 Found 615 hits with Last Name = 'cabral' and Initial = 's'
Found 615 hits with Last Name = 'cabral' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50386955
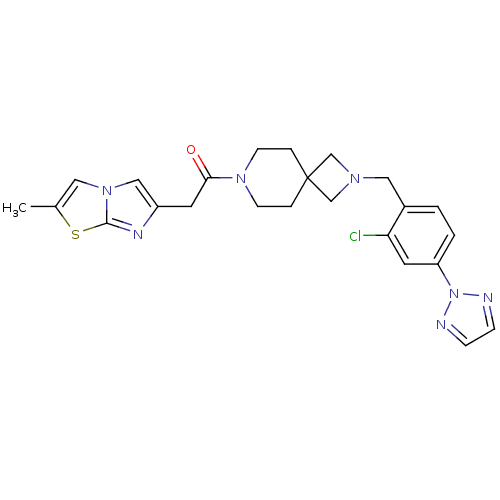
(CHEMBL2048820)Show SMILES Cc1cn2cc(CC(=O)N3CCC4(CN(Cc5ccc(cc5Cl)-n5nccn5)C4)CC3)nc2s1 Show InChI InChI=1S/C24H26ClN7OS/c1-17-12-31-14-19(28-23(31)34-17)10-22(33)30-8-4-24(5-9-30)15-29(16-24)13-18-2-3-20(11-21(18)25)32-26-6-7-27-32/h2-3,6-7,11-12,14H,4-5,8-10,13,15-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA |
Bioorg Med Chem Lett 22: 4281-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.024
BindingDB Entry DOI: 10.7270/Q2P2705D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50386949
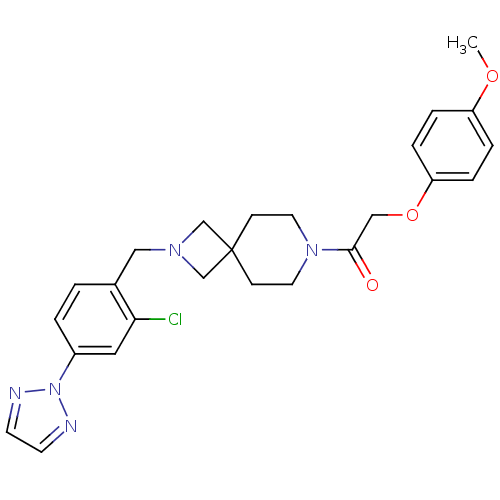
(CHEMBL2048814)Show SMILES COc1ccc(OCC(=O)N2CCC3(CN(Cc4ccc(cc4Cl)-n4nccn4)C3)CC2)cc1 Show InChI InChI=1S/C25H28ClN5O3/c1-33-21-4-6-22(7-5-21)34-16-24(32)30-12-8-25(9-13-30)17-29(18-25)15-19-2-3-20(14-23(19)26)31-27-10-11-28-31/h2-7,10-11,14H,8-9,12-13,15-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA |
Bioorg Med Chem Lett 22: 4281-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.024
BindingDB Entry DOI: 10.7270/Q2P2705D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50386952

