 Found 1077 hits with Last Name = 'hollenbach' and Initial = 'sj'
Found 1077 hits with Last Name = 'hollenbach' and Initial = 'sj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142111
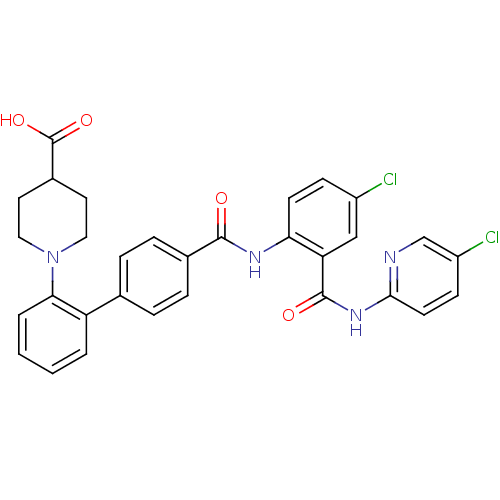
(1-{4'-[4-Chloro-2-(5-chloro-pyridin-2-ylcarbamoyl)...)Show SMILES OC(=O)C1CCN(CC1)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C31H26Cl2N4O4/c32-22-9-11-26(25(17-22)30(39)36-28-12-10-23(33)18-34-28)35-29(38)20-7-5-19(6-8-20)24-3-1-2-4-27(24)37-15-13-21(14-16-37)31(40)41/h1-12,17-18,21H,13-16H2,(H,35,38)(H,40,41)(H,34,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against coagulation factor Xa. |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50193861
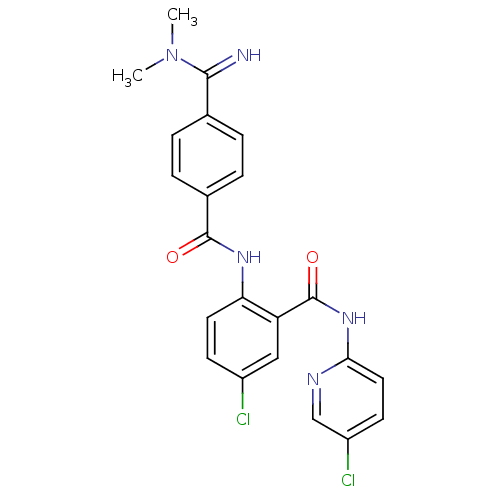
(5-chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N-dimet...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H19Cl2N5O2/c1-29(2)20(25)13-3-5-14(6-4-13)21(30)27-18-9-7-15(23)11-17(18)22(31)28-19-10-8-16(24)12-26-19/h3-12,25H,1-2H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249120
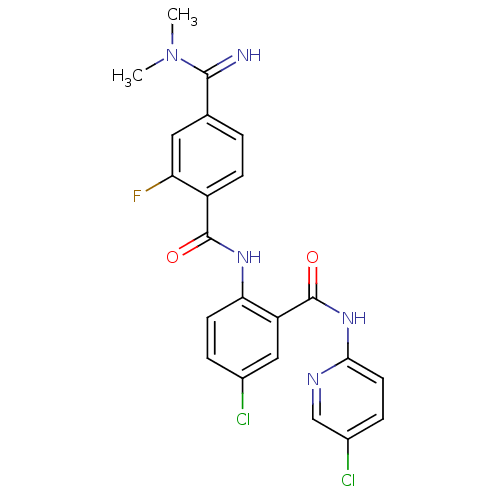
(CHEMBL472967 | N-(4-chloro-2-(5-chloropyridin-2-yl...)Show SMILES CN(C)C(=N)c1ccc(C(=O)Nc2ccc(Cl)cc2C(=O)Nc2ccc(Cl)cn2)c(F)c1 Show InChI InChI=1S/C22H18Cl2FN5O2/c1-30(2)20(26)12-3-6-15(17(25)9-12)21(31)28-18-7-4-13(23)10-16(18)22(32)29-19-8-5-14(24)11-27-19/h3-11,26H,1-2H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM19023

(1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...)Show SMILES COc1ccc(cc1)-n1nc(C(N)=O)c2CCN(C(=O)c12)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142090
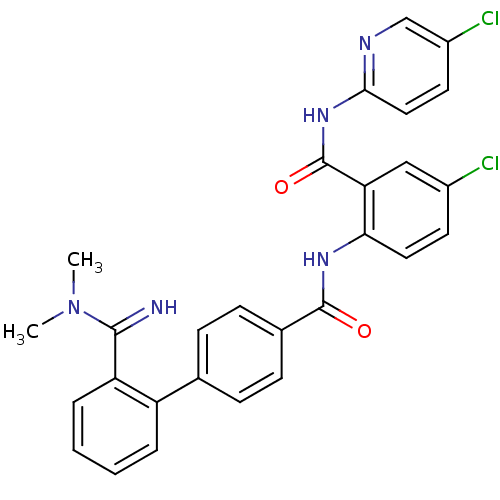
(2'-(N,N-Dimethyl-carbamimidoyl)-biphenyl-4-carboxy...)Show SMILES CN(C)C(=N)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C28H23Cl2N5O2/c1-35(2)26(31)22-6-4-3-5-21(22)17-7-9-18(10-8-17)27(36)33-24-13-11-19(29)15-23(24)28(37)34-25-14-12-20(30)16-32-25/h3-16,31H,1-2H3,(H,33,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142139
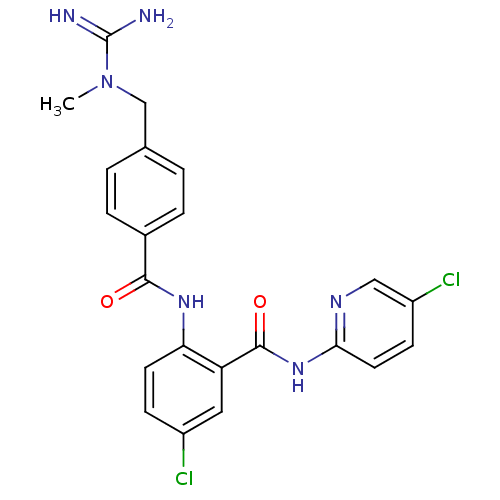
