

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451372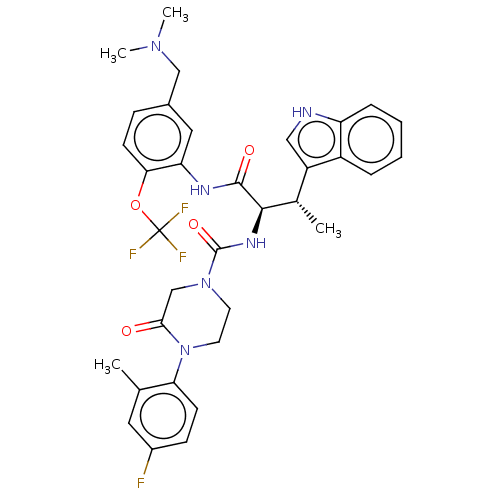 (CHEMBL4217405) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451373 (CHEMBL4206924) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451394 (CHEMBL4205606) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451392 (CHEMBL4214725) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451391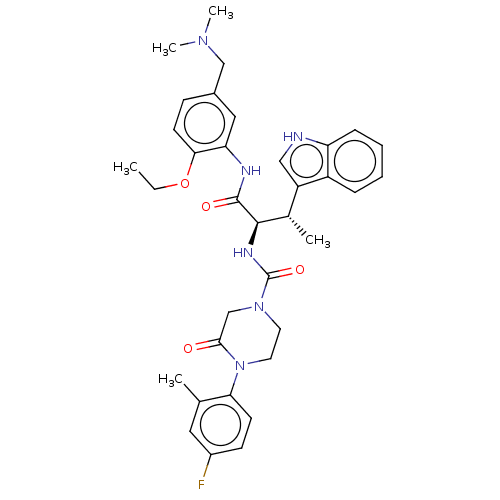 (CHEMBL4212088) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451384 (CHEMBL4210064) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451385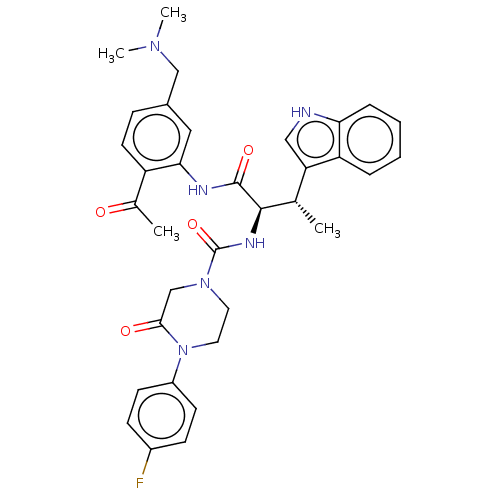 (CHEMBL4205969) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4136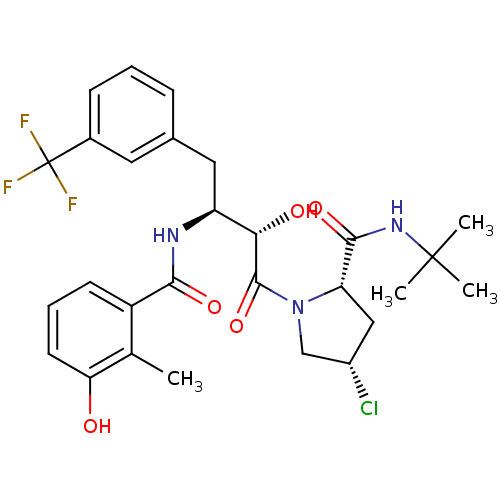 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451382 (CHEMBL4205696) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451399 (CHEMBL4208528) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4126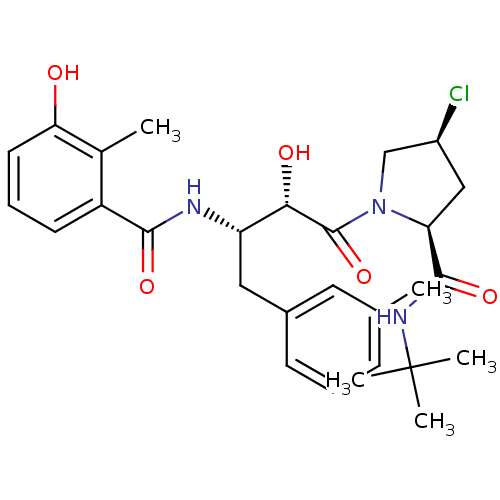 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4134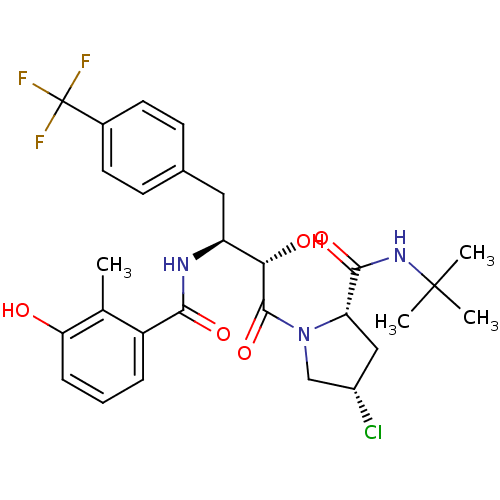 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4129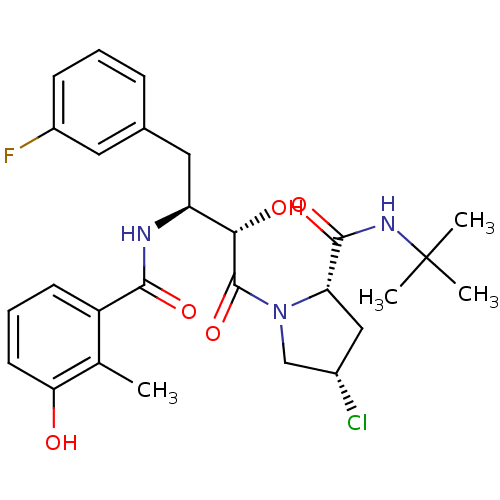 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-4-(3-fluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451395 (CHEMBL4211422) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451390 (CHEMBL4210627) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451388 (CHEMBL4215053) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4130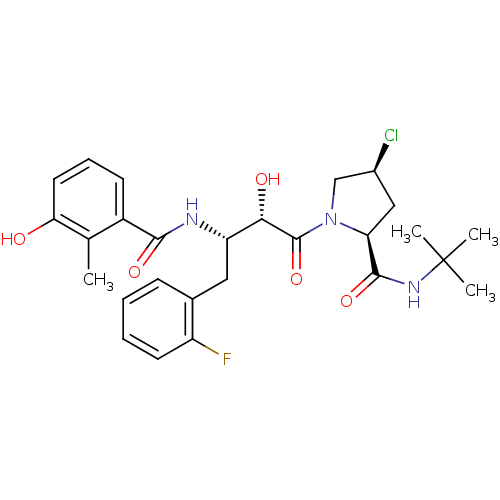 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-4-(2-fluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4132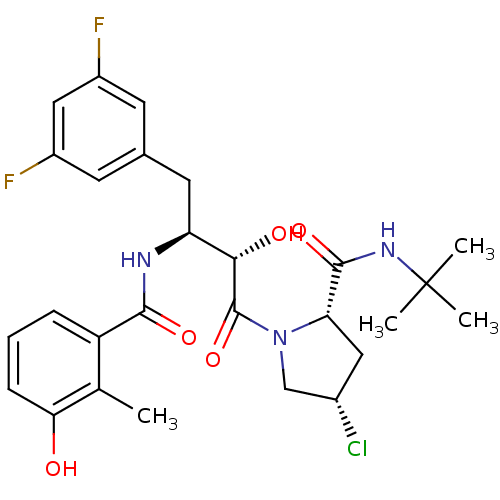 