(CHEMBL2048817)Show SMILES CC(C)COc1ccc(CN2CC3(C2)CCN(CC3)C(=O)Cc2n[nH]c3ccccc23)cc1 Show InChI InChI=1S/C27H34N4O2/c1-20(2)17-33-22-9-7-21(8-10-22)16-30-18-27(19-30)11-13-31(14-12-27)26(32)15-25-23-5-3-4-6-24(23)28-29-25/h3-10,20H,11-19H2,1-2H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA |
Bioorg Med Chem Lett 22: 4281-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.024
BindingDB Entry DOI: 10.7270/Q2P2705D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50386948
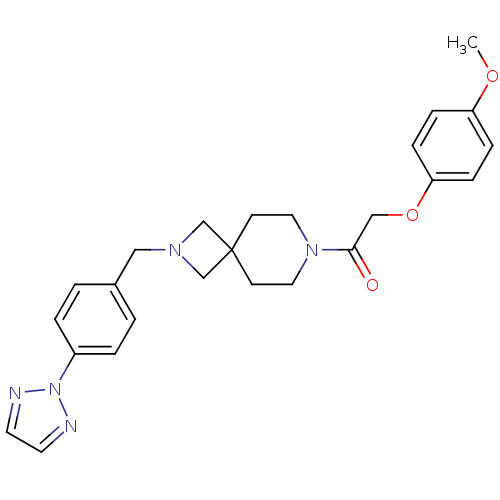
(CHEMBL2048813)Show SMILES COc1ccc(OCC(=O)N2CCC3(CN(Cc4ccc(cc4)-n4nccn4)C3)CC2)cc1 Show InChI InChI=1S/C25H29N5O3/c1-32-22-6-8-23(9-7-22)33-17-24(31)29-14-10-25(11-15-29)18-28(19-25)16-20-2-4-21(5-3-20)30-26-12-13-27-30/h2-9,12-13H,10-11,14-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA |
Bioorg Med Chem Lett 22: 4281-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.024
BindingDB Entry DOI: 10.7270/Q2P2705D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50386957
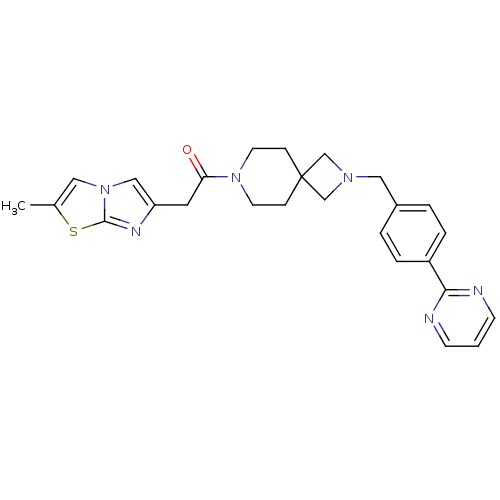
(CHEMBL2048822)Show SMILES Cc1cn2cc(CC(=O)N3CCC4(CN(Cc5ccc(cc5)-c5ncccn5)C4)CC3)nc2s1 Show InChI InChI=1S/C26H28N6OS/c1-19-14-32-16-22(29-25(32)34-19)13-23(33)31-11-7-26(8-12-31)17-30(18-26)15-20-3-5-21(6-4-20)24-27-9-2-10-28-24/h2-6,9-10,14,16H,7-8,11-13,15,17-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA |
Bioorg Med Chem Lett 22: 4281-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.024
BindingDB Entry DOI: 10.7270/Q2P2705D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50386953
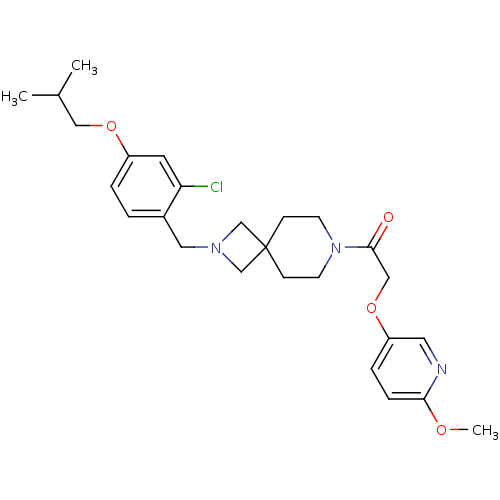
(CHEMBL2048818)Show SMILES COc1ccc(OCC(=O)N2CCC3(CN(Cc4ccc(OCC(C)C)cc4Cl)C3)CC2)cn1 Show InChI InChI=1S/C26H34ClN3O4/c1-19(2)15-33-21-5-4-20(23(27)12-21)14-29-17-26(18-29)8-10-30(11-9-26)25(31)16-34-22-6-7-24(32-3)28-13-22/h4-7,12-13,19H,8-11,14-18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA |
Bioorg Med Chem Lett 22: 4281-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.024
BindingDB Entry DOI: 10.7270/Q2P2705D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50386945
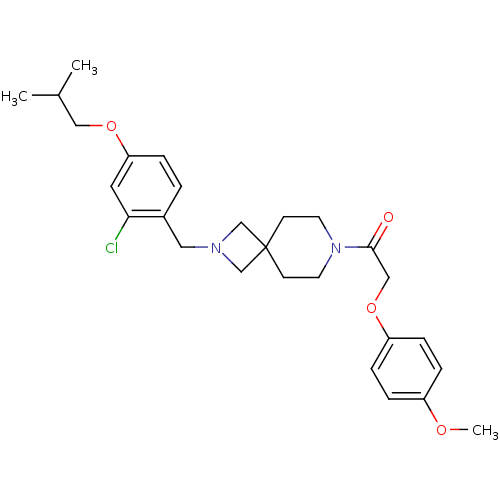
(CHEMBL2048810)Show SMILES COc1ccc(OCC(=O)N2CCC3(CN(Cc4ccc(OCC(C)C)cc4Cl)C3)CC2)cc1 Show InChI InChI=1S/C27H35ClN2O4/c1-20(2)16-33-24-5-4-21(25(28)14-24)15-29-18-27(19-29)10-12-30(13-11-27)26(31)17-34-23-8-6-22(32-3)7-9-23/h4-9,14,20H,10-13,15-19H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA |
Bioorg Med Chem Lett 22: 4281-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.024
BindingDB Entry DOI: 10.7270/Q2P2705D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50386958
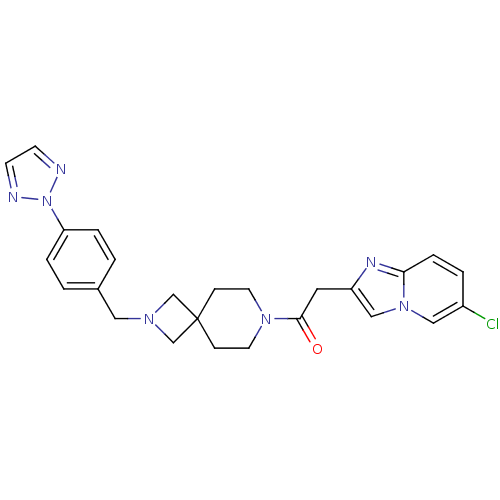
(CHEMBL2048823)Show SMILES Clc1ccc2nc(CC(=O)N3CCC4(CN(Cc5ccc(cc5)-n5nccn5)C4)CC3)cn2c1 Show InChI InChI=1S/C25H26ClN7O/c26-20-3-6-23-29-21(16-32(23)15-20)13-24(34)31-11-7-25(8-12-31)17-30(18-25)14-19-1-4-22(5-2-19)33-27-9-10-28-33/h1-6,9-10,15-16H,7-8,11-14,17-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA |
Bioorg Med Chem Lett 22: 4281-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.024
BindingDB Entry DOI: 10.7270/Q2P2705D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50386951