(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-{4-[(N-methyl...)Show SMILES CN(Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1)C(N)=N Show InChI InChI=1S/C22H20Cl2N6O2/c1-30(22(25)26)12-13-2-4-14(5-3-13)20(31)28-18-8-6-15(23)10-17(18)21(32)29-19-9-7-16(24)11-27-19/h2-11H,12H2,1H3,(H3,25,26)(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against coagulation factor X. |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142125
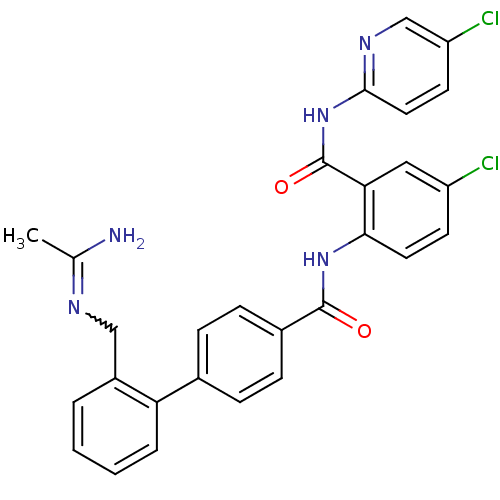
(2'-(Acetimidoylamino-methyl)-biphenyl-4-carboxylic...)Show SMILES CC(N)=NCc1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C28H23Cl2N5O2/c1-17(31)32-15-20-4-2-3-5-23(20)18-6-8-19(9-7-18)27(36)34-25-12-10-21(29)14-24(25)28(37)35-26-13-11-22(30)16-33-26/h2-14,16H,15H2,1H3,(H2,31,32)(H,34,36)(H,33,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against coagulation factor Xa. |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142112
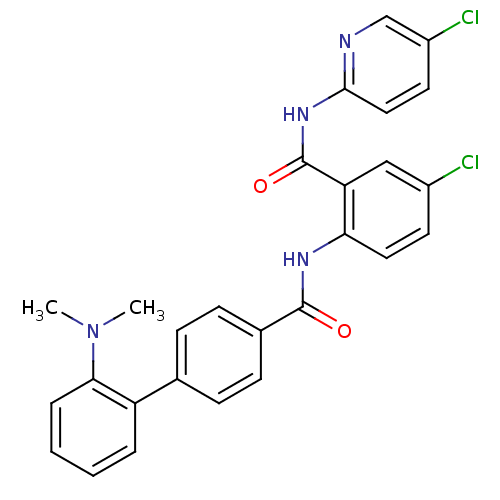
(2'-Dimethylamino-biphenyl-4-carboxylic acid [4-chl...)Show SMILES CN(C)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C27H22Cl2N4O2/c1-33(2)24-6-4-3-5-21(24)17-7-9-18(10-8-17)26(34)31-23-13-11-19(28)15-22(23)27(35)32-25-14-12-20(29)16-30-25/h3-16H,1-2H3,(H,31,34)(H,30,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249423
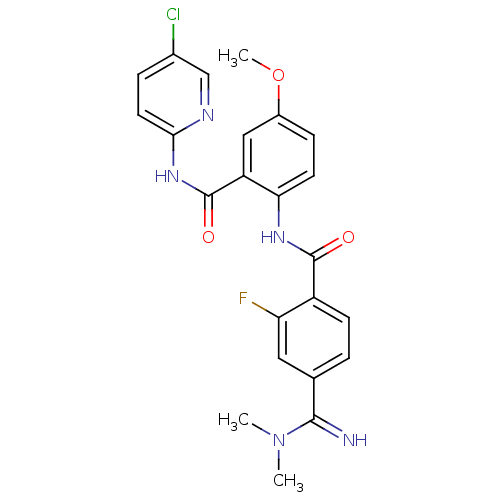
(CHEMBL515919 | N-(2-(5-chloropyridin-2-ylcarbamoyl...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2F)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H21ClFN5O3/c1-30(2)21(26)13-4-7-16(18(25)10-13)22(31)28-19-8-6-15(33-3)11-17(19)23(32)29-20-9-5-14(24)12-27-20/h4-12,26H,1-3H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249298
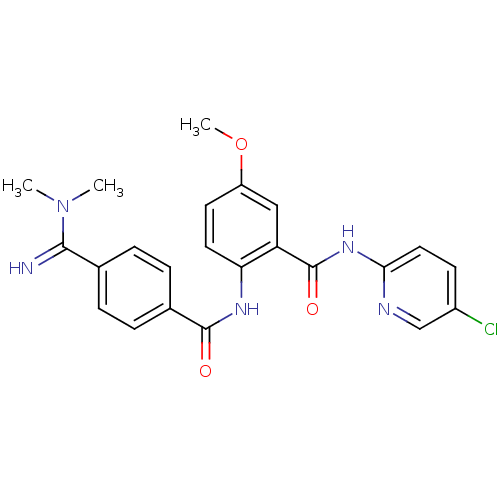
(BEVYXXA | CHEMBL512351 | N-(5-chloropyridin-2-yl)-...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H22ClN5O3/c1-29(2)21(25)14-4-6-15(7-5-14)22(30)27-19-10-9-17(32-3)12-18(19)23(31)28-20-11-8-16(24)13-26-20/h4-13,25H,1-3H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142169
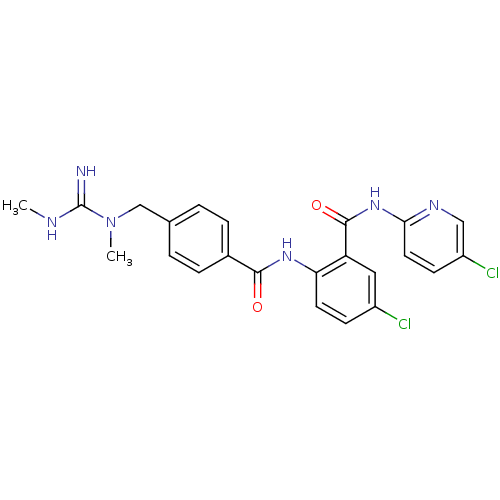
(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N'-dime...)Show SMILES CNC(=N)N(C)Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H22Cl2N6O2/c1-27-23(26)31(2)13-14-3-5-15(6-4-14)21(32)29-19-9-7-16(24)11-18(19)22(33)30-20-10-8-17(25)12-28-20/h3-12H,13H2,1-2H3,(H2,26,27)(H,29,32)(H,28,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142129
