

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Rattus norvegicus) | BDBM50310540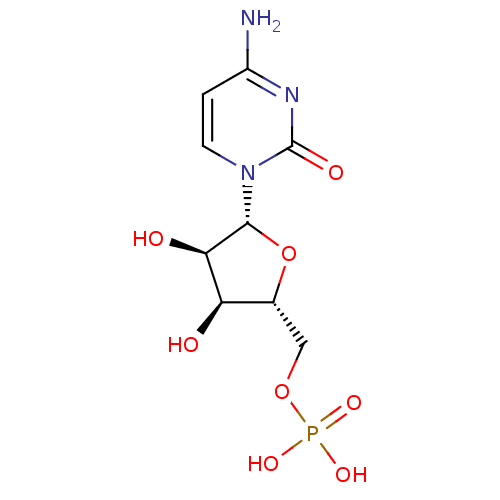 (((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| CMP-N-acetylneuraminate-beta-1,4-galactoside alpha-2,3-sialyltransferase (Rattus norvegicus) | BDBM50310540 (((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,3-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CMP-N-acetylneuraminate-beta-1,4-galactoside alpha-2,3-sialyltransferase (Rattus norvegicus) | BDBM50366833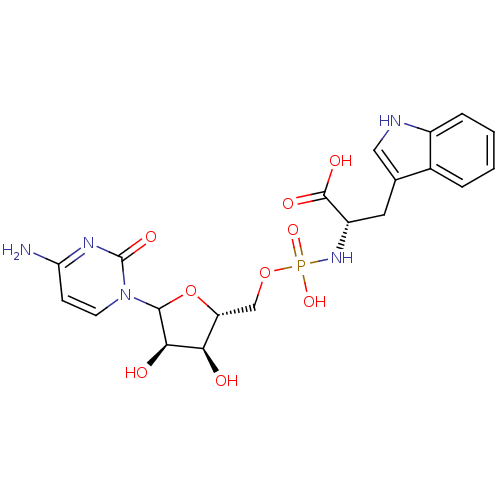 (CHEMBL608928) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,3-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Rattus norvegicus) | BDBM50366837 (CHEMBL608619) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CMP-N-acetylneuraminate-beta-1,4-galactoside alpha-2,3-sialyltransferase (Rattus norvegicus) | BDBM50366837 (CHEMBL608619) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,3-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Rattus norvegicus) | BDBM50366833 (CHEMBL608928) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CMP-N-acetylneuraminate-beta-1,4-galactoside alpha-2,3-sialyltransferase (Rattus norvegicus) | BDBM50366832 (CHEMBL609516) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,3-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Rattus norvegicus) | BDBM50366836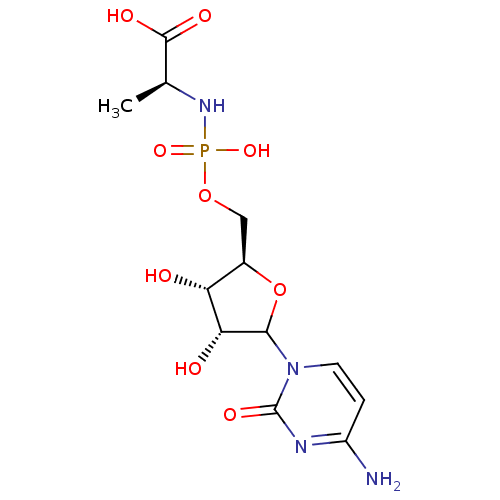 (CHEMBL610554) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Rattus norvegicus) | BDBM50366835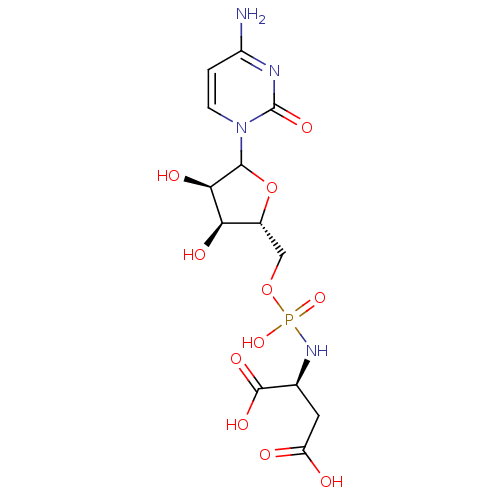 (CHEMBL609514) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CMP-N-acetylneuraminate-beta-1,4-galactoside alpha-2,3-sialyltransferase (Rattus norvegicus) | BDBM50366836 (CHEMBL610554) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,3-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CMP-N-acetylneuraminate-beta-1,4-galactoside alpha-2,3-sialyltransferase (Rattus norvegicus) | BDBM50366834 (CHEMBL609800) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,3-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Rattus norvegicus) | BDBM50366838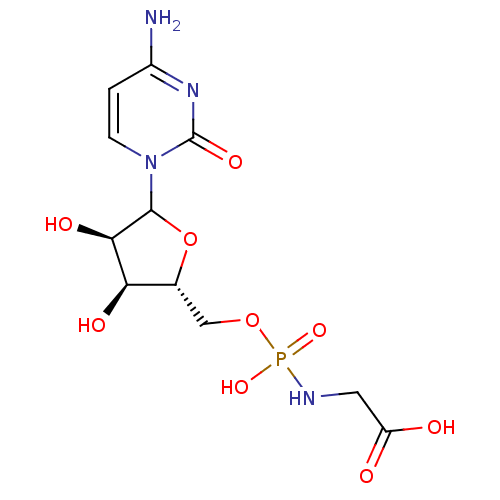 (CHEMBL609215) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Rattus norvegicus) | BDBM50366832 (CHEMBL609516) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Rattus norvegicus) | BDBM50366834 (CHEMBL609800) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CMP-N-acetylneuraminate-beta-1,4-galactoside alpha-2,3-sialyltransferase (Rattus norvegicus) | BDBM50366835 (CHEMBL609514) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,3-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CMP-N-acetylneuraminate-beta-1,4-galactoside alpha-2,3-sialyltransferase (Rattus norvegicus) | BDBM50366838 (CHEMBL609215) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,3-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 7 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of