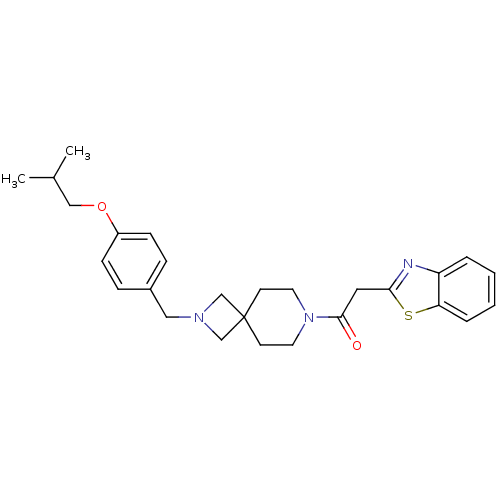
(CHEMBL2048816)Show SMILES CC(C)COc1ccc(CN2CC3(C2)CCN(CC3)C(=O)Cc2nc3ccccc3s2)cc1 Show InChI InChI=1S/C27H33N3O2S/c1-20(2)17-32-22-9-7-21(8-10-22)16-29-18-27(19-29)11-13-30(14-12-27)26(31)15-25-28-23-5-3-4-6-24(23)33-25/h3-10,20H,11-19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA |
Bioorg Med Chem Lett 22: 4281-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.024
BindingDB Entry DOI: 10.7270/Q2P2705D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50386959
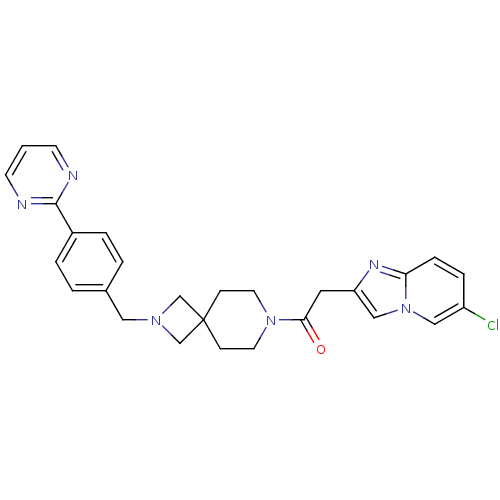
(CHEMBL2048824)Show SMILES Clc1ccc2nc(CC(=O)N3CCC4(CN(Cc5ccc(cc5)-c5ncccn5)C4)CC3)cn2c1 Show InChI InChI=1S/C27H27ClN6O/c28-22-6-7-24-31-23(17-34(24)16-22)14-25(35)33-12-8-27(9-13-33)18-32(19-27)15-20-2-4-21(5-3-20)26-29-10-1-11-30-26/h1-7,10-11,16-17H,8-9,12-15,18-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA |
Bioorg Med Chem Lett 22: 4281-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.024
BindingDB Entry DOI: 10.7270/Q2P2705D |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50140541
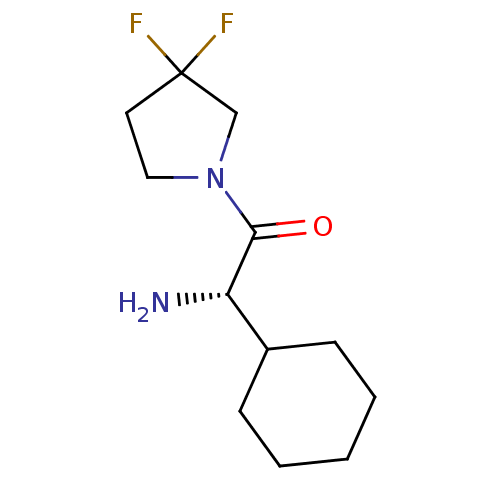
((S)-2-Amino-2-cyclohexyl-1-(3,3-difluoro-pyrrolidi...)Show InChI InChI=1S/C12H20F2N2O/c13-12(14)6-7-16(8-12)11(17)10(15)9-4-2-1-3-5-9/h9-10H,1-8,15H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory constant against Dipeptidylpeptidase IV activity |
Bioorg Med Chem Lett 15: 4770-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.026
BindingDB Entry DOI: 10.7270/Q28K78M0 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50386946
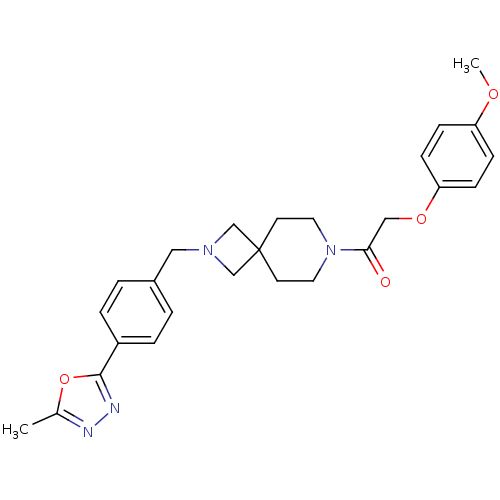
(CHEMBL2048811)Show SMILES COc1ccc(OCC(=O)N2CCC3(CN(Cc4ccc(cc4)-c4nnc(C)o4)C3)CC2)cc1 Show InChI InChI=1S/C26H30N4O4/c1-19-27-28-25(34-19)21-5-3-20(4-6-21)15-29-17-26(18-29)11-13-30(14-12-26)24(31)16-33-23-9-7-22(32-2)8-10-23/h3-10H,11-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA |
Bioorg Med Chem Lett 22: 4281-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.024
BindingDB Entry DOI: 10.7270/Q2P2705D |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50140527
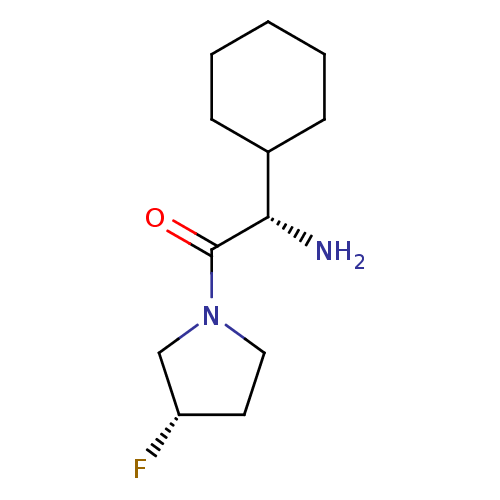
((S)-2-Amino-2-cyclohexyl-1-((S)-3-fluoro-pyrrolidi...)Show InChI InChI=1S/C12H21FN2O/c13-10-6-7-15(8-10)12(16)11(14)9-4-2-1-3-5-9/h9-11H,1-8,14H2/t10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory constant against Dipeptidylpeptidase IV activity |
Bioorg Med Chem Lett 15: 4770-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.026
BindingDB Entry DOI: 10.7270/Q28K78M0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50172141
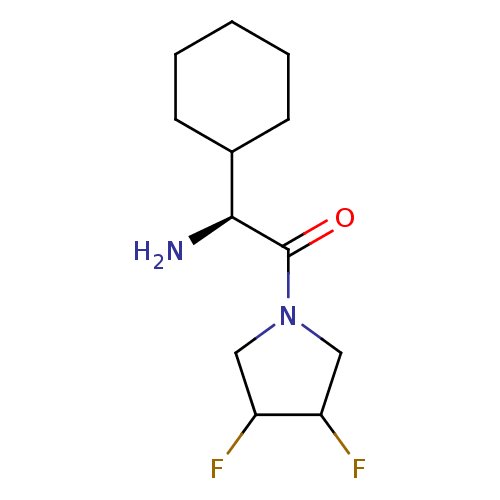
((S)-2-Amino-2-cyclohexyl-1-(3,4-difluoro-pyrrolidi...)Show InChI InChI=1S/C12H20F2N2O/c13-9-6-16(7-10(9)14)12(17)11(15)8-4-2-1-3-5-8/h8-11H,1-7,15H2/t9?,10?,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory constant against Dipeptidylpeptidase IV calculated from IC50 from standard dose inhibition curve using cheng-Prussoff equation; range=61+/... |
Bioorg Med Chem Lett 15: 4770-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.026
BindingDB Entry DOI: 10.7270/Q28K78M0 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50386954