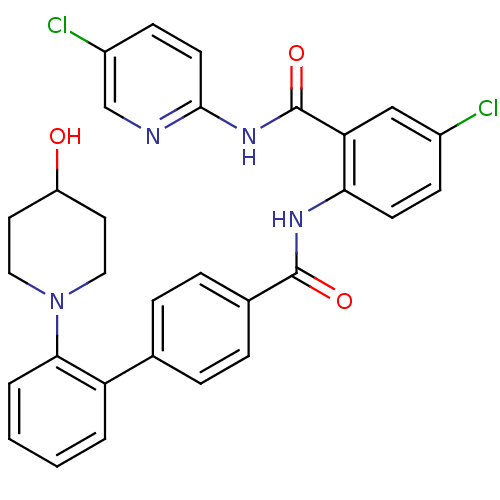
(2'-(4-Hydroxy-piperidin-1-yl)-biphenyl-4-carboxyli...)Show SMILES OC1CCN(CC1)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C30H26Cl2N4O3/c31-21-9-11-26(25(17-21)30(39)35-28-12-10-22(32)18-33-28)34-29(38)20-7-5-19(6-8-20)24-3-1-2-4-27(24)36-15-13-23(37)14-16-36/h1-12,17-18,23,37H,13-16H2,(H,34,38)(H,33,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142118
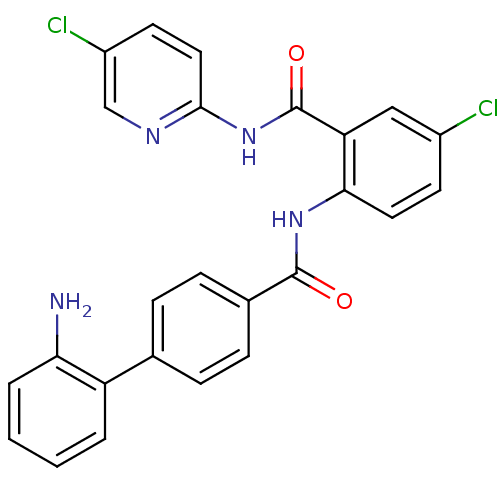
(2'-Amino-biphenyl-4-carboxylic acid [4-chloro-2-(5...)Show SMILES Nc1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C25H18Cl2N4O2/c26-17-9-11-22(20(13-17)25(33)31-23-12-10-18(27)14-29-23)30-24(32)16-7-5-15(6-8-16)19-3-1-2-4-21(19)28/h1-14H,28H2,(H,30,32)(H,29,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142161
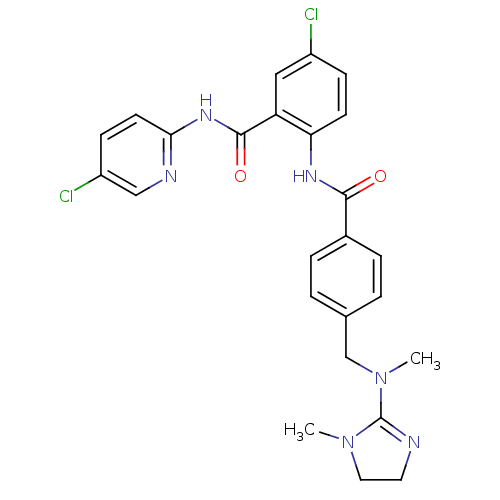
(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-(4-{[methyl-(...)Show SMILES CN(Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1)C1=NCCN1C |t:32| Show InChI InChI=1S/C25H24Cl2N6O2/c1-32-12-11-28-25(32)33(2)15-16-3-5-17(6-4-16)23(34)30-21-9-7-18(26)13-20(21)24(35)31-22-10-8-19(27)14-29-22/h3-10,13-14H,11-12,15H2,1-2H3,(H,30,34)(H,29,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142126
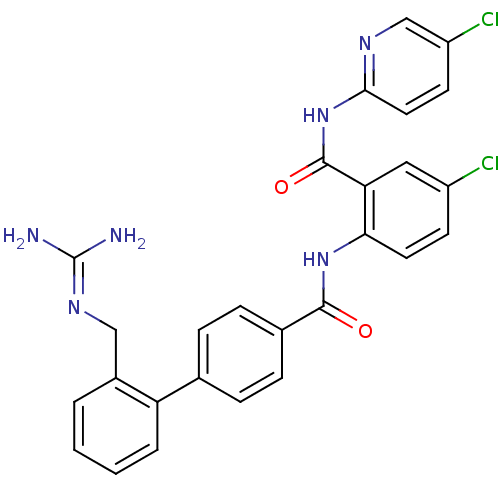
(2'-Guanidinomethyl-biphenyl-4-carboxylic acid [4-c...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-c1ccccc1-c1ccc(cc1)-[#6](=O)-[#7]-c1ccc(Cl)cc1-[#6](=O)-[#7]-c1ccc(Cl)cn1 Show InChI InChI=1S/C27H22Cl2N6O2/c28-19-9-11-23(22(13-19)26(37)35-24-12-10-20(29)15-32-24)34-25(36)17-7-5-16(6-8-17)21-4-2-1-3-18(21)14-33-27(30)31/h1-13,15H,14H2,(H,34,36)(H4,30,31,33)(H,32,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against coagulation factor Xa. |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142142

(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N',N'-dim...)Show SMILES CN(C)NCc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H21Cl2N5O2/c1-29(2)26-12-14-3-5-15(6-4-14)21(30)27-19-9-7-16(23)11-18(19)22(31)28-20-10-8-17(24)13-25-20/h3-11,13,26H,12H2,1-2H3,(H,27,30)(H,25,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM7840