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-4-(3,5-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4131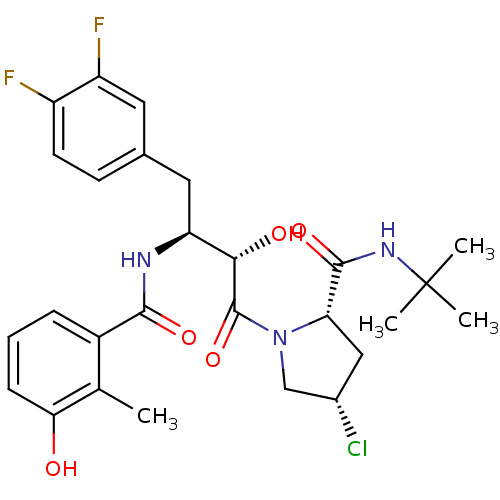 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-4-(3,4-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4124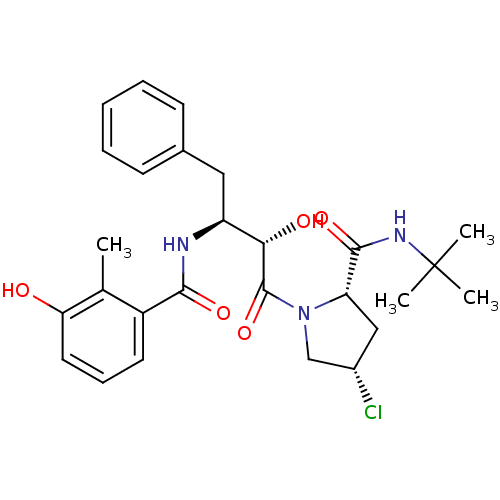 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 6: 595-604 (1998) Article DOI: 10.1016/s0968-0896(98)00004-2 BindingDB Entry DOI: 10.7270/Q2QN64XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4163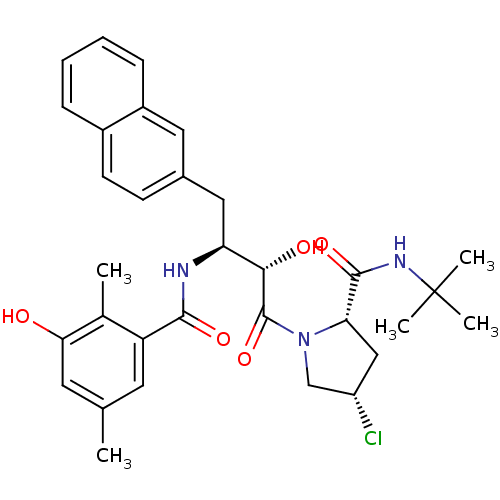 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451396 (CHEMBL4216387) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4265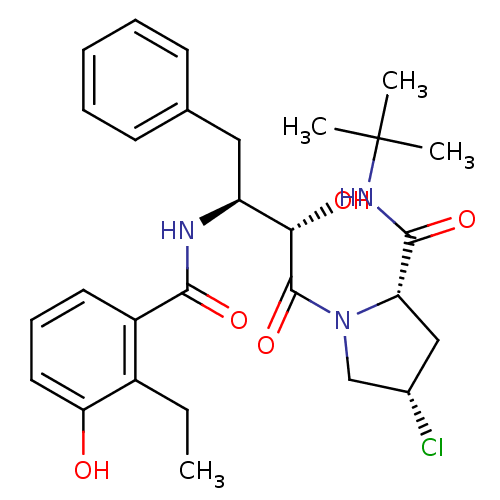 ((2-Ethyl-3-hydroxy)benzoyl-(2S,3S)-AHPBA-4(S)-Cl-P...