

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50426684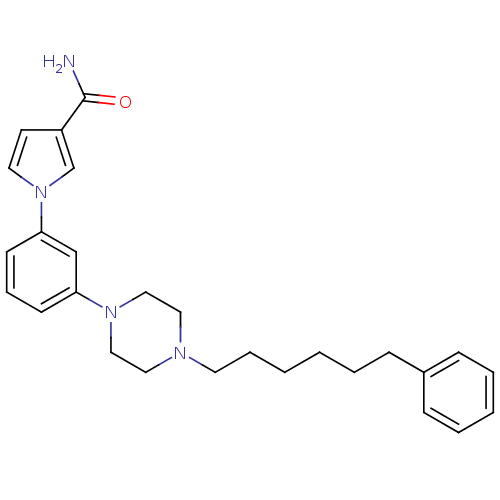 (CHEMBL2326494) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of FAAH in mouse brain homogenate using [3Hethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition measured after ... | Bioorg Med Chem Lett 23: 492-5 (2012) Article DOI: 10.1016/j.bmcl.2012.11.035 BindingDB Entry DOI: 10.7270/Q22B90BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50426683 (CHEMBL2326500) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of FAAH in mouse brain homogenate using [3Hethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition measured after ... | Bioorg Med Chem Lett 23: 492-5 (2012) Article DOI: 10.1016/j.bmcl.2012.11.035 BindingDB Entry DOI: 10.7270/Q22B90BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426683 (CHEMBL2326500) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of human FAAH using [3Hethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition measured after 15 mins by beta coun... | Bioorg Med Chem Lett 23: 492-5 (2012) Article DOI: 10.1016/j.bmcl.2012.11.035 BindingDB Entry DOI: 10.7270/Q22B90BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50426688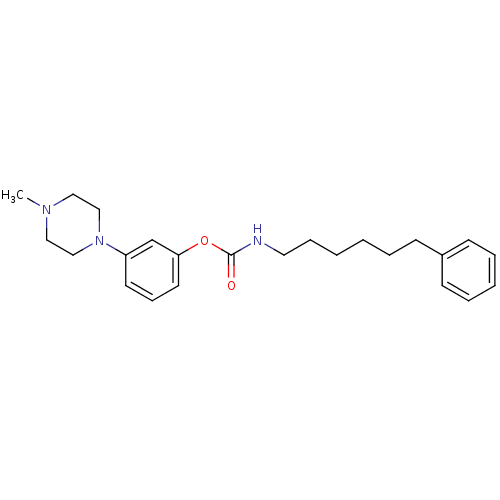 (CHEMBL2326499) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of FAAH in mouse brain homogenate using [3Hethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition measured after ... | Bioorg Med Chem Lett 23: 492-5 (2012) Article DOI: 10.1016/j.bmcl.2012.11.035 BindingDB Entry DOI: 10.7270/Q22B90BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50426692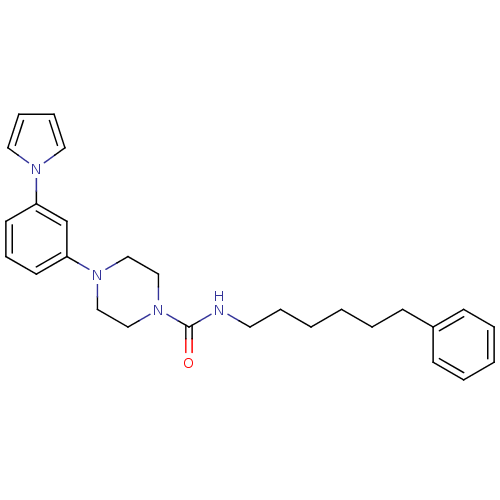 (CHEMBL2326495) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 263 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of FAAH in mouse brain homogenate using [3Hethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition measured after ... | Bioorg Med Chem Lett 23: 492-5 (2012) Article DOI: 10.1016/j.bmcl.2012.11.035 BindingDB Entry DOI: 10.7270/Q22B90BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50426693 (CHEMBL2326493) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of FAAH in mouse brain homogenate using [3Hethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition measured after ... | Bioorg Med Chem Lett 23: 492-5 (2012) Article DOI: 10.1016/j.bmcl.2012.11.035 BindingDB Entry DOI: 10.7270/Q22B90BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50426686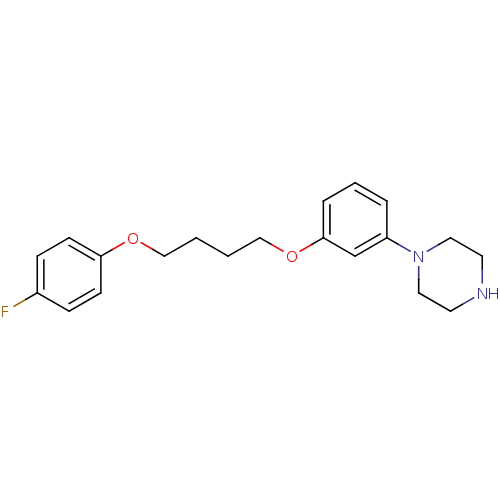 (CHEMBL2326502) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of FAAH in mouse brain homogenate using [3Hethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition measured after ... | Bioorg Med Chem Lett 23: 492-5 (2012) Article DOI: 10.1016/j.bmcl.2012.11.035 BindingDB Entry DOI: 10.7270/Q22B90BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50426689 (CHEMBL2326498) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of FAAH in mouse brain homogenate using [3Hethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition measured after ... | Bioorg Med Chem Lett 23: 492-5 (2012) Article DOI: 10.1016/j.bmcl.2012.11.035 BindingDB Entry DOI: 10.7270/Q22B90BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50426690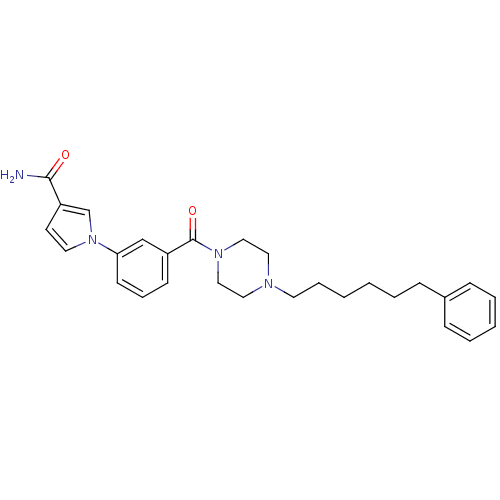 (CHEMBL2326497) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of FAAH in mouse brain homogenate using [3Hethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition measured after ... | Bioorg Med Chem Lett 23: 492-5 (2012) Article DOI: 10.1016/j.bmcl.2012.11.035 BindingDB Entry DOI: 10.7270/Q22B90BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50426691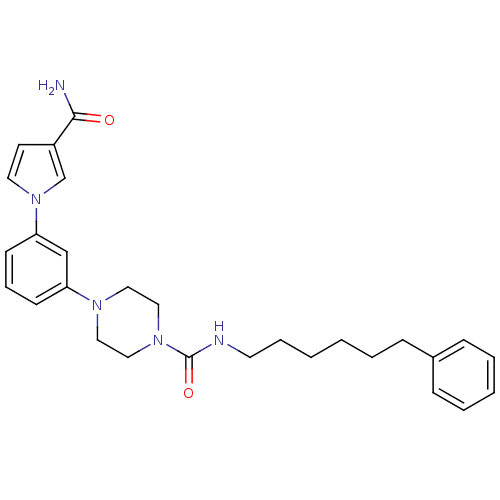 (CHEMBL2326496) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of FAAH in mouse brain homogenate using [3Hethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition measured after ... | Bioorg Med Chem Lett 23: 492-5 (2012) Article DOI: 10.1016/j.bmcl.2012.11.035 BindingDB Entry DOI: 10.7270/Q22B90BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50426685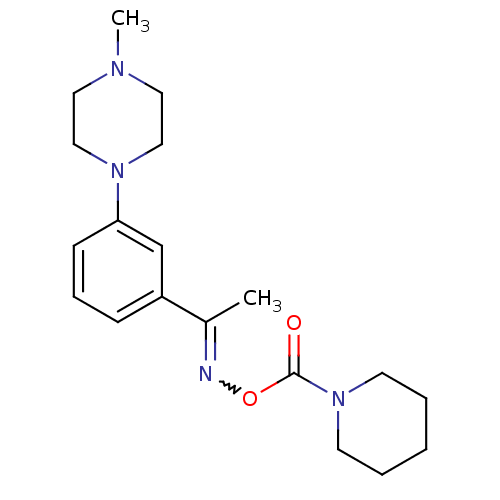 (CHEMBL2326503) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of FAAH in mouse brain homogenate using [3Hethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition measured after ... | Bioorg Med Chem Lett 23: 492-5 (2012) Article DOI: 10.1016/j.bmcl.2012.11.035 BindingDB Entry DOI: 10.7270/Q22B90BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50426687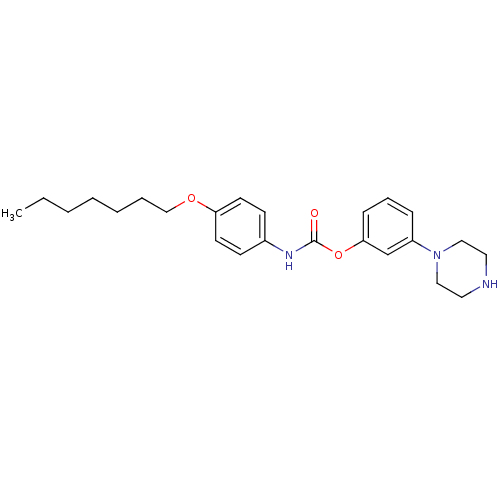 (CHEMBL2326501) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of FAAH in mouse brain homogenate using [3Hethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition measured after ... | Bioorg Med Chem Lett 23: 492-5 (2012) Article DOI: 10.1016/j.bmcl.2012.11.035 BindingDB Entry DOI: 10.7270/Q22B90BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-5 (Homo sapiens (Human)) | BDBM50308730 (4-((2S,5S,11S,14R)-14-benzyl-11-(carboxymethyl)-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche Chimiche e Biochimiche G.Ronzoni Curated by ChEMBL | Assay Description Displacement of [125I]echistatin from integrin alphaVbeta5 receptor after 3 hrs by gamma counting | Bioorg Med Chem 18: 64-72 (2010) Article DOI: 10.1016/j.bmc.2009.11.019 BindingDB Entry DOI: 10.7270/Q2FF3SG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-5 (Homo sapiens (Human)) | BDBM50308728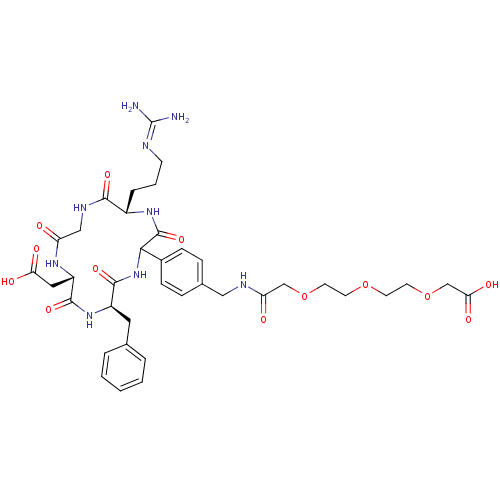 (1-(4-((5S,11S,14R)-14-benzyl-11-(carboxymethyl)-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche Chimiche e Biochimiche G.Ronzoni Curated by ChEMBL | Assay Description Displacement of [125I]echistatin from integrin alphaVbeta5 receptor after 3 hrs by gamma counting | Bioorg Med Chem 18: 64-72 (2010) Article DOI: 10.1016/j.bmc.2009.11.019 BindingDB Entry DOI: 10.7270/Q2FF3SG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-5 (Homo sapiens (Human)) | BDBM50308726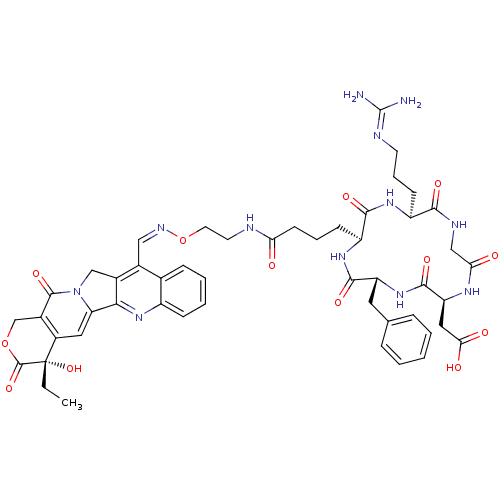 (2-[(2S,5R,8S,11S)-5-benzyl-11-(3-carbamimidamidopr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche Chimiche e Biochimiche G.Ronzoni Curated by ChEMBL | Assay Description Displacement of [125I]echistatin from integrin alphaVbeta5 receptor after 3 hrs by gamma counting | Bioorg Med Chem 18: 64-72 (2010) Article DOI: 10.1016/j.bmc.2009.11.019 BindingDB Entry DOI: 10.7270/Q2FF3SG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Milano Curated by ChEMBL | Assay Description Inhibition of human HDAC6 using fluorogenic tetrapeptide RHKKAc as substrate | Eur J Med Chem 79: 251-9 (2014) Article DOI: 10.1016/j.ejmech.2014.04.021 BindingDB Entry DOI: 10.7270/Q2V989KV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50308726 (2-[(2S,5R,8S,11S)-5-benzyl-11-(3-carbamimidamidopr...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche Chimiche e Biochimiche G.Ronzoni Curated by ChEMBL | Assay Description Displacement of [125I]echistatin from integrin alphaVbeta3 receptor after 3 hrs by gamma counting | Bioorg Med Chem 18: 64-72 (2010) Article DOI: 10.1016/j.bmc.2009.11.019 BindingDB Entry DOI: 10.7270/Q2FF3SG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-5 (Homo sapiens (Human)) | BDBM50308727 (2-((2S,5R,8S,11S)-5-benzyl-11-(3-guanidinopropyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche Chimiche e Biochimiche G.Ronzoni Curated by ChEMBL | Assay Description Displacement of [125I]echistatin from integrin alphaVbeta5 receptor after 3 hrs by gamma counting | Bioorg Med Chem 18: 64-72 (2010) Article DOI: 10.1016/j.bmc.2009.11.019 BindingDB Entry DOI: 10.7270/Q2FF3SG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50323040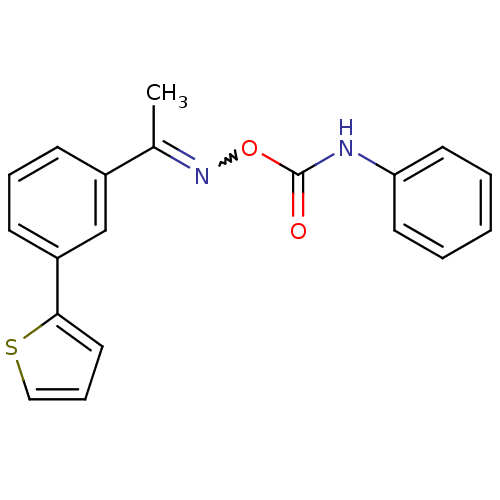 (1-(3-Thiophen-2-yl-phenyl)-ethanone, O-(phenylamin...