CCR7 (unknown origin) | J Med Chem 63: 5031-5073 (2020) Article DOI: 10.1021/acs.jmedchem.9b01701 BindingDB Entry DOI: 10.7270/Q2DJ5JXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50069756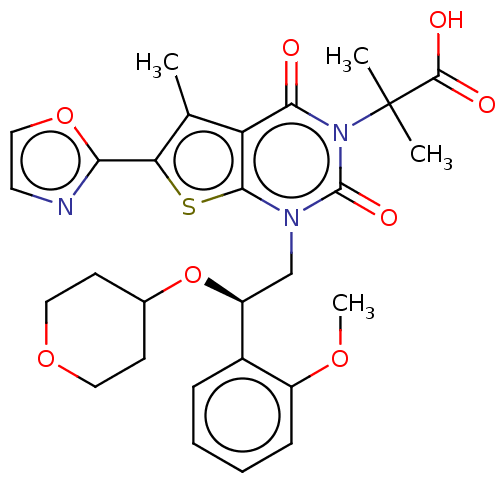 (CHEMBL3407547 | Firsocostat | ND-630 | NDI-010976 ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of ACC1 (unknown origin) | J Med Chem 63: 5031-5073 (2020) Article DOI: 10.1021/acs.jmedchem.9b01701 BindingDB Entry DOI: 10.7270/Q2DJ5JXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50069756 (CHEMBL3407547 | Firsocostat | ND-630 | NDI-010976 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of ACC2 (unknown origin) | J Med Chem 63: 5031-5073 (2020) Article DOI: 10.1021/acs.jmedchem.9b01701 BindingDB Entry DOI: 10.7270/Q2DJ5JXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50511117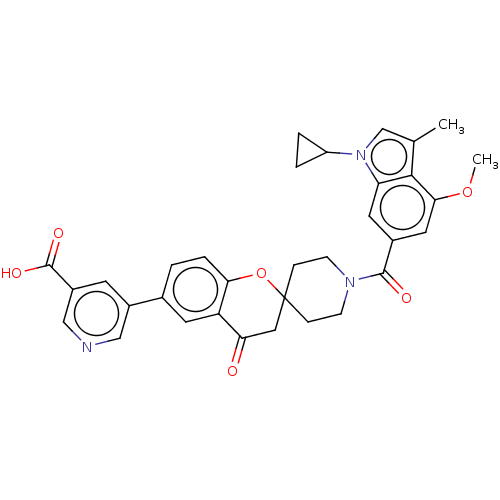 (CHEMBL4557289) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of ACC1 (unknown origin) | J Med Chem 63: 5031-5073 (2020) Article DOI: 10.1021/acs.jmedchem.9b01701 BindingDB Entry DOI: 10.7270/Q2DJ5JXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50511117 (CHEMBL4557289) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of ACC2 (unknown origin) | J Med Chem 63: 5031-5073 (2020) Article DOI: 10.1021/acs.jmedchem.9b01701 BindingDB Entry DOI: 10.7270/Q2DJ5JXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 7 (Homo sapiens (Human)) | BDBM50306033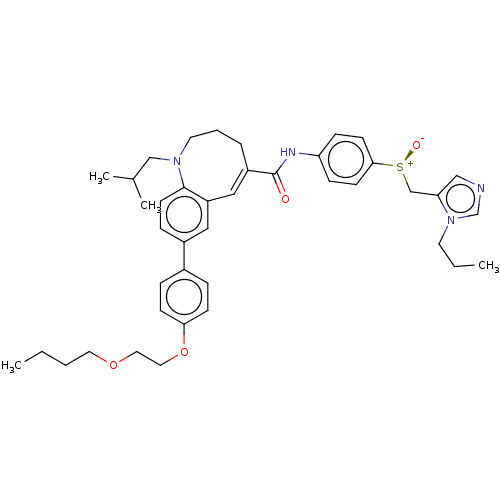 (Cenicriviroc | TAK-652 | TBR-652) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of CCR7 (unknown origin) | J Med Chem 63: 5031-5073 (2020) Article DOI: 10.1021/acs.jmedchem.9b01701 BindingDB Entry DOI: 10.7270/Q2DJ5JXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50306033 (Cenicriviroc | TAK-652 | TBR-652) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of CCR2 (unknown origin) | J Med Chem 63: 5031-5073 (2020) Article DOI: 10.1021/acs.jmedchem.9b01701 BindingDB Entry DOI: 10.7270/Q2DJ5JXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319585 (US10174007, Example 4 | US10787438, Example 4 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged human KHK expressed in Escherichia coli in presence of NADPH | J Med Chem 63: 5031-5073 (2020) Article DOI: 10.1021/acs.jmedchem.9b01701 BindingDB Entry DOI: 10.7270/Q2DJ5JXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM50511118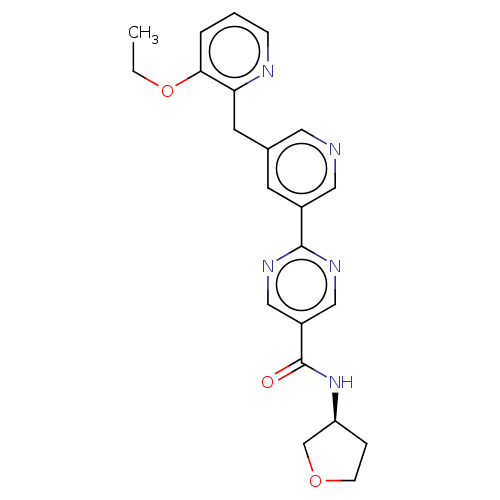 (CHEMBL4456029) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant FLAG-tagged human DGAT2 expressed in SF9 cells after 1 hr by TopCount assay | J Med Chem 63: 5031-5073 (2020) Article DOI: 10.1021/acs.jmedchem.9b01701 BindingDB Entry DOI: 10.7270/Q2DJ5JXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of CCR2 (unknown origin) | J Med Chem 63: 5031-5073 (2020) Article DOI: 10.1021/acs.jmedchem.9b01701 BindingDB Entry DOI: 10.7270/Q2DJ5JXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50511112 (CHEMBL4567446) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of ACC2 (unknown origin) | J Med Chem 63: 5031-5073 (2020) Article DOI: 10.1021/acs.jmedchem.9b01701 BindingDB Entry DOI: 10.7270/Q2DJ5JXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50511112 (CHEMBL4567446) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of ACC1 (unknown origin) | J Med Chem 63: 5031-5073 (2020) Article DOI: 10.1021/acs.jmedchem.9b01701 BindingDB Entry DOI: 10.7270/Q2DJ5JXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM448736 ((R)-2-pivalamido-4-((R)-3-(2-(5,6,7,8- | US1069667...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics, Inc. US Patent | Assay Description Microplates were coated with recombinant human integrin αvβ6 (2 ug/ml) in PBS (100 ul/well 25° C., overnight). The coating solution was rem... | US Patent US10696672 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM448743 ((S)-2-((tert-butoxycarbonyl)amino)-4- | US10696672...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics, Inc. US Patent | Assay Description Microplates were coated with recombinant human integrin αvβ6 (2 ug/ml) in PBS (100 ul/well 25° C., overnight). The coating solution was rem... | US Patent US10696672 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM448745 ((S)-2-benzamido-4-((R)-3-(2-(5,6,7,8- | US10696672...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics, Inc. US Patent | Assay Description Microplates were coated with recombinant human integrin αvβ6 (2 ug/ml) in PBS (100 ul/well 25° C., overnight). The coating solution was rem... | US Patent US10696672 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM448745 ((S)-2-benzamido-4-((R)-3-(2-(5,6,7,8- | US10696672...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics, Inc. US Patent | Assay Description Microplates were coated with recombinant human integrin αvβ6 (2 ug/ml) in PBS (100 ul/well 25° C., overnight). The coating solution was rem... | US Patent US10696672 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM448757 ((S)-2-(2,6-dimethylbenzamido)-4-((R)- | US10696672...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics, Inc. US Patent | Assay Description Microplates were coated with recombinant human integrin αvβ6 (2 ug/ml) in PBS (100 ul/well 25° C., overnight). The coating solution was rem... | US Patent US10696672 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM448759 ((R)-2-(2,6-dimethylbenzamido)-4-((R)- | US10696672...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics, Inc. US Patent | Assay Description Microplates were coated with recombinant human integrin αvβ6 (2 ug/ml) in PBS (100 ul/well 25° C., overnight). The coating solution was rem... | US Patent US10696672 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM448760 ((S)-2-(2-chloro-3-fluorobenzamido)-4- | US10696672...