(CHEMBL2048819)Show SMILES Cc1cn2cc(CC(=O)N3CCC4(CN(Cc5ccc(cc5)-n5nccn5)C4)CC3)nc2s1 Show InChI InChI=1S/C24H27N7OS/c1-18-13-30-15-20(27-23(30)33-18)12-22(32)29-10-6-24(7-11-29)16-28(17-24)14-19-2-4-21(5-3-19)31-25-8-9-26-31/h2-5,8-9,13,15H,6-7,10-12,14,16-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA |
Bioorg Med Chem Lett 22: 4281-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.024
BindingDB Entry DOI: 10.7270/Q2P2705D |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50172144
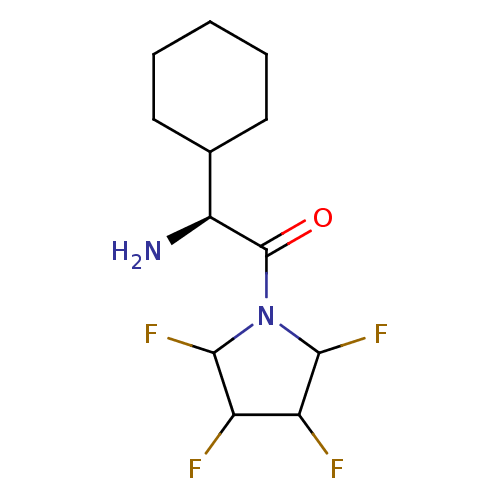
((S)-2-Amino-2-cyclohexyl-1-(2,3,4,5-tetrafluoro-py...)Show InChI InChI=1S/C12H18F4N2O/c13-7-8(14)11(16)18(10(7)15)12(19)9(17)6-4-2-1-3-5-6/h6-11H,1-5,17H2/t7?,8?,9-,10?,11?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory constant against Dipeptidylpeptidase IV activity |
Bioorg Med Chem Lett 15: 4770-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.026
BindingDB Entry DOI: 10.7270/Q28K78M0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50172143
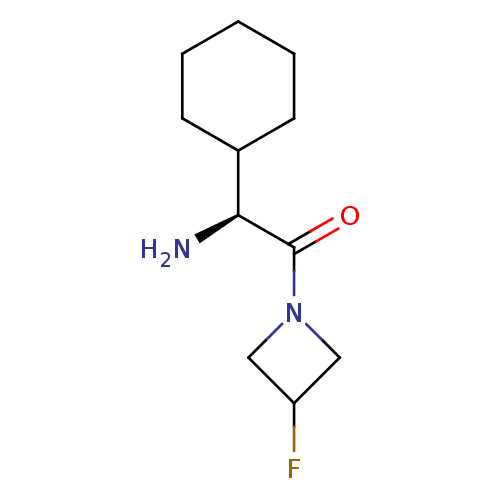
((S)-2-Amino-2-cyclohexyl-1-(3-fluoro-azetidin-1-yl...)Show InChI InChI=1S/C11H19FN2O/c12-9-6-14(7-9)11(15)10(13)8-4-2-1-3-5-8/h8-10H,1-7,13H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory constant against Dipeptidylpeptidase IV activity |
Bioorg Med Chem Lett 15: 4770-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.026
BindingDB Entry DOI: 10.7270/Q28K78M0 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50386950
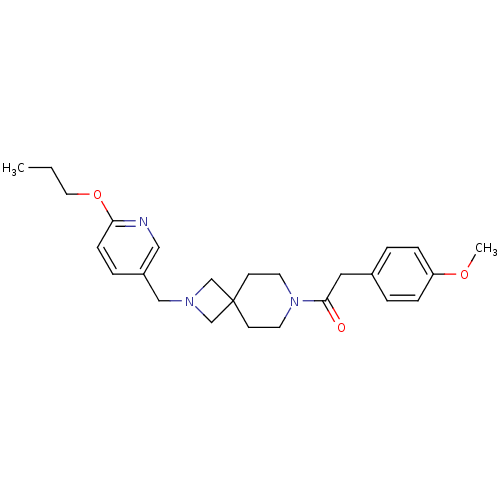
(CHEMBL2048815)Show SMILES CCCOc1ccc(CN2CC3(C2)CCN(CC3)C(=O)Cc2ccc(OC)cc2)cn1 Show InChI InChI=1S/C25H33N3O3/c1-3-14-31-23-9-6-21(16-26-23)17-27-18-25(19-27)10-12-28(13-11-25)24(29)15-20-4-7-22(30-2)8-5-20/h4-9,16H,3,10-15,17-19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA |
Bioorg Med Chem Lett 22: 4281-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.024
BindingDB Entry DOI: 10.7270/Q2P2705D |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50172139
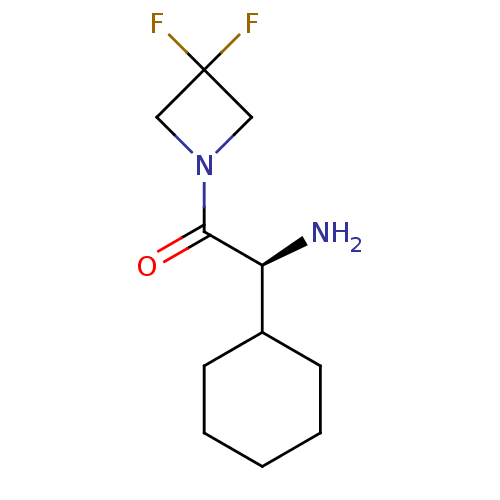
((S)-2-Amino-2-cyclohexyl-1-(3,3-difluoro-azetidin-...)Show InChI InChI=1S/C11H18F2N2O/c12-11(13)6-15(7-11)10(16)9(14)8-4-2-1-3-5-8/h8-9H,1-7,14H2/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory constant against Dipeptidylpeptidase IV activity |