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT5A receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 4406-11 (2010) Article DOI: 10.1016/j.bmcl.2010.06.050 BindingDB Entry DOI: 10.7270/Q24Q7V5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50323040 (1-(3-Thiophen-2-yl-phenyl)-ethanone, O-(phenylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8 from human recombinant CCKA receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 4406-11 (2010) Article DOI: 10.1016/j.bmcl.2010.06.050 BindingDB Entry DOI: 10.7270/Q24Q7V5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50323040 (1-(3-Thiophen-2-yl-phenyl)-ethanone, O-(phenylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [3H]AB-MECA from human recombinant A3 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 4406-11 (2010) Article DOI: 10.1016/j.bmcl.2010.06.050 BindingDB Entry DOI: 10.7270/Q24Q7V5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50323040 (1-(3-Thiophen-2-yl-phenyl)-ethanone, O-(phenylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 4406-11 (2010) Article DOI: 10.1016/j.bmcl.2010.06.050 BindingDB Entry DOI: 10.7270/Q24Q7V5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using RHKK(Ac)AMC as substrate after 1 to 2 hrs by fluorescence assay | Eur J Med Chem 143: 2005-2014 (2018) Article DOI: 10.1016/j.ejmech.2017.11.021 BindingDB Entry DOI: 10.7270/Q2PK0JT2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50308727 (2-((2S,5R,8S,11S)-5-benzyl-11-(3-guanidinopropyl)-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche Chimiche e Biochimiche G.Ronzoni Curated by ChEMBL | Assay Description Displacement of [125I]echistatin from integrin alphaVbeta3 receptor after 3 hrs by gamma counting | Bioorg Med Chem 18: 64-72 (2010) Article DOI: 10.1016/j.bmc.2009.11.019 BindingDB Entry DOI: 10.7270/Q2FF3SG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50308728 (1-(4-((5S,11S,14R)-14-benzyl-11-(carboxymethyl)-5-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche Chimiche e Biochimiche G.Ronzoni Curated by ChEMBL | Assay Description Displacement of [125I]echistatin from integrin