

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM310195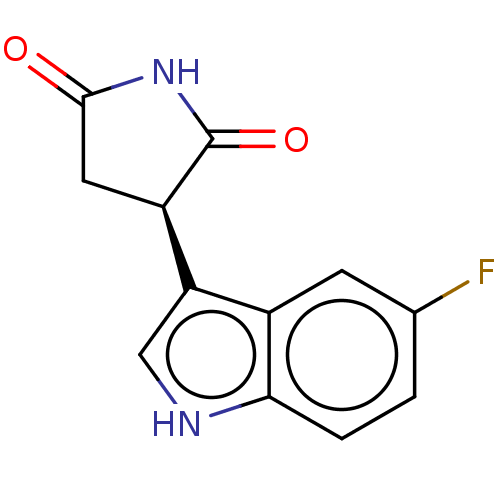 ((-)-(R)-3-(5-fluoro-1H- indol-3-yl)pyrrolidine- 2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description The compounds of the present invention inhibit the enzymatic activity of human IDO1.To measure enzymatic activity of human IDO1, the reaction mixture... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2959KW7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM310195 ((-)-(R)-3-(5-fluoro-1H- indol-3-yl)pyrrolidine- 2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
ITEOS THERAPEUTICS US Patent | Assay Description To measure enzymatic activity of human IDO1, the reaction mixture contained (final concentrations) potassium phosphate buffer (50 mM, pH 6.5), ascorb... | US Patent US9603836 (2017) BindingDB Entry DOI: 10.7270/Q2MK6FZ8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM310195 ((-)-(R)-3-(5-fluoro-1H- indol-3-yl)pyrrolidine- 2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.; iTEOS THERAPEUTICS US Patent | Assay Description To measure enzymatic activity of human IDO1, the reaction mixture contained (final concentrations) potassium phosphate buffer (50 mM, pH 6.5), ascorb... | US Patent US10945994 (2021) BindingDB Entry DOI: 10.7270/Q25D8W03 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM310195 ((-)-(R)-3-(5-fluoro-1H- indol-3-yl)pyrrolidine- 2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay | J Med Chem 60: 9617-9629 (2017) Article DOI: 10.1021/acs.jmedchem.7b00974 BindingDB Entry DOI: 10.7270/Q2ZP48M3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM309529 (3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description The compounds of the present invention inhibit the enzymatic activity of human IDO1.To measure enzymatic activity of human IDO1, the reaction mixture... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2959KW7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM309529 (3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
ITEOS THERAPEUTICS US Patent | Assay Description To measure enzymatic activity of human IDO1, the reaction mixture contained (final concentrations) potassium phosphate buffer (50 mM, pH 6.5), ascorb... | US Patent US9603836 (2017) BindingDB Entry DOI: 10.7270/Q2MK6FZ8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM309529 (3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.; iTEOS THERAPEUTICS US Patent | Assay Description To measure enzymatic