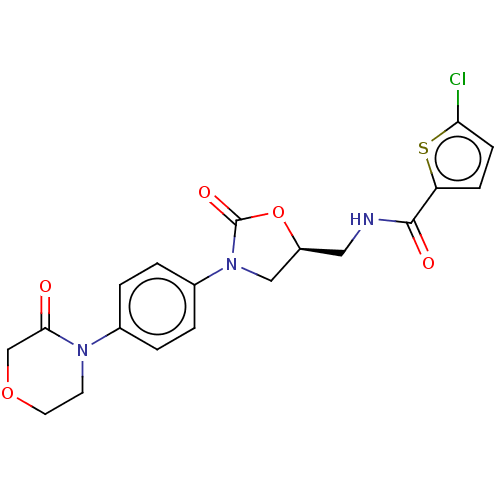
(RIVAROXABAN | US8822458, 44 | US8822458, 97)Show SMILES Clc1ccc(s1)C(=O)NC[C@H]1CN(C(=O)O1)c1ccc(cc1)N1CCOCC1=O |r| Show InChI InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142158

(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(2-imino-i...)Show SMILES NC1=NCCN1Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |t:1| Show InChI InChI=1S/C23H20Cl2N6O2/c24-16-5-7-19(18(11-16)22(33)30-20-8-6-17(25)12-28-20)29-21(32)15-3-1-14(2-4-15)13-31-10-9-27-23(31)26/h1-8,11-12H,9-10,13H2,(H2,26,27)(H,29,32)(H,28,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142177
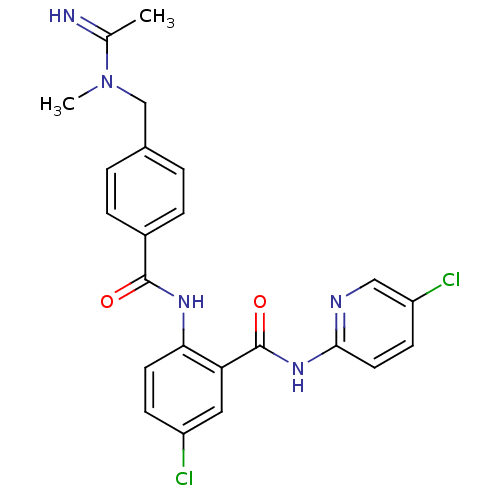
(2-{4-[(Acetimidoyl-methyl-amino)-methyl]-benzoylam...)Show SMILES CN(Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1)C(C)=N Show InChI InChI=1S/C23H21Cl2N5O2/c1-14(26)30(2)13-15-3-5-16(6-4-15)22(31)28-20-9-7-17(24)11-19(20)23(32)29-21-10-8-18(25)12-27-21/h3-12,26H,13H2,1-2H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142089
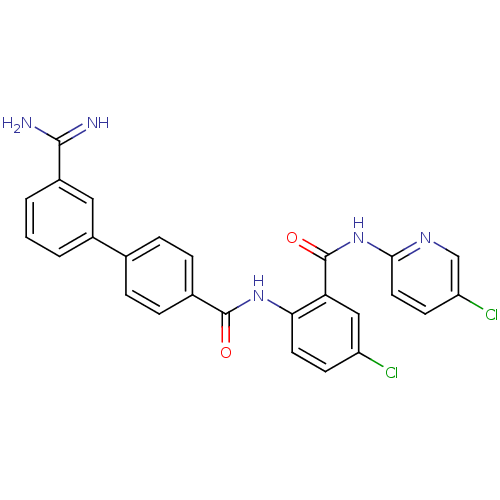
(3'-Carbamimidoyl-biphenyl-4-carboxylic acid [4-chl...)Show SMILES NC(=N)c1cccc(c1)-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H19Cl2N5O2/c27-19-8-10-22(21(13-19)26(35)33-23-11-9-20(28)14-31-23)32-25(34)16-6-4-15(5-7-16)17-2-1-3-18(12-17)24(29)30/h1-14H,(H3,29,30)(H,32,34)(H,31,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50123431
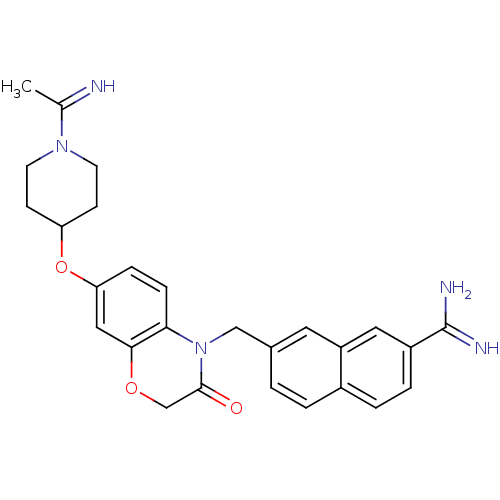
(7-{7-[1-(1-Imino-ethyl)-piperidin-4-yloxy]-3-oxo-2...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc2N(Cc3ccc4ccc(cc4c3)C(N)=N)C(=O)COc2c1 Show InChI InChI=1S/C27H29N5O3/c1-17(28)31-10-8-22(9-11-31)35-23-6-7-24-25(14-23)34-16-26(33)32(24)15-18-2-3-19-4-5-20(27(29)30)13-21(19)12-18/h2-7,12-14,22,28H,8-11,15-16H2,1H3,(H3,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards factor Xa |
Bioorg Med Chem Lett 13: 561-6 (2003)
BindingDB Entry DOI: 10.7270/Q2DB816X |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142100
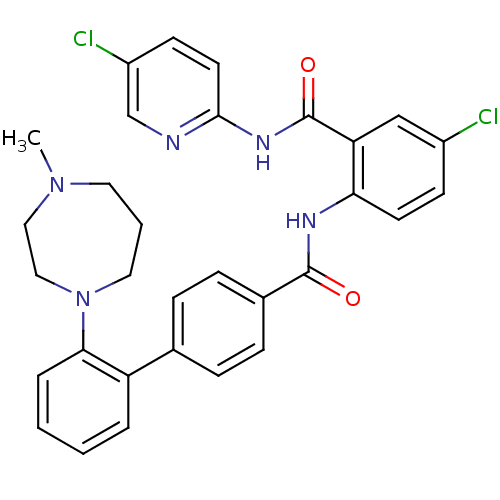
