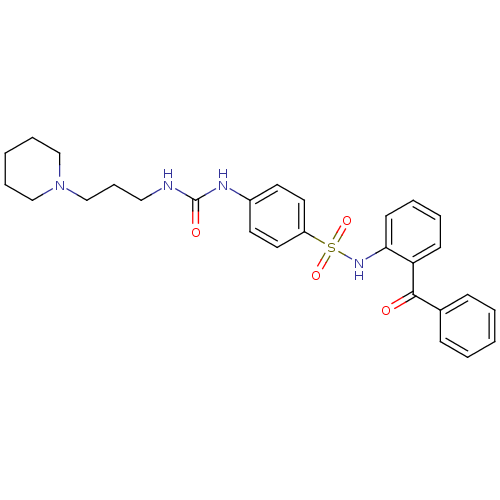 (CHEMBL487445 | N-(2-Benzoyl-phenyl)-4-[3-(3-piperi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human bradykinin B1 receptor | J Med Chem 51: 3946-52 (2008) Article DOI: 10.1021/jm800199h BindingDB Entry DOI: 10.7270/Q2JH3N33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090014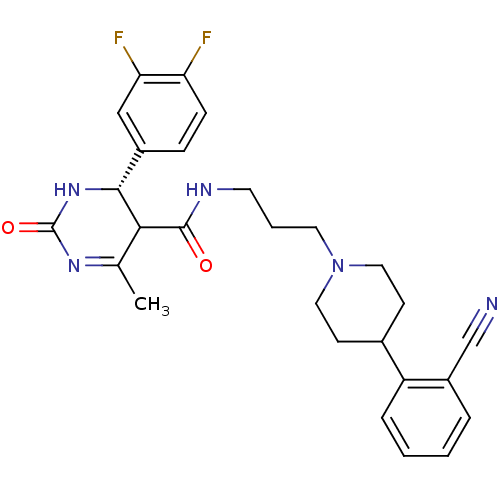 (4-(3,4-Difluoro-phenyl)-6-methyl-2-oxo-1,2,3,4-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 2 (Homo sapiens) | BDBM50458771 (CHEMBL4214046) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50059090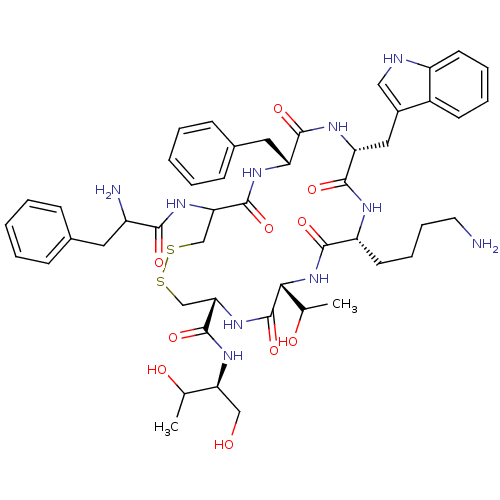 (10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionyla...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 95: 10836-41 (1998) Article DOI: 10.1073/pnas.95.18.10836 BindingDB Entry DOI: 10.7270/Q2XW4HCM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090012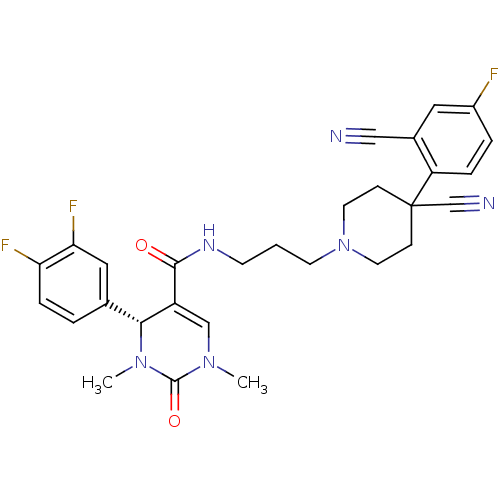 (4-(3,4-Difluoro-phenyl)-1,3-dimethyl-2-oxo-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50369584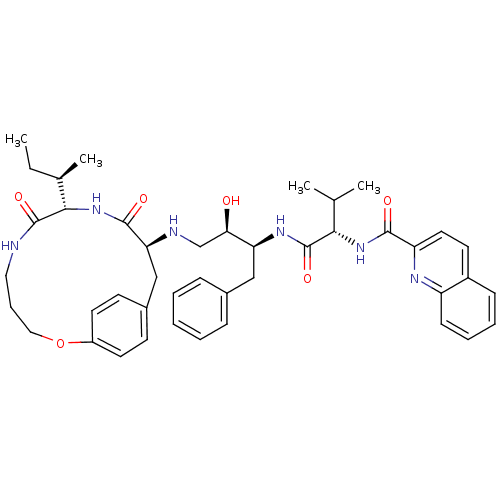 (CHEMBL1790230) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay | J Med Chem 47: 1641-51 (2004) Article DOI: 10.1021/jm030337m BindingDB Entry DOI: 10.7270/Q2JD4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50369584 (CHEMBL1790230) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 protease | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [491-589,Q7K,L33I,C67B,C95B] (Human immunodeficiency virus type 