activity of human IDO1, the reaction mixture contained (final concentrations) potassium phosphate buffer (50 mM, pH 6.5), ascorb... | US Patent US10945994 (2021) BindingDB Entry DOI: 10.7270/Q25D8W03 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM309529 (3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay | J Med Chem 60: 9617-9629 (2017) Article DOI: 10.1021/acs.jmedchem.7b00974 BindingDB Entry DOI: 10.7270/Q2ZP48M3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM310195 ((-)-(R)-3-(5-fluoro-1H- indol-3-yl)pyrrolidine- 2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.; iTEOS THERAPEUTICS US Patent | Assay Description To measure enzymatic activity of human IDO1, the reaction mixture contained (final concentrations) potassium phosphate buffer (50 mM, pH 6.5), ascorb... | US Patent US10945994 (2021) BindingDB Entry DOI: 10.7270/Q25D8W03 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Dog) | BDBM310195 ((-)-(R)-3-(5-fluoro-1H- indol-3-yl)pyrrolidine- 2,...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.; iTEOS THERAPEUTICS US Patent | Assay Description To measure enzymatic activity of human IDO1, the reaction mixture contained (final concentrations) potassium phosphate buffer (50 mM, pH 6.5), ascorb... | US Patent US10945994 (2021) BindingDB Entry DOI: 10.7270/Q25D8W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM310195 ((-)-(R)-3-(5-fluoro-1H- indol-3-yl)pyrrolidine- 2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO-1 using tryptophan as substrate after 22 mins by LC-MS/MS method | J Med Chem 60: 9617-9629 (2017) Article DOI: 10.1021/acs.jmedchem.7b00974 BindingDB Entry DOI: 10.7270/Q2ZP48M3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM309529 (3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
ITEOS THERAPEUTICS US Patent | Assay Description To measure enzymatic activity of human IDO1, the reaction mixture contained (final concentrations) potassium phosphate buffer (50 mM, pH 6.5), ascorb... | US Patent US9603836 (2017) BindingDB Entry DOI: 10.7270/Q2MK6FZ8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM309529 (3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description The compounds of the present invention inhibit the enzymatic activity of human IDO1.To measure enzymatic activity of human IDO1, the reaction mixture... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2959KW7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM310197 (US10945994, Compound 2a | US9603836, Compound 2a |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.; iTEOS THERAPEUTICS US Patent | Assay Description To measure enzymatic activity of human IDO1, the reaction mixture contained (final concentrations) potassium phosphate buffer (50 mM, pH 6.5), ascorb... | US Patent US10945994 (2021) BindingDB Entry DOI: 10.7270/Q25D8W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM312073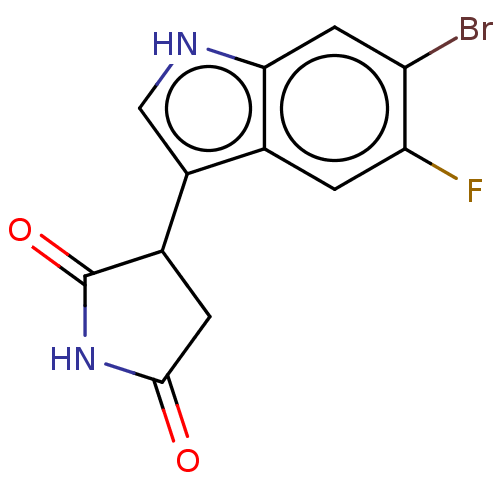 (3-(6-bromo-5-fluoro- 1H-indol-3-yl)- pyrrolidine-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
ITEOS THERAPEUTICS US Patent | Assay Description To measure enzymatic activity of human IDO1, the reaction mixture contained (final concentrations) potassium phosphate buffer (50 mM, pH 6.5), ascorb... | US Patent US9603836 (2017) BindingDB Entry DOI: 10.7270/Q2MK6FZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM312073 (3-(6-bromo-5-fluoro- 1H-indol-3-yl)- pyrrolidine-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description The compounds of the present invention inhibit the enzymatic activity of human IDO1.To measure enzymatic activity of human IDO1, the reaction mixture... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2959KW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM339961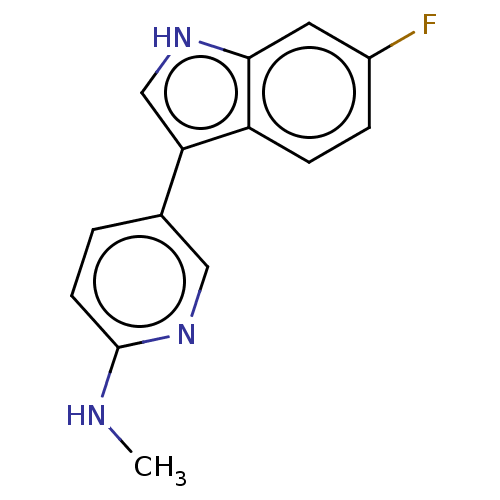 (5-(6-fluoro-1H-indol-3-yl)-N- methylpyridin-2-amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics US Patent | Assay Description The compounds of formula I inhibit the enzymatic activity of human TDO2.To measure the TDO2 activity, the procedure described in Dolusic et al. J. Me... | US Patent US9758505 (2017) BindingDB Entry DOI: 10.7270/Q20V8FW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM312073 (3-(6-bromo-5-fluoro- 1H-indol-3-yl)- pyrrolidine-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay | J Med Chem 60: 9617-9629 (2017) Article DOI: 10.1021/acs.jmedchem.7b00974 BindingDB Entry DOI: 10.7270/Q2ZP48M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM312076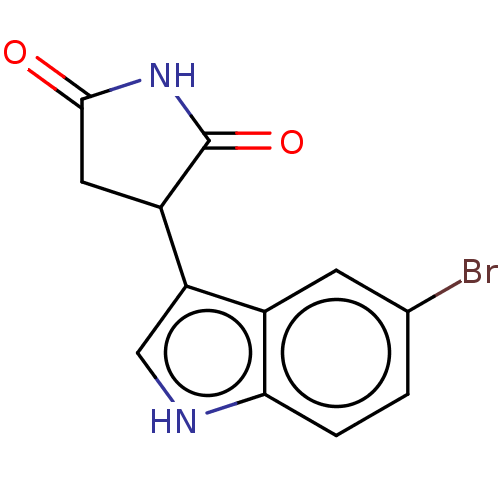 (3-(5-bromo-1H-indol-3- yl)pyrrolidine-2,5-dione | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay | J Med Chem 60: 9617-9629 (2017) Article DOI: 10.1021/acs.jmedchem.7b00974 BindingDB Entry DOI: 10.7270/Q2ZP48M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM339928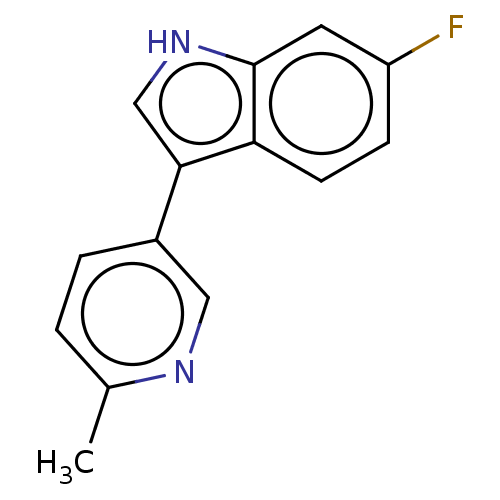 (6-fluoro-3-(6-methylpyridin-3- yl)-1H-indole | US9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics US Patent | Assay Description The compounds of formula I