(2'-(4-Methyl-[1,4]diazepan-1-yl)-biphenyl-4-carbox...)Show SMILES CN1CCCN(CC1)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C31H29Cl2N5O2/c1-37-15-4-16-38(18-17-37)28-6-3-2-5-25(28)21-7-9-22(10-8-21)30(39)35-27-13-11-23(32)19-26(27)31(40)36-29-14-12-24(33)20-34-29/h2-3,5-14,19-20H,4,15-18H2,1H3,(H,35,39)(H,34,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142103
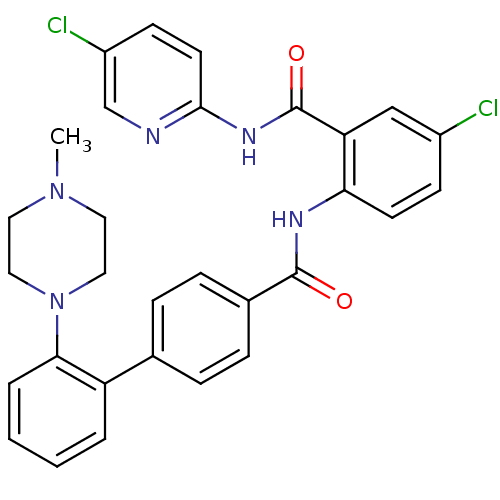
(2'-(4-Methyl-piperazin-1-yl)-biphenyl-4-carboxylic...)Show SMILES CN1CCN(CC1)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C30H27Cl2N5O2/c1-36-14-16-37(17-15-36)27-5-3-2-4-24(27)20-6-8-21(9-7-20)29(38)34-26-12-10-22(31)18-25(26)30(39)35-28-13-11-23(32)19-33-28/h2-13,18-19H,14-17H2,1H3,(H,34,38)(H,33,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142106
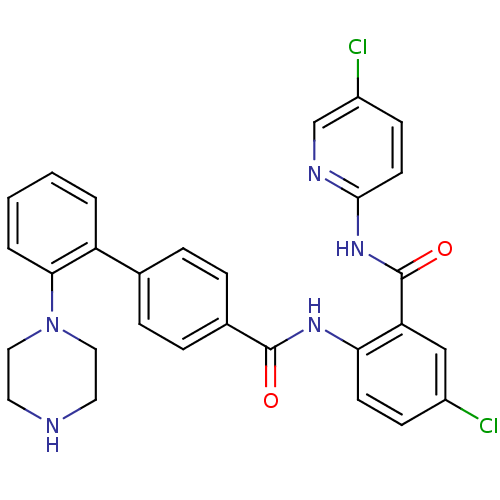
(2'-Piperazin-1-yl-biphenyl-4-carboxylic acid [4-ch...)Show SMILES Clc1ccc(NC(=O)c2cc(Cl)ccc2NC(=O)c2ccc(cc2)-c2ccccc2N2CCNCC2)nc1 Show InChI InChI=1S/C29H25Cl2N5O2/c30-21-9-11-25(24(17-21)29(38)35-27-12-10-22(31)18-33-27)34-28(37)20-7-5-19(6-8-20)23-3-1-2-4-26(23)36-15-13-32-14-16-36/h1-12,17-18,32H,13-16H2,(H,34,37)(H,33,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
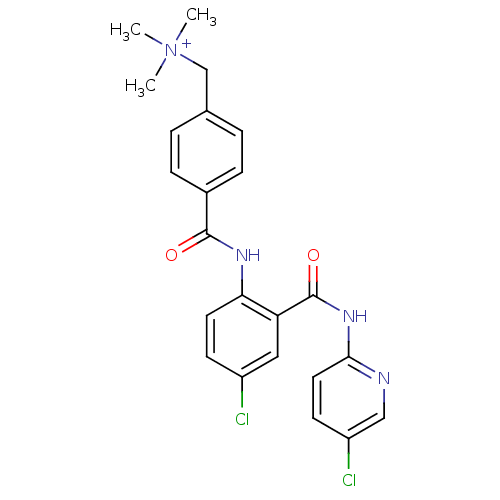
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142154
(CHEMBL174499 | {4-[4-Chloro-2-(5-chloro-pyridin-2-...)Show SMILES C[N+](C)(C)Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H22Cl2N4O2/c1-29(2,3)14-15-4-6-16(7-5-15)22(30)27-20-10-8-17(24)12-19(20)23(31)28-21-11-9-18(25)13-26-21/h4-13H,14H2,1-3H3,(H-,26,27,28,30,31)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
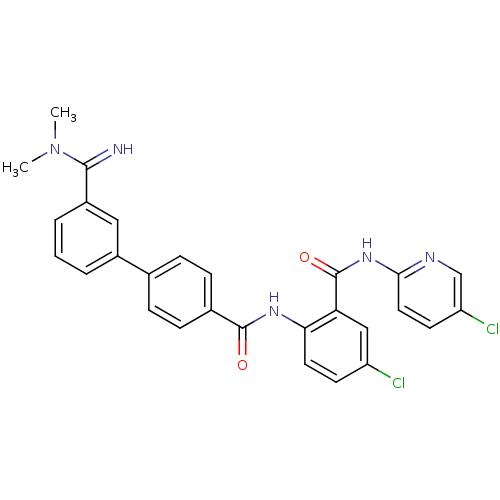
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142096
(3'-(N,N-Dimethyl-carbamimidoyl)-biphenyl-4-carboxy...)Show SMILES CN(C)C(=N)c1cccc(c1)-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C28H23Cl2N5O2/c1-35(2)26(31)20-5-3-4-19(14-20)17-6-8-18(9-7-17)27(36)33-24-12-10-21(29)15-23(24)28(37)34-25-13-11-22(30)16-32-25/h3-16,31H,1-2H3,(H,33,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against coagulation factor Xa. |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142146