alphaVbeta3 receptor after 3 hrs by gamma counting | Bioorg Med Chem 18: 64-72 (2010) Article DOI: 10.1016/j.bmc.2009.11.019 BindingDB Entry DOI: 10.7270/Q2FF3SG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27566 (4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Milano Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-fused PARP-1 expressed in Escherichia coli after 30 mins by fluorescence assay | Bioorg Med Chem 22: 1089-103 (2014) Article DOI: 10.1016/j.bmc.2013.12.031 BindingDB Entry DOI: 10.7270/Q2JS9RX4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50608725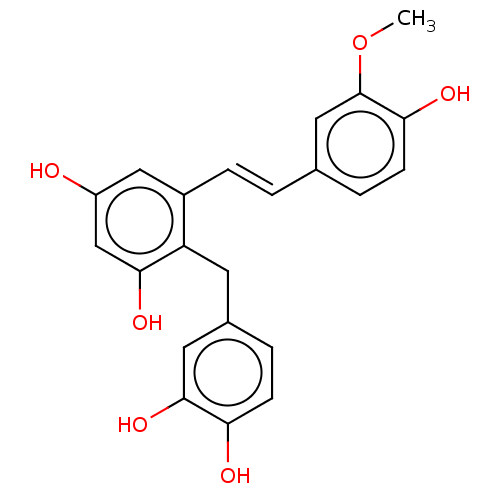 (CHEMBL5269587) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-5 (Homo sapiens (Human)) | BDBM50308729 (4-(4-((2S,5S,11S,14R)-14-benzyl-11-(carboxymethyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche Chimiche e Biochimiche G.Ronzoni Curated by ChEMBL | Assay Description Displacement of [125I]echistatin from integrin alphaVbeta5 receptor after 3 hrs by gamma counting | Bioorg Med Chem 18: 64-72 (2010) Article DOI: 10.1016/j.bmc.2009.11.019 BindingDB Entry DOI: 10.7270/Q2FF3SG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27135 (2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Milano Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-fused PARP-1 expressed in Escherichia coli after 30 mins by fluorescence assay | Bioorg Med Chem 22: 1089-103 (2014) Article DOI: 10.1016/j.bmc.2013.12.031 BindingDB Entry DOI: 10.7270/Q2JS9RX4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50308722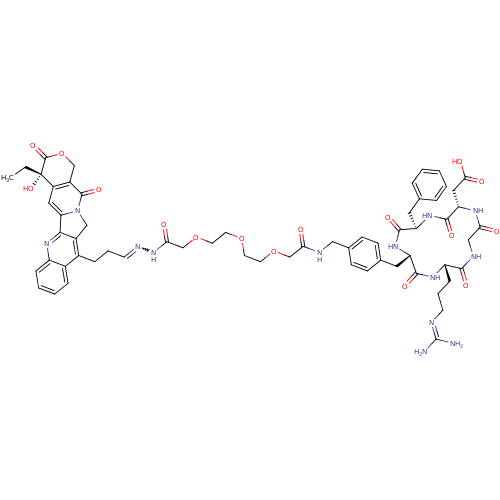 (2-[(2S,5R,8S,11S)-5-benzyl-11-(3-carbamimidamidopr...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche Chimiche e Biochimiche G.Ronzoni Curated by ChEMBL | Assay Description Displacement of [125I]echistatin from integrin alphaVbeta3 receptor after 3 hrs by gamma counting | Bioorg Med Chem 18: 64-72 (2010) Article DOI: 10.1016/j.bmc.2009.11.019 BindingDB Entry DOI: 10.7270/Q2FF3SG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-5 (Homo sapiens (Human)) | BDBM50308724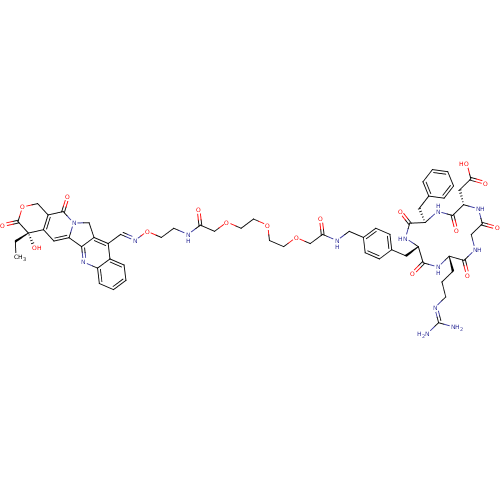 (2-[(2S,5R,8S,11S)-5-benzyl-11-(3-carbamimidamidopr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche Chimiche e Biochimiche G.Ronzoni Curated by ChEMBL | Assay Description Displacement of [125I]echistatin from integrin alphaVbeta5 receptor after 3 hrs by gamma counting | Bioorg Med Chem 18: 64-72 (2010) Article DOI: 10.1016/j.bmc.2009.11.019 