Bioorg Med Chem Lett 15: 4770-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.026
BindingDB Entry DOI: 10.7270/Q28K78M0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM12191
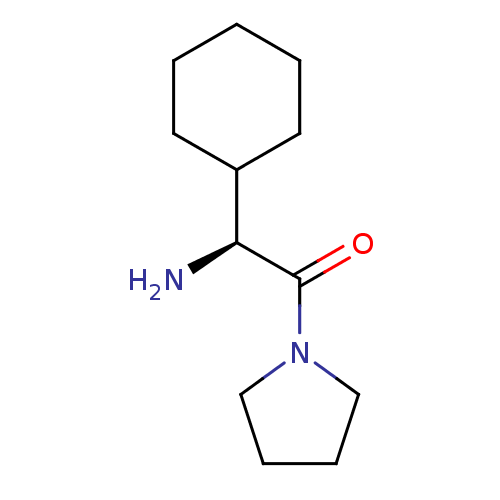
((2S)-2-amino-2-cyclohexyl-1-(pyrrolidin-1-yl)ethan...)Show InChI InChI=1S/C12H22N2O/c13-11(10-6-2-1-3-7-10)12(15)14-8-4-5-9-14/h10-11H,1-9,13H2/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory constant against Dipeptidylpeptidase IV activity |
Bioorg Med Chem Lett 15: 4770-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.026
BindingDB Entry DOI: 10.7270/Q28K78M0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50140539
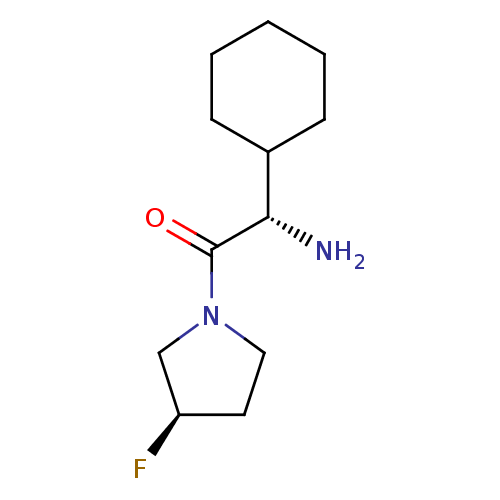
((S)-2-Amino-2-cyclohexyl-1-((R)-3-fluoro-pyrrolidi...)Show InChI InChI=1S/C12H21FN2O/c13-10-6-7-15(8-10)12(16)11(14)9-4-2-1-3-5-9/h9-11H,1-8,14H2/t10-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory constant against Dipeptidylpeptidase IV activity |
Bioorg Med Chem Lett 15: 4770-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.026
BindingDB Entry DOI: 10.7270/Q28K78M0 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50386947
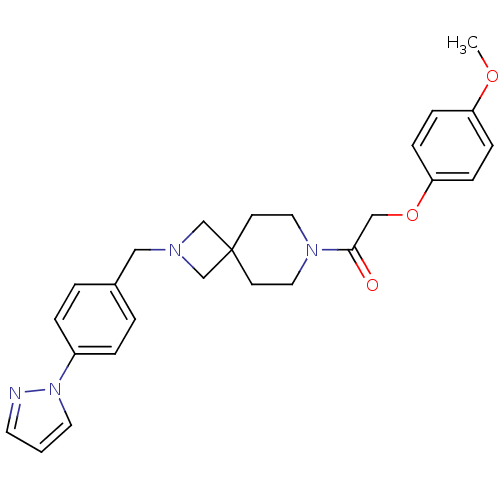
(CHEMBL2048812)Show SMILES COc1ccc(OCC(=O)N2CCC3(CN(Cc4ccc(cc4)-n4cccn4)C3)CC2)cc1 Show InChI InChI=1S/C26H30N4O3/c1-32-23-7-9-24(10-8-23)33-18-25(31)29-15-11-26(12-16-29)19-28(20-26)17-21-3-5-22(6-4-21)30-14-2-13-27-30/h2-10,13-14H,11-12,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA |
Bioorg Med Chem Lett 22: 4281-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.024
BindingDB Entry DOI: 10.7270/Q2P2705D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50386956
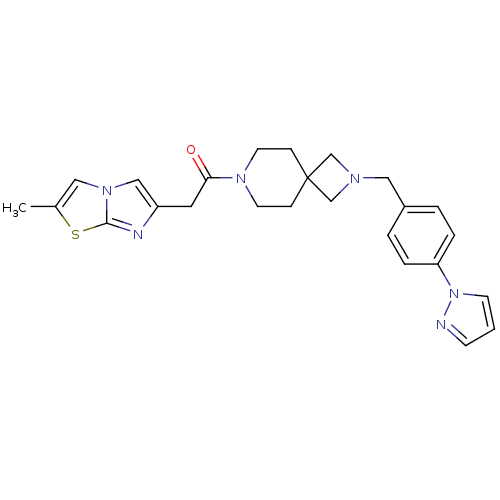
(CHEMBL2048821)Show SMILES Cc1cn2cc(CC(=O)N3CCC4(CN(Cc5ccc(cc5)-n5cccn5)C4)CC3)nc2s1 Show InChI InChI=1S/C25H28N6OS/c1-19-14-30-16-21(27-24(30)33-19)13-23(32)29-11-7-25(8-12-29)17-28(18-25)15-20-3-5-22(6-4-20)31-10-2-9-26-31/h2-6,9-10,14,16H,7-8,11-13,15,17-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 206 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA |
Bioorg Med Chem Lett 22: 4281-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.024
BindingDB Entry DOI: 10.7270/Q2P2705D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50386942