(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-(4-dimethylam...)Show SMILES CN(C)Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H20Cl2N4O2/c1-28(2)13-14-3-5-15(6-4-14)21(29)26-19-9-7-16(23)11-18(19)22(30)27-20-10-8-17(24)12-25-20/h3-12H,13H2,1-2H3,(H,26,29)(H,25,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
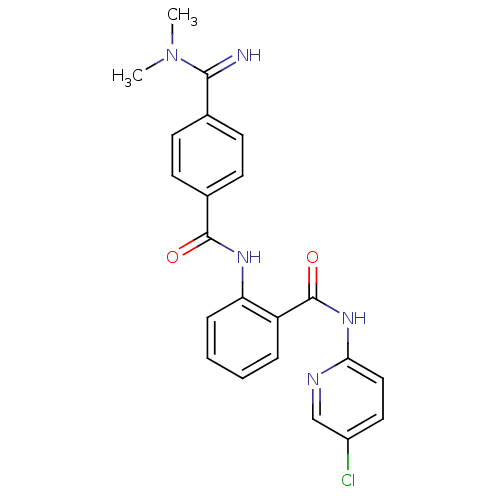
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249295
(CHEMBL471725 | N-(5-chloropyridin-2-yl)-2-(4-(N,N-...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccccc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H20ClN5O2/c1-28(2)20(24)14-7-9-15(10-8-14)21(29)26-18-6-4-3-5-17(18)22(30)27-19-12-11-16(23)13-25-19/h3-13,24H,1-2H3,(H,26,29)(H,25,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142163

(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(2-imino-o...)Show SMILES Clc1ccc(NC(=O)c2cc(Cl)ccc2NC(=O)c2ccc(CN3CCOC3=N)cc2)nc1 Show InChI InChI=1S/C23H19Cl2N5O3/c24-16-5-7-19(18(11-16)22(32)29-20-8-6-17(25)12-27-20)28-21(31)15-3-1-14(2-4-15)13-30-9-10-33-23(30)26/h1-8,11-12,26H,9-10,13H2,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against coagulation factor X. |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142094
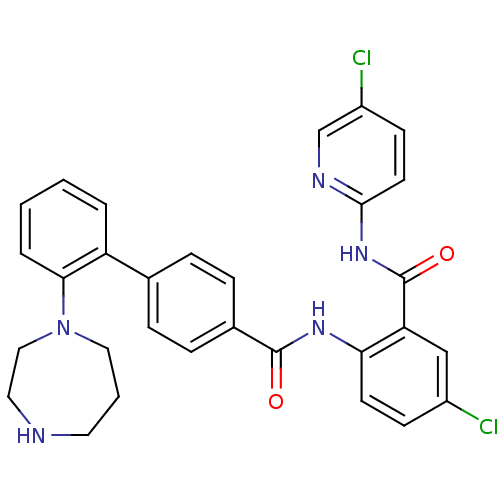
(2'-[1,4]Diazepan-1-yl-biphenyl-4-carboxylic acid [...)Show SMILES Clc1ccc(NC(=O)c2cc(Cl)ccc2NC(=O)c2ccc(cc2)-c2ccccc2N2CCCNCC2)nc1 Show InChI InChI=1S/C30H27Cl2N5O2/c31-22-10-12-26(25(18-22)30(39)36-28-13-11-23(32)19-34-28)35-29(38)21-8-6-20(7-9-21)24-4-1-2-5-27(24)37-16-3-14-33-15-17-37/h1-2,4-13,18-19,33H,3,14-17H2,(H,35,38)(H,34,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142091
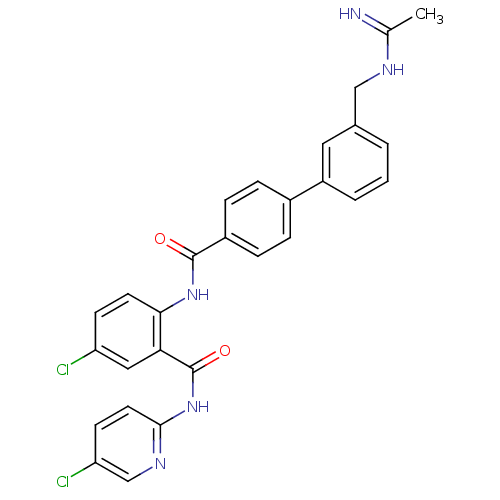
(3'-(Acetimidoylamino-methyl)-biphenyl-4-carboxylic...)Show SMILES CC(=N)NCc1cccc(c1)-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C28H23Cl2N5O2/c1-17(31)32-15-18-3-2-4-21(13-18)19-5-7-20(8-6-19)27(36)34-25-11-9-22(29)14-24(25)28(37)35-26-12-10-23(30)16-33-26/h2-14,16H,15H2,1H3,(H2,31,32)(H,34,36)(H,33,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144092
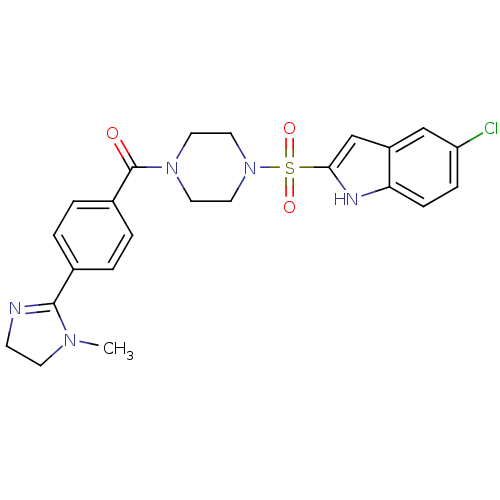
(CHEMBL294121 | [4-(5-Chloro-1H-indole-2-sulfonyl)-...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |c:4| Show InChI InChI=1S/C23H24ClN5O3S/c1-27-9-8-25-22(27)16-2-4-17(5-3-16)23(30)28-10-12-29(13-11-28)33(31,32)21-15-18-14-19(24)6-7-20(18)26-21/h2-7,14-15,26H,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50193861