BindingDB Entry DOI: 10.7270/Q2FF3SG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Milano Curated by ChEMBL | Assay Description Inhibition of human HDAC1 using fluorogenic tetrapeptide RHKKAc as substrate | Eur J Med Chem 79: 251-9 (2014) Article DOI: 10.1016/j.ejmech.2014.04.021 BindingDB Entry DOI: 10.7270/Q2V989KV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50308725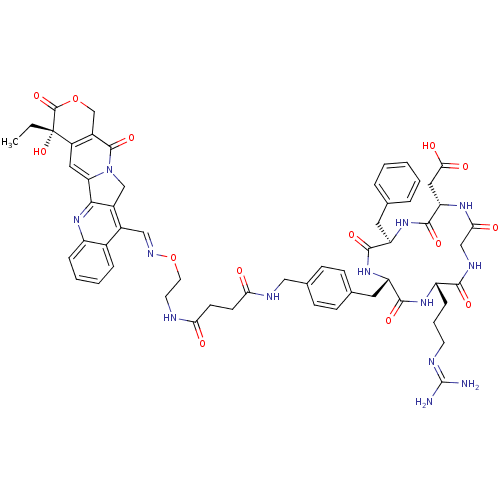 (2-[(2S,5R,8S,11S)-5-benzyl-11-(3-carbamimidamidopr...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche Chimiche e Biochimiche G.Ronzoni Curated by ChEMBL | Assay Description Displacement of [125I]echistatin from integrin alphaVbeta3 receptor after 3 hrs by gamma counting | Bioorg Med Chem 18: 64-72 (2010) Article DOI: 10.1016/j.bmc.2009.11.019 BindingDB Entry DOI: 10.7270/Q2FF3SG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM50464536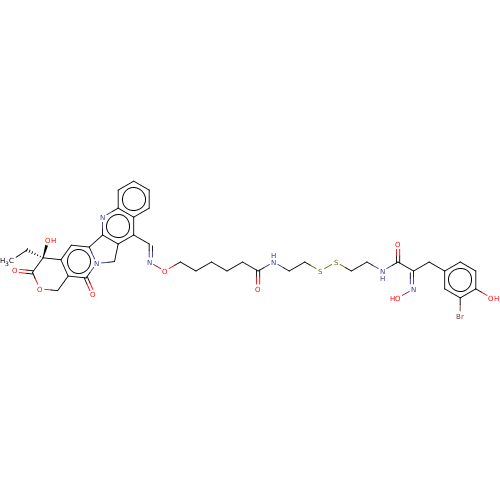 (CHEMBL4290191) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of HDAC10 (unknown origin) using RHKK(Ac)AMC as substrate after 1 to 2 hrs by fluorescence assay | Eur J Med Chem 143: 2005-2014 (2018) Article DOI: 10.1016/j.ejmech.2017.11.021 BindingDB Entry DOI: 10.7270/Q2PK0JT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50323040 (1-(3-Thiophen-2-yl-phenyl)-ethanone, O-(phenylamin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of mouse brain FAAH assessed as conversion of [3H-ethanolamine]AEA to [3H]ethanolamine by scintillation counting | Bioorg Med Chem Lett 20: 4406-11 (2010) Article DOI: 10.1016/j.bmcl.2010.06.050 BindingDB Entry DOI: 10.7270/Q24Q7V5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-5 (Homo sapiens (Human)) | BDBM50308725 (2-[(2S,5R,8S,11S)-5-benzyl-11-(3-carbamimidamidopr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche Chimiche e Biochimiche G.Ronzoni Curated by ChEMBL | Assay Description Displacement of [125I]echistatin from integrin alphaVbeta5 receptor after 3 hrs by gamma counting | Bioorg Med Chem 18: 64-72 (2010) Article DOI: 10.1016/j.bmc.2009.11.019 BindingDB Entry DOI: 10.7270/Q2FF3SG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM50464537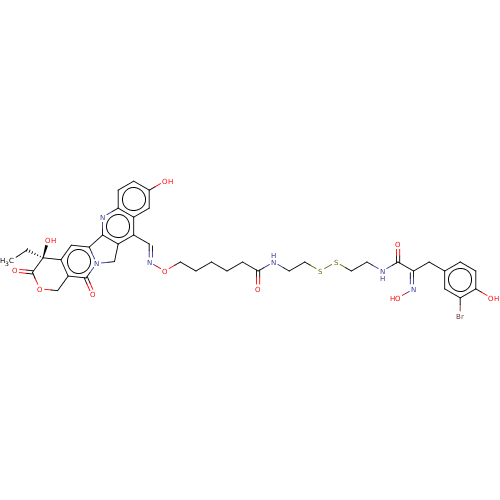 (CHEMBL4280303) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of HDAC10 (unknown origin) using RHKK(Ac)AMC as substrate after 1 to 2 hrs by fluorescence assay | Eur J Med Chem 143: 2005-2014 (2018) Article DOI: 10.1016/j.ejmech.2017.11.021 BindingDB Entry DOI: 10.7270/Q2PK0JT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50445826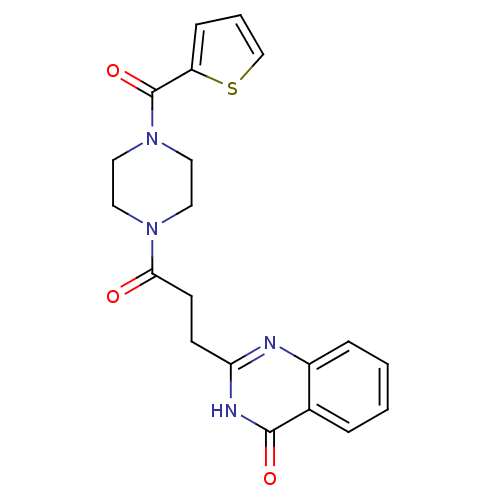 (CHEMBL3105402) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite S.p.A. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human PARP-1 expressed in Escherichia coli after 30 mins by fluorescence-based assay | Bioorg Med Chem Lett 24: 462-6 (2014) Article DOI: 10.1016/j.bmcl.2013.12.048 BindingDB Entry DOI: 10.7270/Q26H4JV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27135 (2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite S.p.A. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human PARP-1 expressed in Escherichia coli after 30 mins by fluorescence-based assay | Bioorg Med Chem Lett 24: 462-6 (2014) Article DOI: 10.1016/j.bmcl.2013.12.048 BindingDB Entry DOI: 10.7270/Q26H4JV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Milano Curated by ChEMBL | Assay Description Inhibition of human HDAC3 complexed with NCOR2 using fluorogenic tetrapeptide RHKKAc as susbtrate | Eur J Med Chem 79: 251-9 (2014) Article DOI: 10.1016/j.ejmech.2014.04.021 BindingDB Entry DOI: 10.7270/Q2V989KV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50323051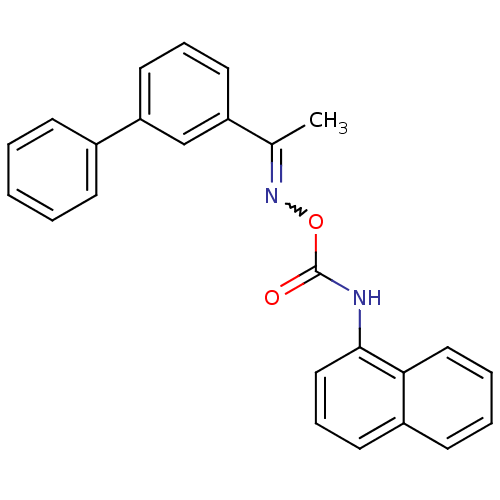 (1-Biphenyl-3-yl-ethanone, O-(1-naphthylaminocarbon...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of mouse brain FAAH assessed as conversion of [3H-ethanolamine]AEA to [3H]ethanolamine by scintillation counting | Bioorg Med Chem Lett 20: 4406-11 (2010) Article DOI: 10.1016/j.bmcl.2010.06.050 BindingDB Entry DOI: 10.7270/Q24Q7V5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27566 (4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite S.p.A. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human PARP-1 expressed in Escherichia coli after 30 mins by fluorescence-based assay | Bioorg Med Chem Lett 24: 462-6 (2014) Article DOI: 10.1016/j.bmcl.2013.12.048 BindingDB Entry DOI: 10.7270/Q26H4JV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Milano Curated by ChEMBL | Assay Description Inhibition of human HDAC4 using fluorogenic tetrapeptide RHKKAc as substrate | Eur J Med Chem 79: 251-9 (2014) Article DOI: 10.1016/j.ejmech.2014.04.021 BindingDB Entry DOI: 10.7270/Q2V989KV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50308723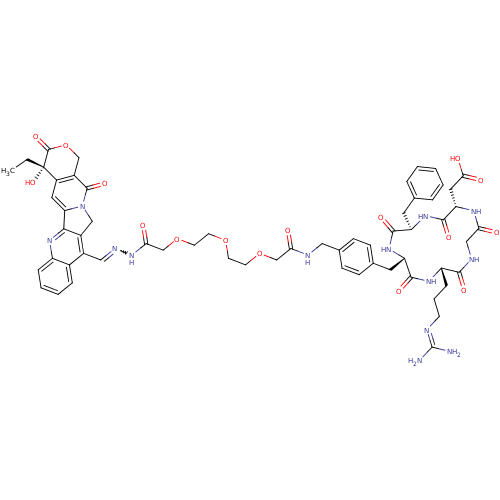 (2-[(2S,5R,8S,11S)-5-benzyl-11-(3-carbamimidamidopr...