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics, Inc. US Patent | Assay Description Microplates were coated with recombinant human integrin αvβ6 (2 ug/ml) in PBS (100 ul/well 25° C., overnight). The coating solution was rem... | US Patent US10696672 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM448761 ((S)-2-(2,6-dichlorobenzamido)-4-((R)- | US10696672...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics, Inc. US Patent | Assay Description Microplates were coated with recombinant human integrin αvβ6 (2 ug/ml) in PBS (100 ul/well 25° C., overnight). The coating solution was rem... | US Patent US10696672 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM448766 (US10696672, Compound 7a | US11634418, Compound 7a) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics, Inc. US Patent | Assay Description Microplates were coated with recombinant human integrin αvβ6 (2 ug/ml) in PBS (100 ul/well 25° C., overnight). The coating solution was rem... | US Patent US10696672 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM448767 (US10696672, Compound 7c | US11634418, Compound 7c) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics, Inc. US Patent | Assay Description Microplates were coated with recombinant human integrin αvβ6 (2 ug/ml) in PBS (100 ul/well 25° C., overnight). The coating solution was rem... | US Patent US10696672 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM448768 ((S)-4-((R)-3-(2-(5,6,7,8-tetrahydro-1,8- | US10696...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics, Inc. US Patent | Assay Description Microplates were coated with recombinant human integrin αvβ6 (2 ug/ml) in PBS (100 ul/well 25° C., overnight). The coating solution was rem... | US Patent US10696672 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM448771 ((S)-4-((R)-3-(2-(5,6,7,8-tetrahydro-1,8- | US10696...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics, Inc. US Patent | Assay Description Microplates were coated with recombinant human integrin αvβ6 (2 ug/ml) in PBS (100 ul/well 25° C., overnight). The coating solution was rem... | US Patent US10696672 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM448772 ((S)-4-((R)-3-(2-(5,6,7,8-tetrahydro-1,8- | US10696...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics, Inc. US Patent | Assay Description Microplates were coated with recombinant human integrin αvβ6 (2 ug/ml) in PBS (100 ul/well 25° C., overnight). The coating solution was rem... | US Patent US10696672 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM448774 ((S)-2-(2,6-dichlorobenzamido)-4-((R)- | US10696672...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics, Inc. US Patent | Assay Description Microplates were coated with recombinant human integrin αvβ6 (2 ug/ml) in PBS (100 ul/well 25° C., overnight). The coating solution was rem... | US Patent US10696672 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM448776 ((S)-2-(3-(3,5-dimethyl-1H-pyrazol-1- | US10696672,...