(5-chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N-dimet...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H19Cl2N5O2/c1-29(2)20(25)13-3-5-14(6-4-13)21(30)27-18-9-7-15(23)11-17(18)22(31)28-19-10-8-16(24)12-26-19/h3-12,25H,1-2H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50193849

(CHEMBL219106 | N-(2-(5-chloropyridin-2-ylcarbamoyl...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2N2CCCCC2)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C28H31ClN6O3/c1-34(2)26(30)18-7-10-21(24(15-18)35-13-5-4-6-14-35)27(36)32-23-11-9-20(38-3)16-22(23)28(37)33-25-12-8-19(29)17-31-25/h7-12,15-17,30H,4-6,13-14H2,1-3H3,(H,32,36)(H,31,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50249120

(CHEMBL472967 | N-(4-chloro-2-(5-chloropyridin-2-yl...)Show SMILES CN(C)C(=N)c1ccc(C(=O)Nc2ccc(Cl)cc2C(=O)Nc2ccc(Cl)cn2)c(F)c1 Show InChI InChI=1S/C22H18Cl2FN5O2/c1-30(2)20(26)12-3-6-15(17(25)9-12)21(31)28-18-7-4-13(23)10-16(18)22(32)29-19-8-5-14(24)11-27-19/h3-11,26H,1-2H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50249298

(BEVYXXA | CHEMBL512351 | N-(5-chloropyridin-2-yl)-...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H22ClN5O3/c1-29(2)21(25)14-4-6-15(7-5-14)22(30)27-19-10-9-17(32-3)12-18(19)23(31)28-20-11-8-16(24)13-26-20/h4-13,25H,1-3H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50249423

(CHEMBL515919 | N-(2-(5-chloropyridin-2-ylcarbamoyl...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2F)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H21ClFN5O3/c1-30(2)21(26)13-4-7-16(18(25)10-13)22(31)28-19-8-6-15(33-3)11-17(19)23(32)29-20-9-5-14(24)12-27-20/h4-12,26H,1-3H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50193850
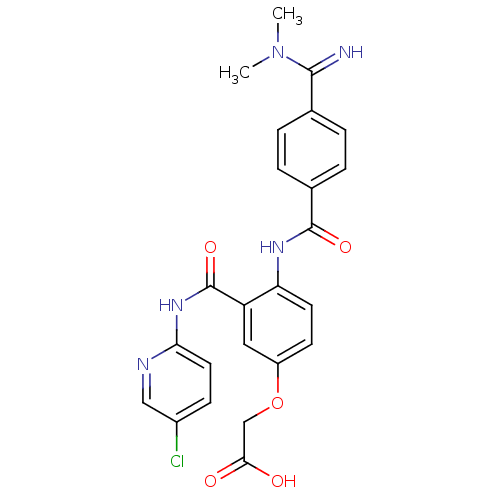
(2-(3-(5-chloropyridin-2-ylcarbamoyl)-4-(4-(N,N-dim...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccc(OCC(O)=O)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C24H22ClN5O5/c1-30(2)22(26)14-3-5-15(6-4-14)23(33)28-19-9-8-17(35-13-21(31)32)11-18(19)24(34)29-20-10-7-16(25)12-27-20/h3-12,26H,13H2,1-2H3,(H,28,33)(H,31,32)(H,27,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50193842