inhibit the enzymatic activity of human TDO2.To measure the TDO2 activity, the procedure described in Dolusic et al. J. Me... | US Patent US9758505 (2017) BindingDB Entry DOI: 10.7270/Q20V8FW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM312076 (3-(5-bromo-1H-indol-3- yl)pyrrolidine-2,5-dione | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description The compounds of the present invention inhibit the enzymatic activity of human IDO1.To measure enzymatic activity of human IDO1, the reaction mixture... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2959KW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM312076 (3-(5-bromo-1H-indol-3- yl)pyrrolidine-2,5-dione | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
ITEOS THERAPEUTICS US Patent | Assay Description To measure enzymatic activity of human IDO1, the reaction mixture contained (final concentrations) potassium phosphate buffer (50 mM, pH 6.5), ascorb... | US Patent US9603836 (2017) BindingDB Entry DOI: 10.7270/Q2MK6FZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM339940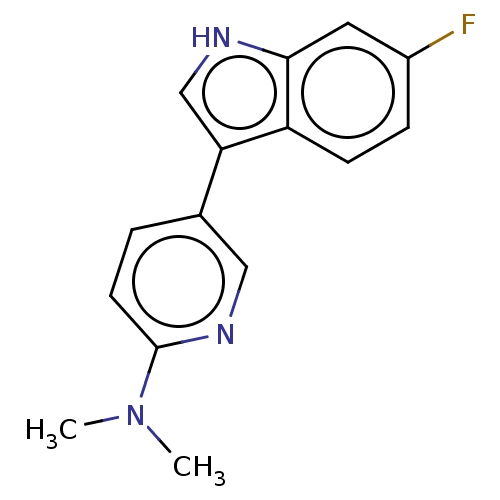 (5-(6-fluoro-1H-indol-3-yl)- N,N-dimethylpyridin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics US Patent | Assay Description The compounds of formula I inhibit the enzymatic activity of human TDO2.To measure the TDO2 activity, the procedure described in Dolusic et al. J. Me... | US Patent US9758505 (2017) BindingDB Entry DOI: 10.7270/Q20V8FW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM309529 (3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.; iTEOS THERAPEUTICS US Patent | Assay Description To measure enzymatic activity of human IDO1, the reaction mixture contained (final concentrations) potassium phosphate buffer (50 mM, pH 6.5), ascorb... | US Patent US10945994 (2021) BindingDB Entry DOI: 10.7270/Q25D8W03 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM309529 (3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO-1 using tryptophan as substrate after 22 mins by LC-MS/MS method | J Med Chem 60: 9617-9629 (2017) Article DOI: 10.1021/acs.jmedchem.7b00974 BindingDB Entry DOI: 10.7270/Q2ZP48M3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM312084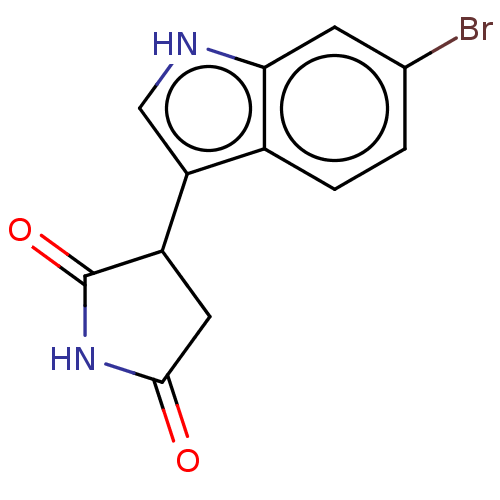 (3-(6-bromo-1H-indol-3- yl)pyrrolidine-2,5-dione | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