(CHEMBL2048807)Show SMILES COc1ccc(OCC(=O)N2CCC3(CN(Cc4ccc5OC(C)(C)CCc5c4)C3)CC2)cc1 Show InChI InChI=1S/C28H36N2O4/c1-27(2)11-10-22-16-21(4-9-25(22)34-27)17-29-19-28(20-29)12-14-30(15-13-28)26(31)18-33-24-7-5-23(32-3)6-8-24/h4-9,16H,10-15,17-20H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 408 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA |
Bioorg Med Chem Lett 22: 4281-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.024
BindingDB Entry DOI: 10.7270/Q2P2705D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50386944
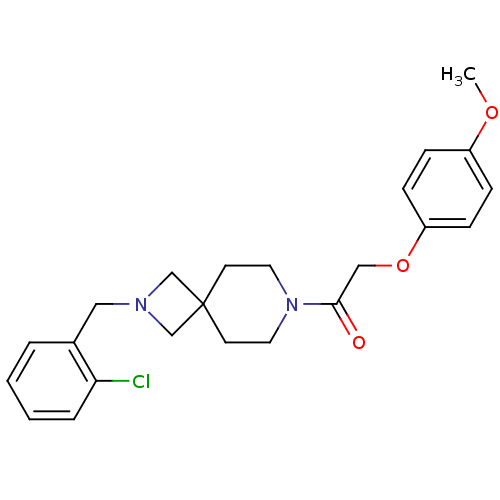
(CHEMBL2048809)Show SMILES COc1ccc(OCC(=O)N2CCC3(CN(Cc4ccccc4Cl)C3)CC2)cc1 Show InChI InChI=1S/C23H27ClN2O3/c1-28-19-6-8-20(9-7-19)29-15-22(27)26-12-10-23(11-13-26)16-25(17-23)14-18-4-2-3-5-21(18)24/h2-9H,10-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 445 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA |
Bioorg Med Chem Lett 22: 4281-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.024
BindingDB Entry DOI: 10.7270/Q2P2705D |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50172145
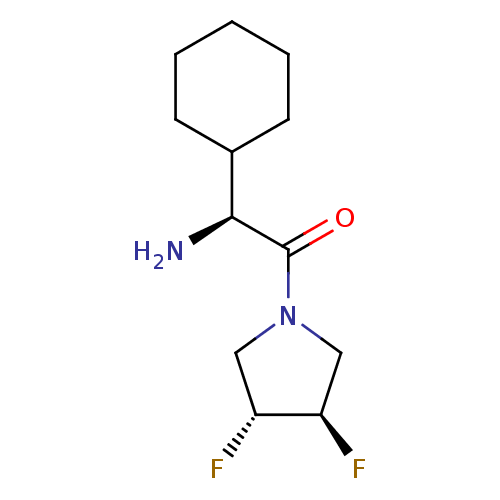
((S)-2-Amino-2-cyclohexyl-1-((3R,4R)-3,4-difluoro-p...)Show SMILES N[C@@H](C1CCCCC1)C(=O)N1C[C@@H](F)[C@H](F)C1 Show InChI InChI=1S/C12H20F2N2O/c13-9-6-16(7-10(9)14)12(17)11(15)8-4-2-1-3-5-8/h8-11H,1-7,15H2/t9-,10-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 504 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory constant against Dipeptidylpeptidase IV activity |
Bioorg Med Chem Lett 15: 4770-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.026
BindingDB Entry DOI: 10.7270/Q28K78M0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50172140
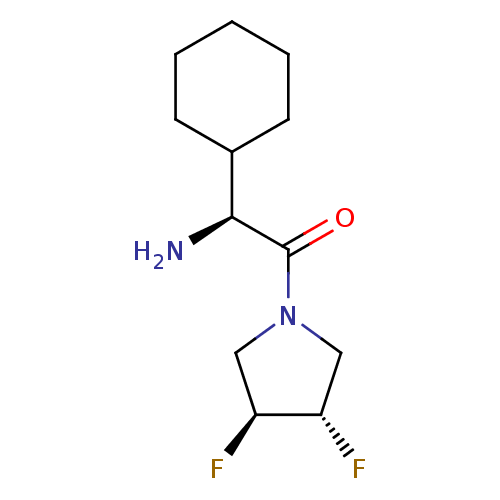
((S)-2-Amino-2-cyclohexyl-1-((3S,4S)-3,4-difluoro-p...)Show SMILES N[C@@H](C1CCCCC1)C(=O)N1C[C@H](F)[C@@H](F)C1 Show InChI InChI=1S/C12H20F2N2O/c13-9-6-16(7-10(9)14)12(17)11(15)8-4-2-1-3-5-8/h8-11H,1-7,15H2/t9-,10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory constant against Dipeptidylpeptidase IV activity |
Bioorg Med Chem Lett 15: 4770-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.026
BindingDB Entry DOI: 10.7270/Q28K78M0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50172142
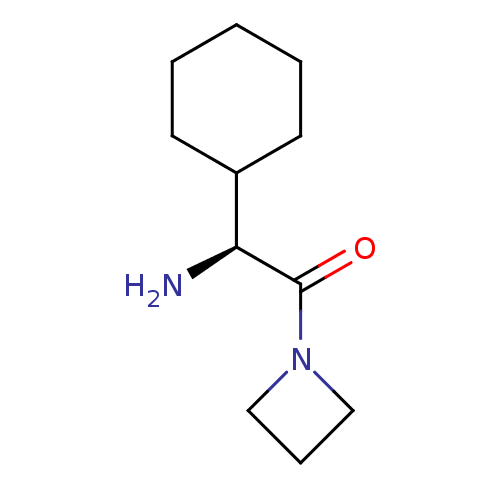
((S)-2-Amino-1-azetidin-1-yl-2-cyclohexyl-ethanone ...)Show InChI InChI=1S/C11H20N2O/c12-10(9-5-2-1-3-6-9)11(14)13-7-4-8-13/h9-10H,1-8,12H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory constant against Dipeptidylpeptidase IV activity |
Bioorg Med Chem Lett 15: 4770-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.026
BindingDB Entry DOI: 10.7270/Q28K78M0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565931
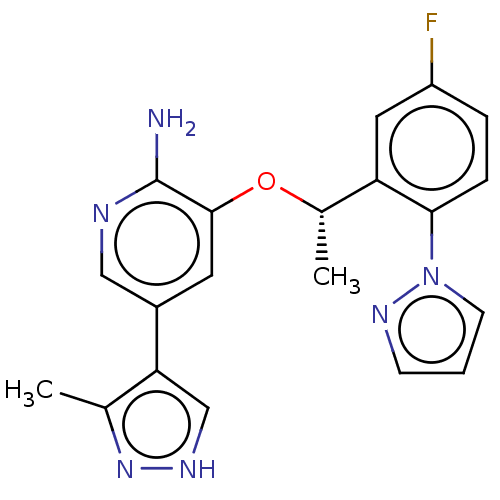
(CHEMBL4787096)Show SMILES C[C@H](Oc1cc(cnc1N)-c1c[nH]nc1C)c1cc(F)ccc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565919
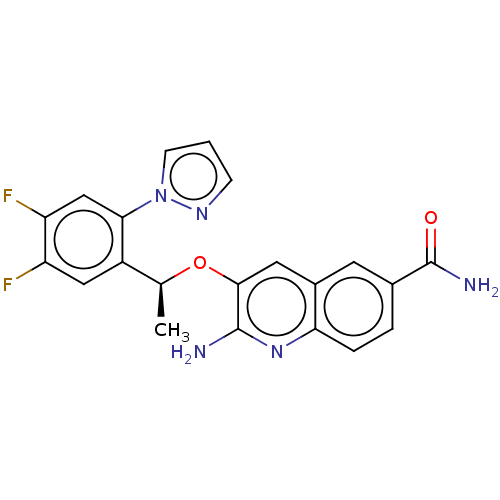
(CHEMBL4794362)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C(N)=O)c1cc(F)c(F)cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565920
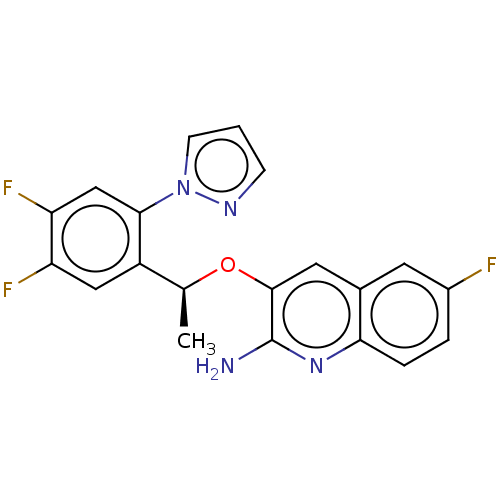
(CHEMBL4784517)Show SMILES C[C@H](Oc1cc2cc(F)ccc2nc1N)c1cc(F)c(F)cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565925
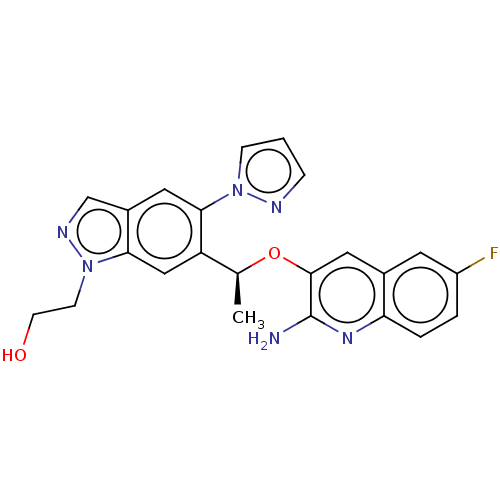
(CHEMBL4778780)Show SMILES C[C@H](Oc1cc2cc(F)ccc2nc1N)c1cc2n(CCO)ncc2cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565921
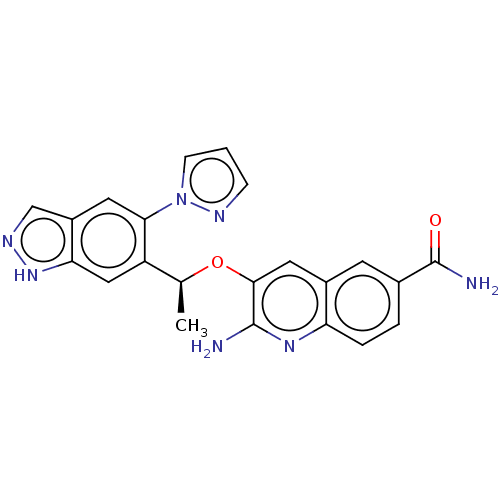
(CHEMBL4781765)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C(N)=O)c1cc2[nH]ncc2cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565918