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics, Inc. US Patent | Assay Description Microplates were coated with recombinant human integrin αvβ6 (2 ug/ml) in PBS (100 ul/well 25° C., overnight). The coating solution was rem... | US Patent US10696672 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM448777 ((R)-2-(3-(3,5-dimethyl-1H-pyrazol-1- | US10696672,...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics, Inc. US Patent | Assay Description Microplates were coated with recombinant human integrin αvβ6 (2 ug/ml) in PBS (100 ul/well 25° C., overnight). The coating solution was rem... | US Patent US10696672 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM448776 ((S)-2-(3-(3,5-dimethyl-1H-pyrazol-1- | US10696672,...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics, Inc. US Patent | Assay Description Microplates were coated with recombinant human integrin αvβ6 (2 ug/ml) in PBS (100 ul/well 25° C., overnight). The coating solution was rem... | US Patent US10696672 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM448777 ((R)-2-(3-(3,5-dimethyl-1H-pyrazol-1- | US10696672,...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics, Inc. US Patent | Assay Description Microplates were coated with recombinant human integrin αvβ6 (2 ug/ml) in PBS (100 ul/well 25° C., overnight). The coating solution was rem... | US Patent US10696672 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM448779 ((S)-2-(3-morpholinobenzamido)-4- | US10696672, Com...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics, Inc. US Patent | Assay Description Microplates were coated with recombinant human integrin αvβ6 (2 ug/ml) in PBS (100 ul/well 25° C., overnight). The coating solution was rem... | US Patent US10696672 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM448782 ((S)-2-(isonicotinamido)-4-((R)-3-(2- | US10696672,...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics, Inc. US Patent | Assay Description Microplates were coated with recombinant human integrin αvβ6 (2 ug/ml) in PBS (100 ul/well 25° C., overnight). The coating solution was rem... | US Patent US10696672 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM448784 ((S)-2-(3,5-dichloroisonicotinamido)-4- | US1069667...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics, Inc. US Patent | Assay Description Microplates were coated with recombinant human integrin αvβ6 (2 ug/ml) in PBS (100 ul/well 25° C., overnight). The coating solution was rem... | US Patent US10696672 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM448785 ((R)-2-(3,5-dichloroisonicotinamido)-4- | US1069667...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics, Inc. US Patent | Assay Description Microplates were coated with recombinant human integrin αvβ6 (2 ug/ml) in PBS (100 ul/well 25° C., overnight). The coating solution was rem... | US Patent US10696672 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 423 total ) | Next | Last >> |