ITEOS THERAPEUTICS US Patent | Assay Description To measure enzymatic activity of human IDO1, the reaction mixture contained (final concentrations) potassium phosphate buffer (50 mM, pH 6.5), ascorb... | US Patent US9603836 (2017) BindingDB Entry DOI: 10.7270/Q2MK6FZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM312084 (3-(6-bromo-1H-indol-3- yl)pyrrolidine-2,5-dione | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description The compounds of the present invention inhibit the enzymatic activity of human IDO1.To measure enzymatic activity of human IDO1, the reaction mixture... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2959KW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM312084 (3-(6-bromo-1H-indol-3- yl)pyrrolidine-2,5-dione | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay | J Med Chem 60: 9617-9629 (2017) Article DOI: 10.1021/acs.jmedchem.7b00974 BindingDB Entry DOI: 10.7270/Q2ZP48M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM312072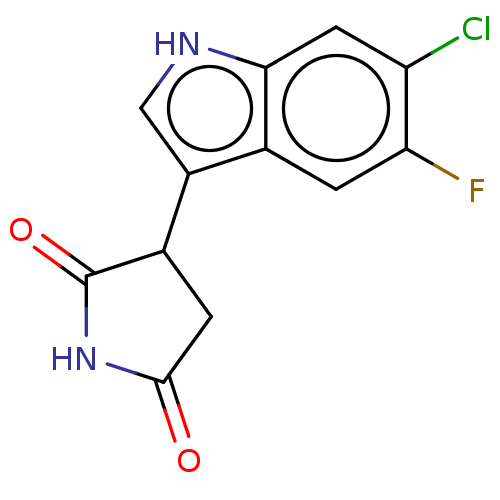 (3-(6-chloro-5-fluoro-1H- indol-3-yl)pyrrolidine- 2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
ITEOS THERAPEUTICS US Patent | Assay Description To measure enzymatic activity of human IDO1, the reaction mixture contained (final concentrations) potassium phosphate buffer (50 mM, pH 6.5), ascorb... | US Patent US9603836 (2017) BindingDB Entry DOI: 10.7270/Q2MK6FZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM339957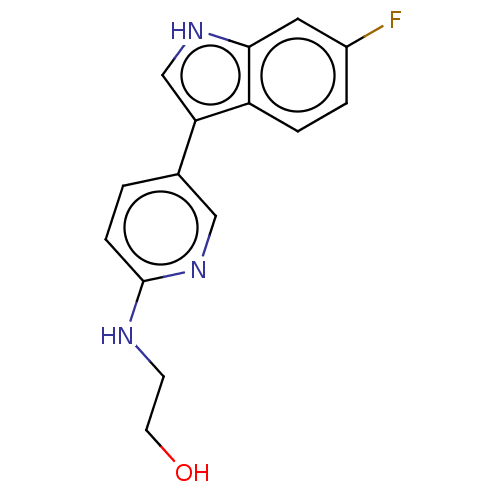 (2-((5-(6-fluoro-1H-indol-3- yl)pyridin-2-yl)amino)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics US Patent | Assay Description The compounds of formula I inhibit the enzymatic activity of human TDO2.To measure the TDO2 activity, the procedure described in Dolusic et al. J. Me... | US Patent US9758505 (2017) BindingDB Entry DOI: 10.7270/Q20V8FW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM312072 (3-(6-chloro-5-fluoro-1H- indol-3-yl)pyrrolidine- 2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description The compounds of the present invention inhibit the enzymatic activity of human IDO1.To measure enzymatic activity of human IDO1, the reaction mixture... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2959KW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM312072 (3-(6-chloro-5-fluoro-1H- indol-3-yl)pyrrolidine- 2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay | J Med Chem 60: 9617-9629 (2017) Article DOI: 10.1021/acs.jmedchem.7b00974 BindingDB Entry DOI: 10.7270/Q2ZP48M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM339941 (4-(5-(6-fluoro-1H-indol-3- yl)pyridin-2-yl)morphol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics US Patent | Assay Description The compounds of formula I inhibit the enzymatic activity of human TDO2.To measure the TDO2 activity, the procedure described in Dolusic et al. J. Me... | US Patent US9758505 (2017) BindingDB Entry DOI: 10.7270/Q20V8FW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM339955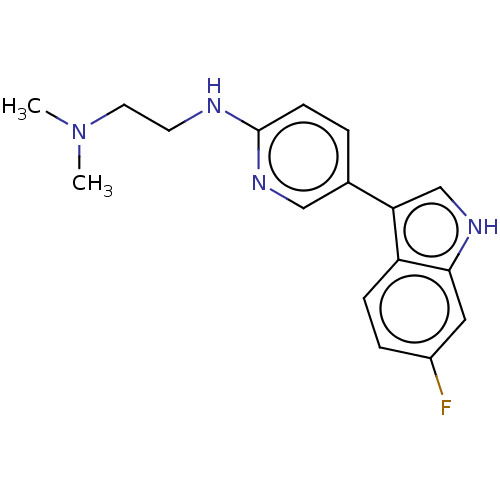 (N1-(5-(6-fluoro-1H-indol-3- yl)pyridin-2-yl)-N2,N2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics US Patent | Assay Description The compounds of formula I inhibit the enzymatic activity of human TDO2.To measure the TDO2 activity, the procedure described in Dolusic et al. J. Me... | US Patent US9758505 (2017) BindingDB Entry DOI: 10.7270/Q20V8FW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM339929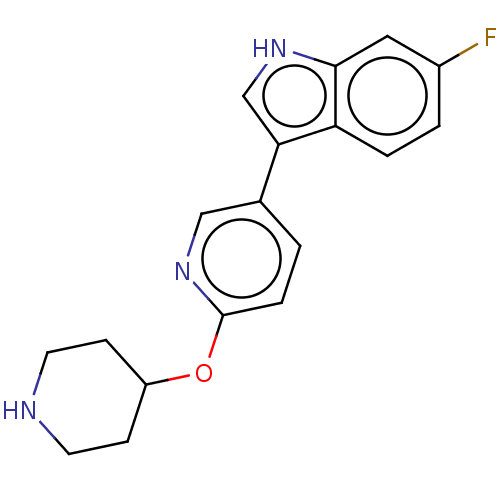 (6-fluoro-3-(6-(piperidin-4- yloxy)pyridin-3-yl)-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics US Patent | Assay Description The compounds of formula I inhibit the enzymatic activity of human TDO2.To measure the TDO2 activity, the procedure described in Dolusic et al. J. Me... | US Patent US9758505 (2017) BindingDB Entry DOI: 10.7270/Q20V8FW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM389161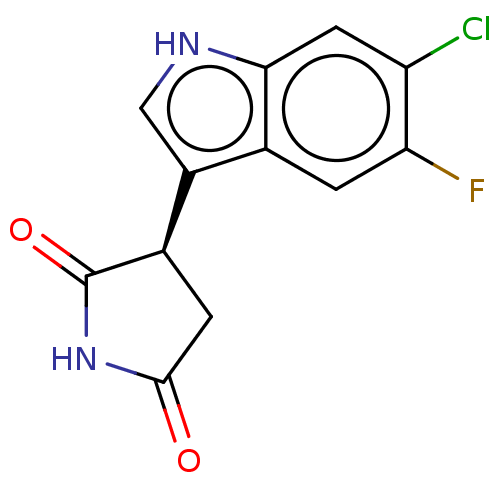 ((R)-3-(6-chloro-5- fluoro-1H-indol-3-yl) pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay | J Med Chem 60: 9617-9629 (2017) Article DOI: 10.1021/acs.jmedchem.7b00974 BindingDB Entry DOI: 10.7270/Q2ZP48M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Dog) | BDBM309529 (3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.; iTEOS THERAPEUTICS US Patent | Assay Description To measure enzymatic activity of human IDO1, the reaction mixture contained (final concentrations) potassium phosphate buffer (50 mM, pH 6.5), ascorb... | US Patent US10945994 (2021) BindingDB Entry DOI: 10.7270/Q25D8W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM312074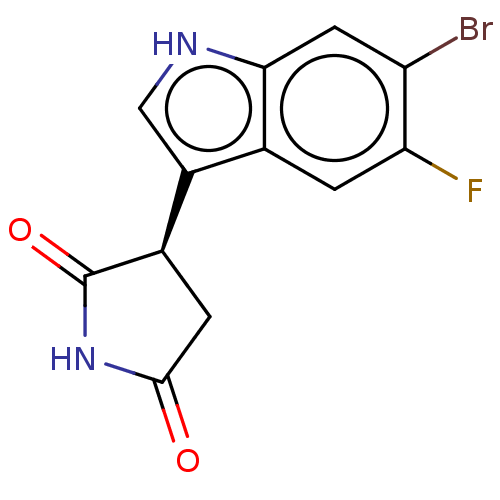 ((R)-3-(6-bromo-5- fluoro-1H-indol-3-yl)- pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description The compounds of the present invention inhibit the enzymatic activity of human IDO1.To measure enzymatic activity of human IDO1, the reaction mixture... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2959KW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM312074 ((R)-3-(6-bromo-5- fluoro-1H-indol-3-yl)- pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay | J Med Chem 60: 9617-9629 (2017) Article DOI: 10.1021/acs.jmedchem.7b00974 BindingDB Entry DOI: 10.7270/Q2ZP48M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM339953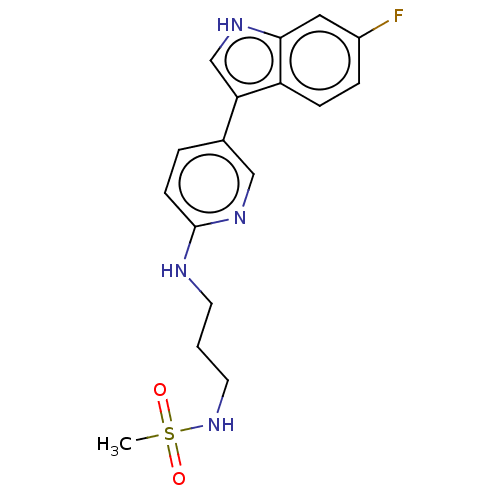 (N-(3-((5-(6-fluoro-1H-indol-3- yl)pyridin-2-yl)ami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics US Patent | Assay Description The compounds of formula I inhibit the enzymatic activity of human TDO2.To measure the TDO2 activity, the procedure described in Dolusic et al. J. Me... | US Patent US9758505 (2017) BindingDB Entry DOI: 10.7270/Q20V8FW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM312074 ((R)-3-(6-bromo-5- fluoro-1H-indol-3-yl)- pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
ITEOS THERAPEUTICS US Patent | Assay Description To measure enzymatic activity of human IDO1, the reaction mixture contained (final concentrations) potassium phosphate buffer (50 mM, pH 6.5), ascorb... | US Patent US9603836 (2017) BindingDB Entry DOI: 10.7270/Q2MK6FZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM339950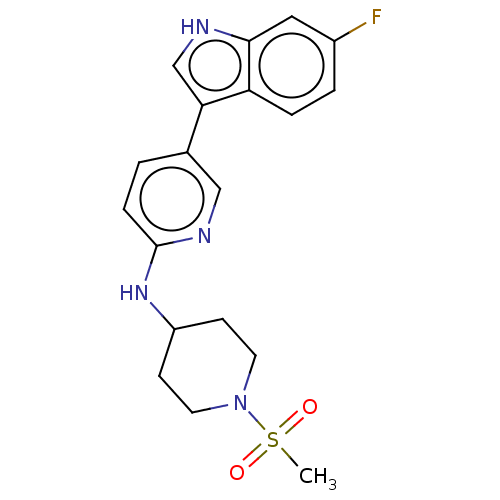 (5-(6-fluoro-1H-indol-3-yl)-N- (1-(methylsulfonyl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics US Patent | Assay Description The compounds of formula I inhibit the enzymatic activity of human TDO2.To measure the TDO2 activity, the procedure described in Dolusic et al. J. Me... | US Patent US9758505 (2017) BindingDB Entry DOI: 10.7270/Q20V8FW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM339943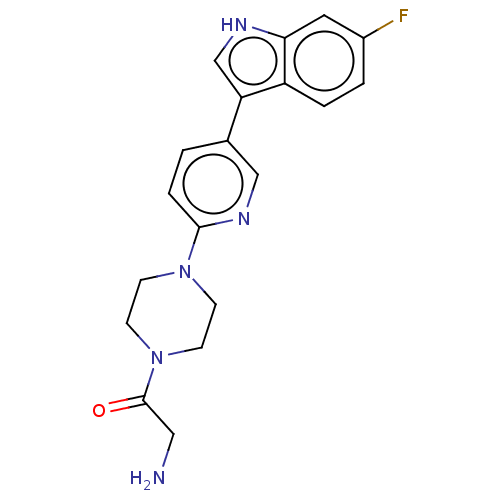 (2-amino-1-(4-(5-(6-fluoro-1H- indol-3-yl)pyridin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics US Patent | Assay Description The compounds of formula I inhibit the enzymatic activity of human TDO2.To measure the TDO2 activity, the procedure described in Dolusic et al. J. Me... | US Patent US9758505 (2017) BindingDB Entry DOI: 10.7270/Q20V8FW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM339956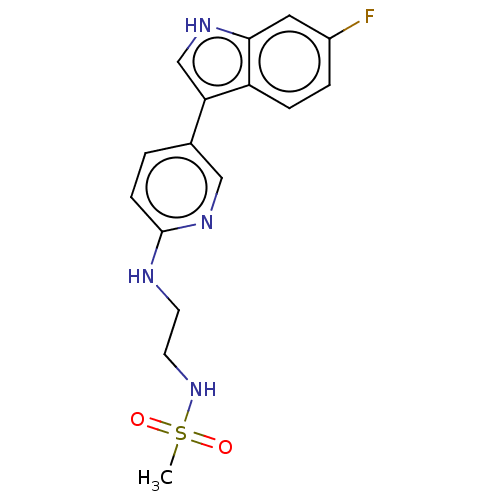 (N-(2-((5-(6-fluoro-1H-indol-3- yl)pyridin-2-yl)ami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics US Patent | Assay Description The compounds of formula I inhibit the enzymatic activity of human TDO2.To measure the TDO2 activity, the procedure described in Dolusic et al. J. Me... | US Patent US9758505 (2017) BindingDB Entry DOI: 10.7270/Q20V8FW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM339942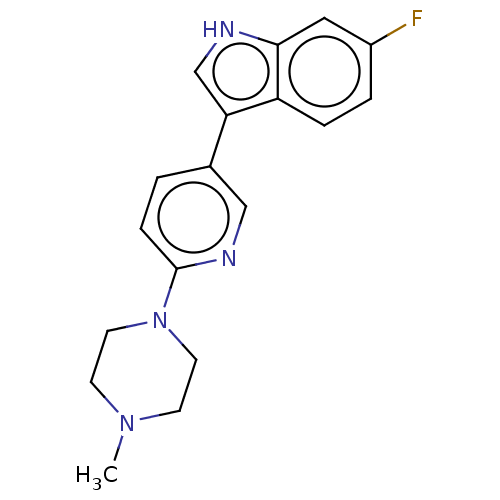 (6-fluoro-3-(6-(4- methylpiperazin-1-yl)pyridin- 