(CHEMBL4778108)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C#N)c1cc(F)c(F)cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565917
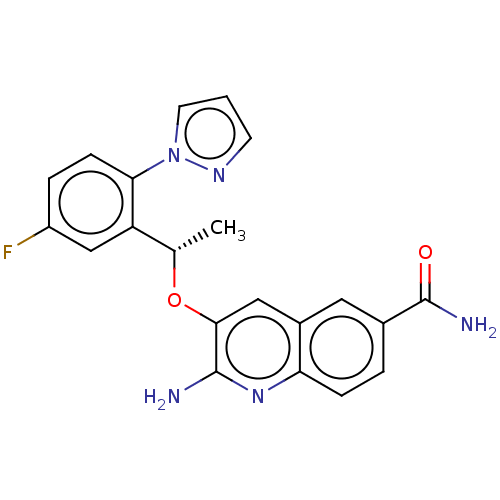
(CHEMBL4783261)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C(N)=O)c1cc(F)ccc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565922

(CHEMBL4797664)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C(N)=O)c1ncc(F)cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565929

(Pf-07059013)Show SMILES C[C@H](Oc1cc2cc(F)ccc2nc1N)c1[nH]c(=O)ccc1-n1cccn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565928

(CHEMBL4785484)Show SMILES C[C@H](Oc1cc2cc(F)cc(F)c2nc1N)c1cc(ccc1-n1cccn1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565927

(CHEMBL4779453)Show SMILES C[C@H](Oc1cc2cc(F)ccc2nc1N)c1cc(ccc1-n1cccn1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565926

(CHEMBL4778770)Show SMILES C[C@H](Oc1cc2cc(F)ccc2nc1N)c1cc2n(CC(O)=O)ncc2cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565924

(CHEMBL4795396)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C(N)=O)c1cc2n(CCO)ncc2cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565923

(CHEMBL4762748)Show SMILES C[C@H](Oc1cc2cc(cc(F)c2nc1N)C(N)=O)c1ncc(F)cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565916

(CHEMBL4777878)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C#N)c1cc(F)ccc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565930