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 6: 595-604 (1998) Article DOI: 10.1016/s0968-0896(98)00004-2 BindingDB Entry DOI: 10.7270/Q2QN64XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4138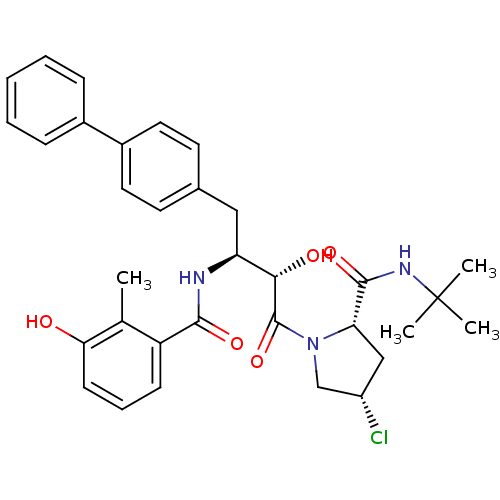 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4135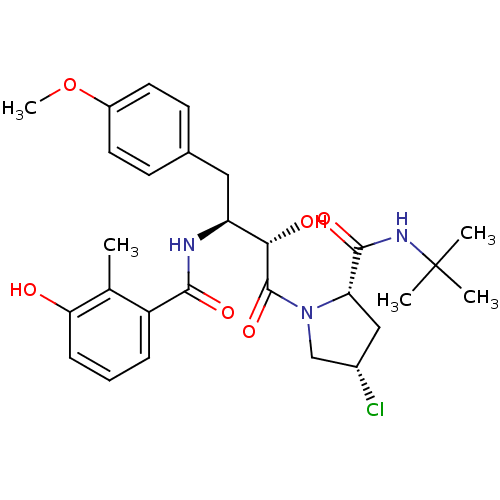 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4128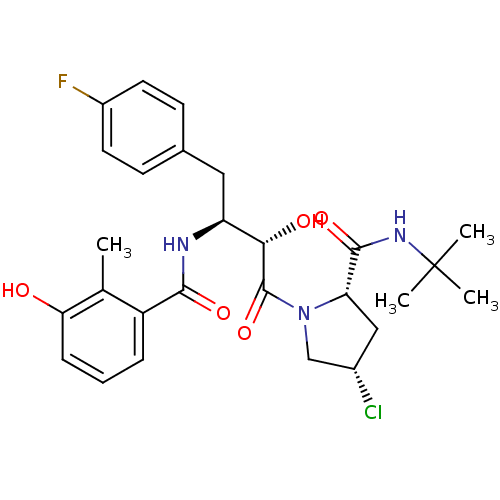 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-4-(4-fluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4124 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4125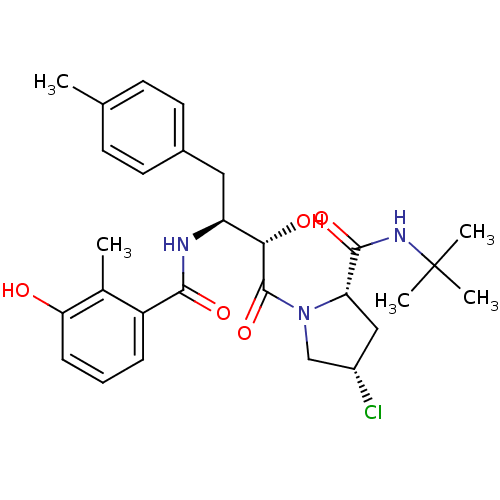 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451386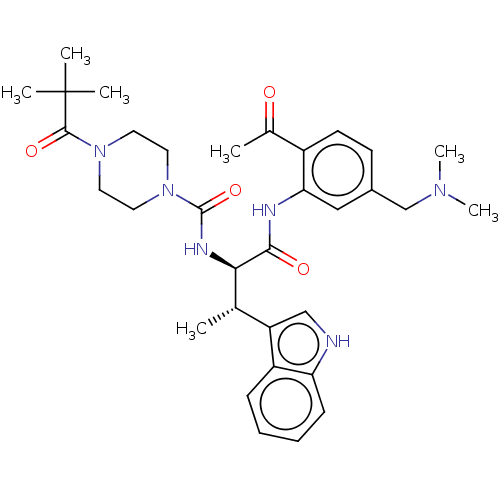 (CHEMBL4203793) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4142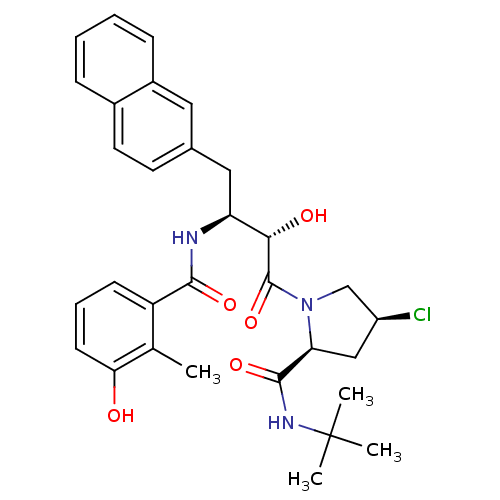 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451397 (CHEMBL4208009) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4161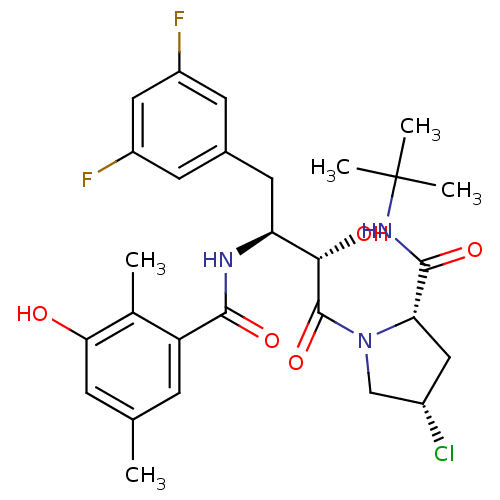 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-4-(3,5-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4165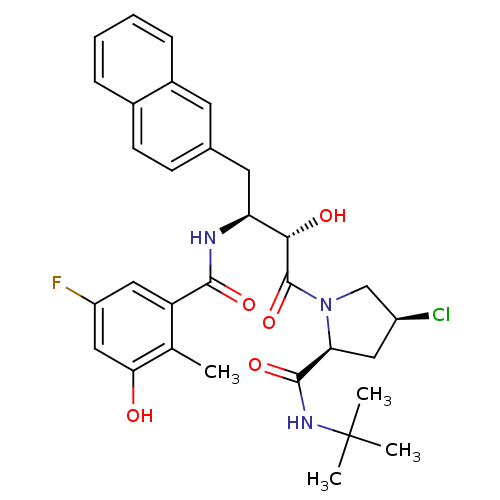 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-3-[(5-flu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4139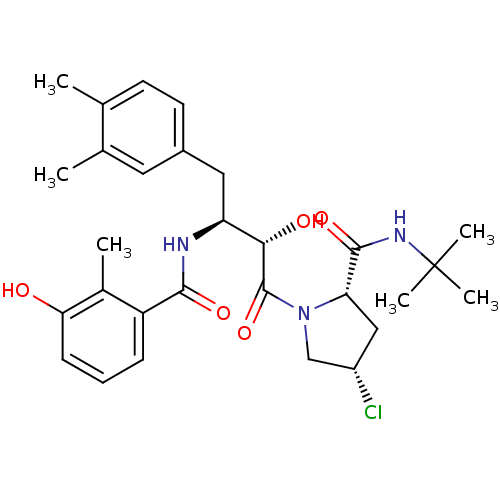 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-4-(3,4-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4137 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4144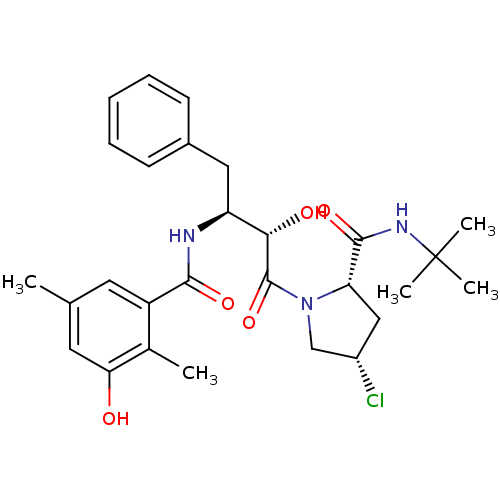 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4154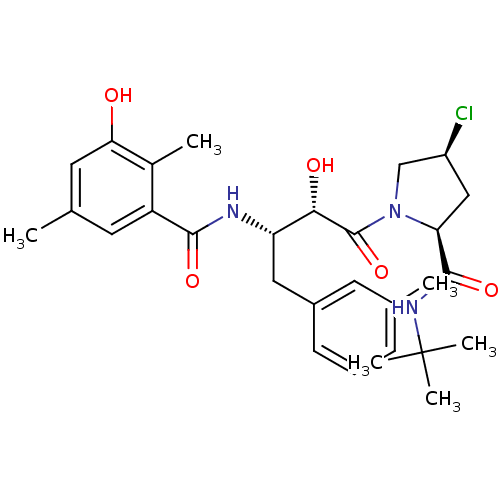 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4160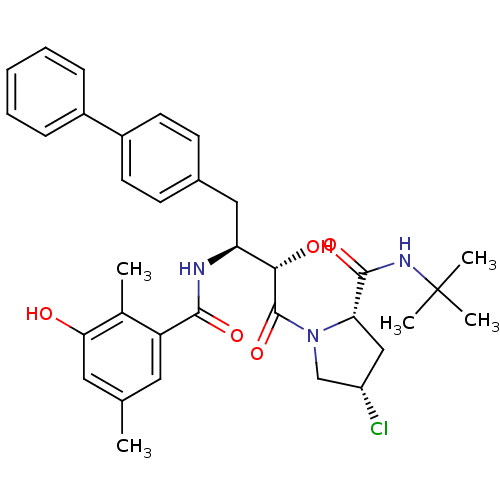 