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche Chimiche e Biochimiche G.Ronzoni Curated by ChEMBL | Assay Description Displacement of [125I]echistatin from integrin alphaVbeta3 receptor after 3 hrs by gamma counting | Bioorg Med Chem 18: 64-72 (2010) Article DOI: 10.1016/j.bmc.2009.11.019 BindingDB Entry DOI: 10.7270/Q2FF3SG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 12.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using RHKK(Ac)AMC as substrate after 1 to 2 hrs by fluorescence assay | Eur J Med Chem 143: 2005-2014 (2018) Article DOI: 10.1016/j.ejmech.2017.11.021 BindingDB Entry DOI: 10.7270/Q2PK0JT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50308724 (2-[(2S,5R,8S,11S)-5-benzyl-11-(3-carbamimidamidopr...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche Chimiche e Biochimiche G.Ronzoni Curated by ChEMBL | Assay Description Displacement of [125I]echistatin from integrin alphaVbeta3 receptor after 3 hrs by gamma counting | Bioorg Med Chem 18: 64-72 (2010) Article DOI: 10.1016/j.bmc.2009.11.019 BindingDB Entry DOI: 10.7270/Q2FF3SG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Milano Curated by ChEMBL | Assay Description Inhibition of human HDAC11 using fluorogenic tetrapeptide RHKKAc as substrate | Eur J Med Chem 79: 251-9 (2014) Article DOI: 10.1016/j.ejmech.2014.04.021 BindingDB Entry DOI: 10.7270/Q2V989KV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50323050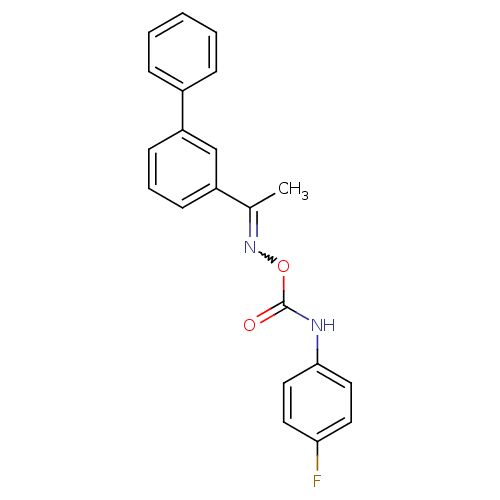 (1-Biphenyl-3-yl-ethanone, O-(4-fluorophenylaminoca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of mouse brain FAAH assessed as conversion of [3H-ethanolamine]AEA to [3H]ethanolamine by scintillation counting | Bioorg Med Chem Lett 20: 4406-11 (2010) Article DOI: 10.1016/j.bmcl.2010.06.050 BindingDB Entry DOI: 10.7270/Q24Q7V5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 5 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Milano Curated by ChEMBL | Assay Description Inhibition of human HDAC5 using fluorogenic tetrapeptide RHKKAc as substrate | Eur J Med Chem 79: 251-9 (2014) Article DOI: 10.1016/j.ejmech.2014.04.021 BindingDB Entry DOI: 10.7270/Q2V989KV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Milano Curated by ChEMBL | Assay Description Inhibition of human HDAC10 using fluorogenic tetrapeptide RHKKAc as substrate | Eur J Med Chem 79: 251-9 (2014) Article DOI: 10.1016/j.ejmech.2014.04.021 BindingDB Entry DOI: 10.7270/Q2V989KV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 409 total ) | Next | Last >> |