1) | BDBM798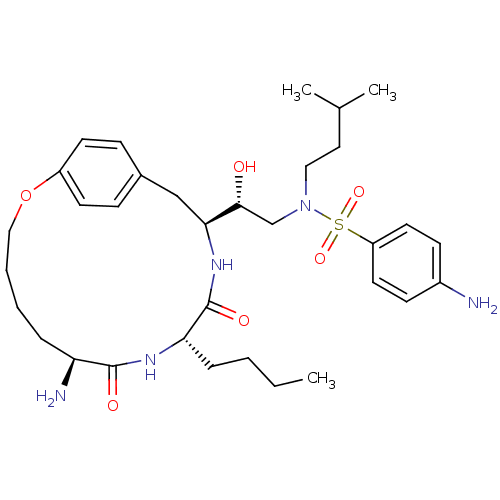 ((2R)-2-[(7S,10S,13S)-7-amino-10-butyl-8,11-dioxo-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.310 | -56.5 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland | Assay Description Inhibition constants were determined by a fluorometric assay. Ki values were calculated from either Dixon plots or Henderson plots in cases where the... | J Med Chem 45: 371-81 (2002) Article DOI: 10.1021/jm010414i BindingDB Entry DOI: 10.7270/Q2FQ9TSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [491-589,Q7K,L33I,C67B,C95B] (Human immunodeficiency virus type 1) | BDBM797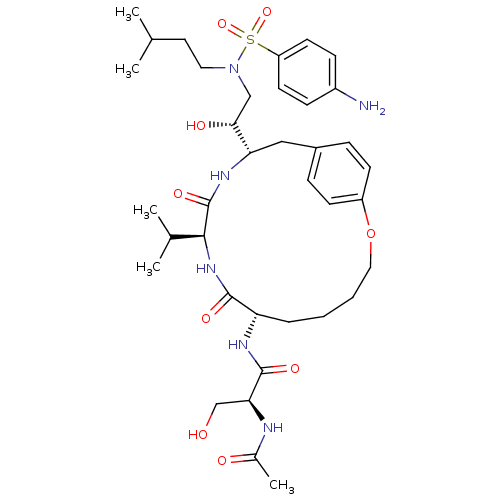 ((2S)-N-[(7S,10S,13S)-13-[(1R)-2-[(4-aminobenzene)(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.340 | -56.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland | Assay Description Inhibition constants were determined by a fluorometric assay. Ki values were calculated from either Dixon plots or Henderson plots in cases where the... | J Med Chem 45: 371-81 (2002) Article DOI: 10.1021/jm010414i BindingDB Entry DOI: 10.7270/Q2FQ9TSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50371333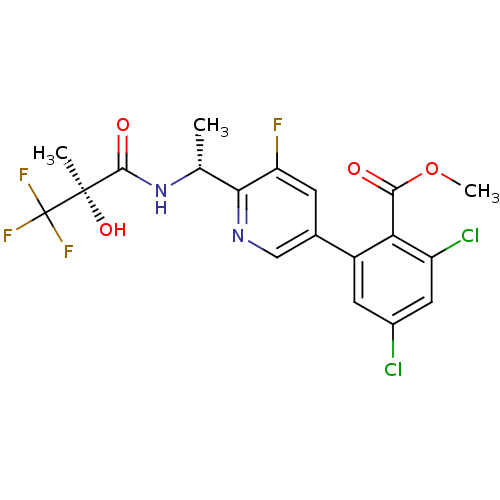 (CHEMBL256671) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human bradikinin B1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 716-20 (2008) Article DOI: 10.1016/j.bmcl.2007.11.050 BindingDB Entry DOI: 10.7270/Q2GM8849 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4020 total ) | Next | Last >> |