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4140 ((2S,4S)-1-[(2S,3S)-4-(1-benzothiophen-5-yl)-2-hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4123 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4141 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4166 ((2S,4S)-1-[(2S,3S)-4-(1-benzothiophen-5-yl)-2-hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4264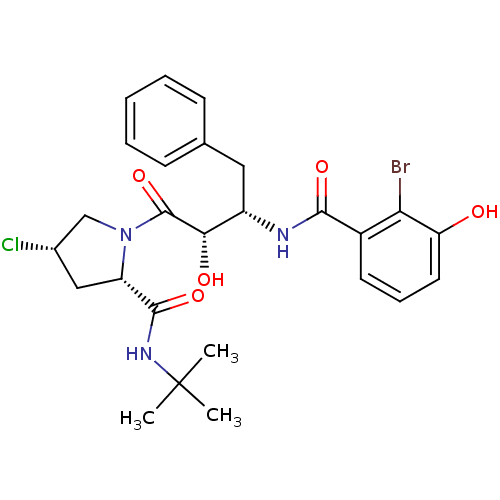 ((2-Bromo-3-hydroxy)benzoyl-(2S,3S)-AHPBA-4(S)-Cl-P...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 6: 595-604 (1998) Article DOI: 10.1016/s0968-0896(98)00004-2 BindingDB Entry DOI: 10.7270/Q2QN64XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4133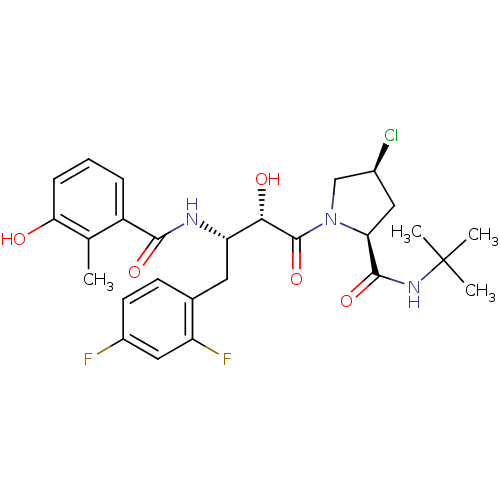 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-4-(2,4-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4266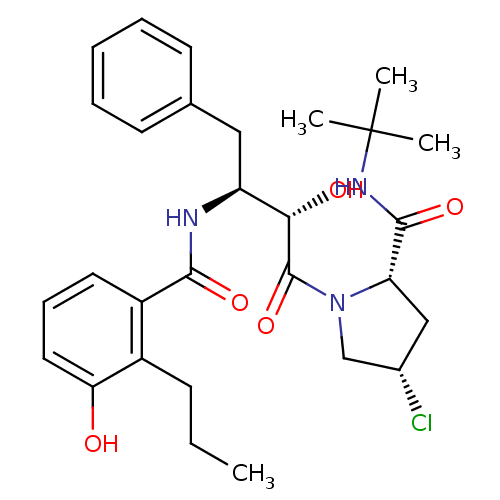 