3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics US Patent | Assay Description The compounds of formula I inhibit the enzymatic activity of human TDO2.To measure the TDO2 activity, the procedure described in Dolusic et al. J. Me... | US Patent US9758505 (2017) BindingDB Entry DOI: 10.7270/Q20V8FW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM310195 ((-)-(R)-3-(5-fluoro-1H- indol-3-yl)pyrrolidine- 2,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.; iTEOS THERAPEUTICS US Patent | Assay Description To measure enzymatic activity of human IDO1, the reaction mixture contained (final concentrations) potassium phosphate buffer (50 mM, pH 6.5), ascorb... | US Patent US10945994 (2021) BindingDB Entry DOI: 10.7270/Q25D8W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM339938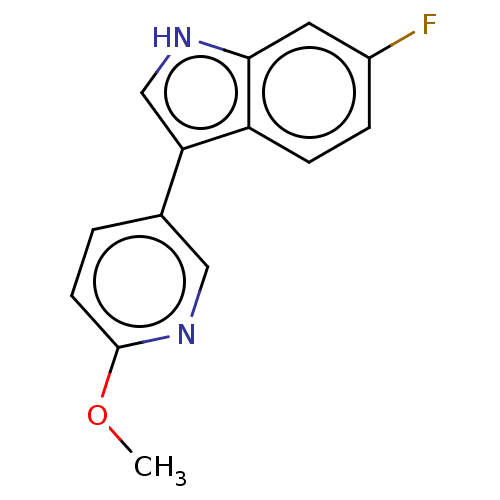 (6-fluoro-3-(6-methoxypyridin- 3-yl)-1H-indole | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics US Patent | Assay Description The compounds of formula I inhibit the enzymatic activity of human TDO2.To measure the TDO2 activity, the procedure described in Dolusic et al. J. Me... | US Patent US9758505 (2017) BindingDB Entry DOI: 10.7270/Q20V8FW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM339960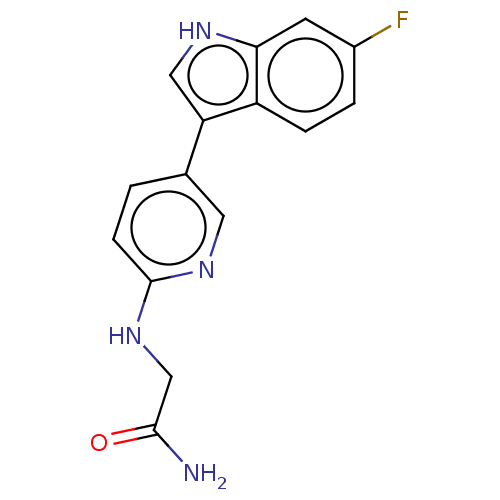 (2-((5-(6-fluoro-1H-indol-3- yl)pyridin-2- yl)amino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics US Patent | Assay Description The compounds of formula I inhibit the enzymatic activity of human TDO2.To measure the TDO2 activity, the procedure described in Dolusic et al. J. Me... | US Patent US9758505 (2017) BindingDB Entry DOI: 10.7270/Q20V8FW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM312069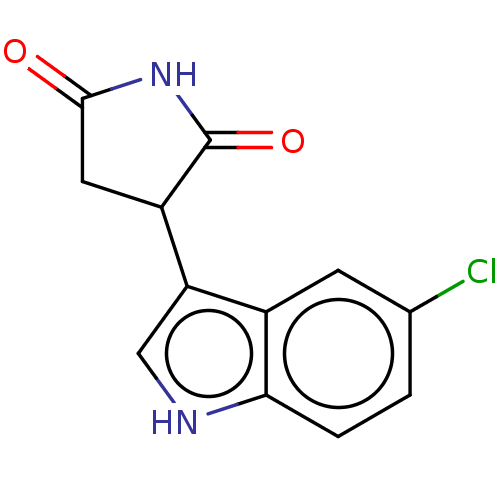 (3-(5-chloro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay | J Med Chem 60: 9617-9629 (2017) Article DOI: 10.1021/acs.jmedchem.7b00974 BindingDB Entry DOI: 10.7270/Q2ZP48M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM339963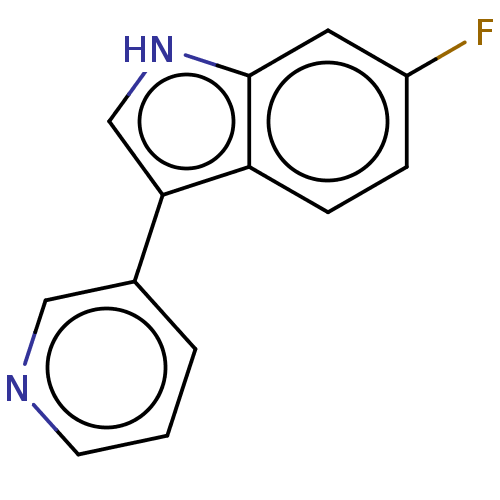 (6-fluoro-3-(pyridin-3-yl)-1H- indole | US9758505, ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics US Patent | Assay Description The compounds of formula I inhibit the enzymatic activity of human TDO2.To measure the TDO2 activity, the procedure described in Dolusic et al. J. Me... | US Patent US9758505 (2017) BindingDB Entry DOI: 10.7270/Q20V8FW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 173 total ) | Next | Last >> |