(CHEMBL4796436)Show SMILES C[C@H](Oc1cc2cc(F)cc(F)c2nc1N)c1[nH]c(=O)ccc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM215446

(US9296745, 1)Show SMILES FC(F)(F)Oc1cccc(c1)-c1nc2ccc(nc2[nH]1)N1CCC[C@H](C1)C(=O)N1CCCC1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
For determination of IC50 values, the reactions were carried out in 384-well white Polyplates (Perkin Elmer) in a total volume of 20 uL. To 1 uL of c... |
US Patent US9296745 (2016)
BindingDB Entry DOI: 10.7270/Q25719VW |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM215552
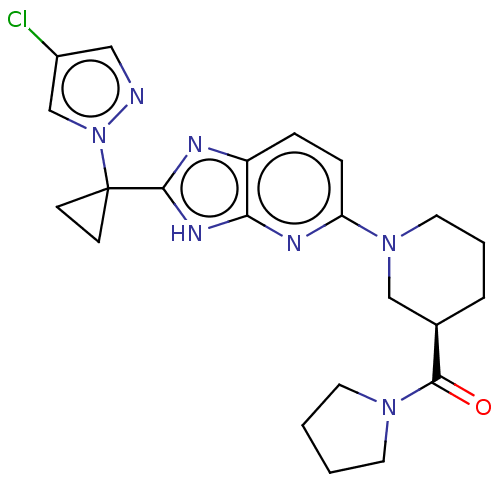
(US9296745, 109-A | US9296745, 109-B | US9296745, 1...)Show SMILES Clc1cnn(c1)C1(CC1)c1nc2ccc(nc2[nH]1)N1CCC[C@H](C1)C(=O)N1CCCC1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
For evaluation of the effects of DGAT2 inhibitors in a cell-based setting, cryopreserved human hepatocytes (Lot QOC, Celsis, Chicago, Ill.) were thaw... |
US Patent US9296745 (2016)
BindingDB Entry DOI: 10.7270/Q25719VW |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM215661

(US9296745, 199)Show SMILES C[C@H](c1nc2cnc(nc2[nH]1)N1CCC[C@H](C1)C(=O)N1CCCC1)n1cc(Cl)cn1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
For evaluation of the effects of DGAT2 inhibitors in a cell-based setting, cryopreserved human hepatocytes (Lot QOC, Celsis, Chicago, Ill.) were thaw... |
US Patent US9296745 (2016)
BindingDB Entry DOI: 10.7270/Q25719VW |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM215447
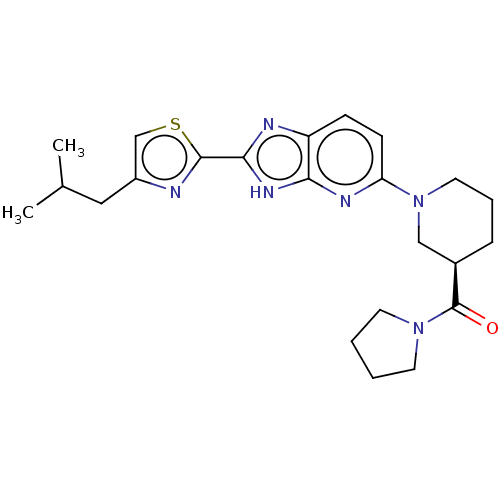
(US9296745, 2)Show SMILES CC(C)Cc1csc(n1)-c1nc2ccc(nc2[nH]1)N1CCC[C@H](C1)C(=O)N1CCCC1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.71 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
For determination of IC50 values, the reactions were carried out in 384-well white Polyplates (Perkin Elmer) in a total volume of 20 uL. To 1 uL of c... |
US Patent US9296745 (2016)
BindingDB Entry DOI: 10.7270/Q25719VW |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM215551
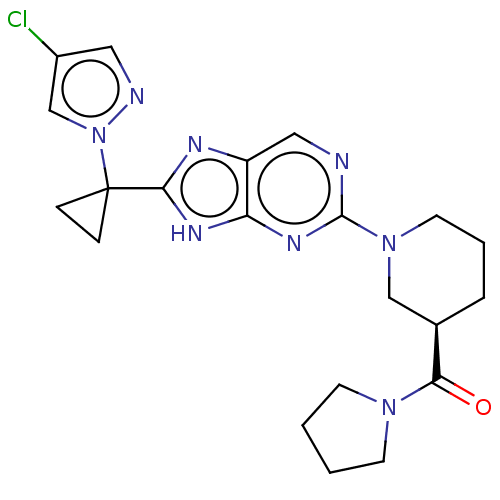
(US9296745, 108)Show SMILES Clc1cnn(c1)C1(CC1)c1nc2cnc(nc2[nH]1)N1CCC[C@H](C1)C(=O)N1CCCC1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
For evaluation of the effects of DGAT2 inhibitors in a cell-based setting, cryopreserved human hepatocytes (Lot QOC, Celsis, Chicago, Ill.) were thaw... |
US Patent US9296745 (2016)
BindingDB Entry DOI: 10.7270/Q25719VW |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM215487
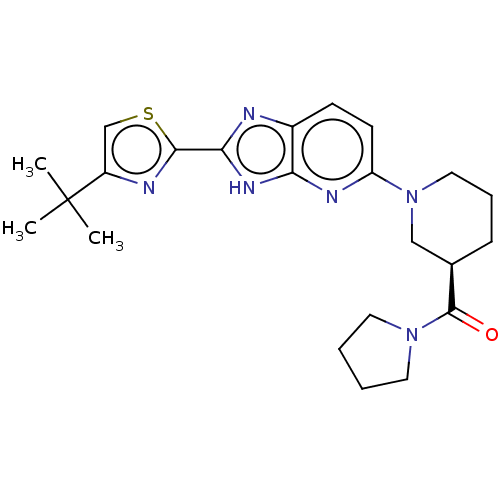
(US9296745, 42)Show SMILES CC(C)(C)c1csc(n1)-c1nc2ccc(nc2[nH]1)N1CCC[C@H](C1)C(=O)N1CCCC1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.18 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
For determination of IC50 values, the reactions were carried out in 384-well white Polyplates (Perkin Elmer) in a total volume of 20 uL. To 1 uL of c... |
US Patent US9296745 (2016)
BindingDB Entry DOI: 10.7270/Q25719VW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data