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 6: 595-604 (1998) Article DOI: 10.1016/s0968-0896(98)00004-2 BindingDB Entry DOI: 10.7270/Q2QN64XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4145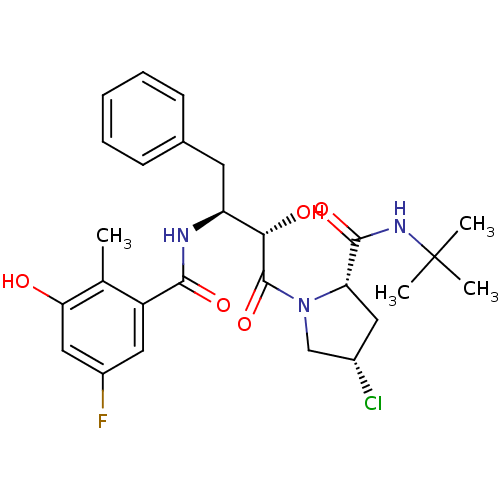 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-3-[(5-flu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451387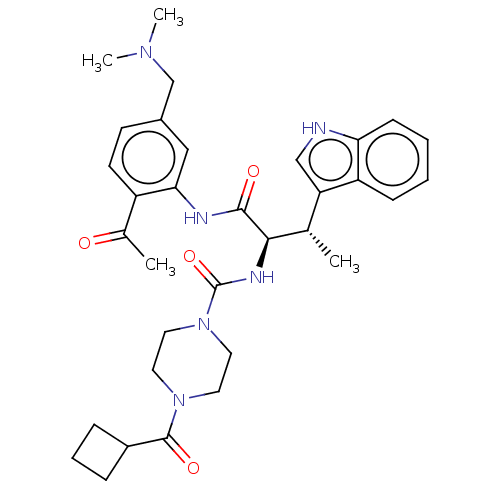 (CHEMBL4203387) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4167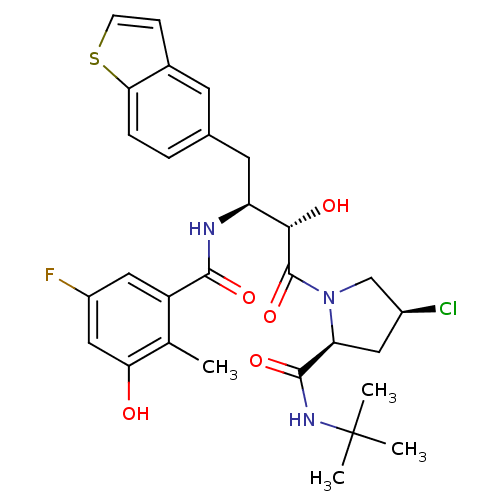 ((2S,4S)-1-[(2S,3S)-4-(1-benzothiophen-5-yl)-3-[(5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4148 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451398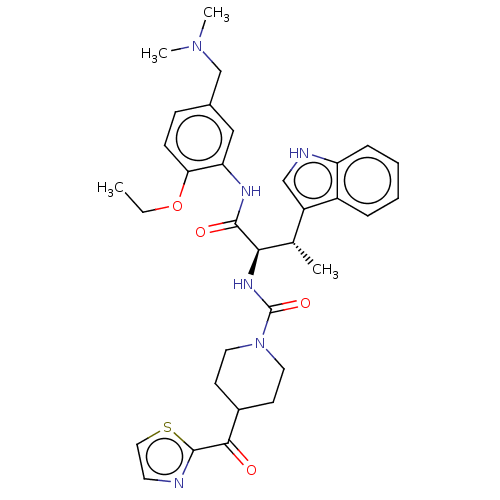 (CHEMBL4203652) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 237 total ) | Next | Last >> |