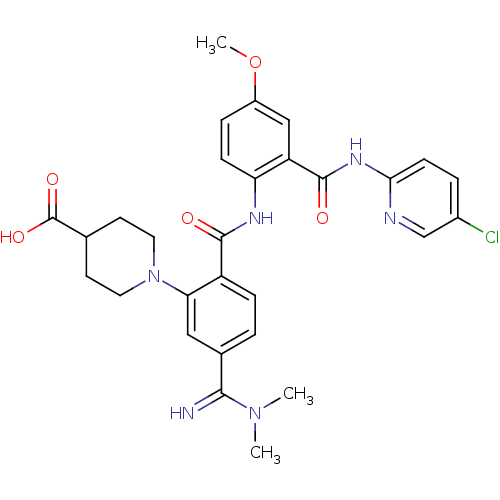
(1-(2-(2-(5-chloropyridin-2-ylcarbamoyl)-4-methoxyp...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2N2CCC(CC2)C(O)=O)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C29H31ClN6O5/c1-35(2)26(31)18-4-7-21(24(14-18)36-12-10-17(11-13-36)29(39)40)27(37)33-23-8-6-20(41-3)15-22(23)28(38)34-25-9-5-19(30)16-32-25/h4-9,14-17,31H,10-13H2,1-3H3,(H,33,37)(H,39,40)(H,32,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition Protein kinase C (PKC) |
J Med Chem 45: 3772-93 (2002)
BindingDB Entry DOI: 10.7270/Q2WW7JD9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142090

(2'-(N,N-Dimethyl-carbamimidoyl)-biphenyl-4-carboxy...)Show SMILES CN(C)C(=N)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C28H23Cl2N5O2/c1-35(2)26(31)22-6-4-3-5-21(22)17-7-9-18(10-8-17)27(36)33-24-13-11-19(29)15-23(24)28(37)34-25-14-12-20(30)16-32-25/h3-16,31H,1-2H3,(H,33,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against coagulation factor Xa. |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142163

(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(2-imino-o...)Show SMILES Clc1ccc(NC(=O)c2cc(Cl)ccc2NC(=O)c2ccc(CN3CCOC3=N)cc2)nc1 Show InChI InChI=1S/C23H19Cl2N5O3/c24-16-5-7-19(18(11-16)22(32)29-20-8-6-17(25)12-27-20)28-21(31)15-3-1-14(2-4-15)13-30-9-10-33-23(30)26/h1-8,11-12,26H,9-10,13H2,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against coagulation factor X. |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142161

(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-(4-{[methyl-(...)Show SMILES CN(Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1)C1=NCCN1C |t:32| Show InChI InChI=1S/C25H24Cl2N6O2/c1-32-12-11-28-25(32)33(2)15-16-3-5-17(6-4-16)23(34)30-21-9-7-18(26)13-20(21)24(35)31-22-10-8-19(27)14-29-22/h3-10,13-14H,11-12,15H2,1-2H3,(H,30,34)(H,29,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against coagulation factor X. |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249166

(2-(azetidin-1-yl)-N-(4-chloro-2-(5-chloropyridin-2...)Show SMILES CN(C)C(=N)c1ccc(C(=O)Nc2ccc(Cl)cc2C(=O)Nc2ccc(Cl)cn2)c(c1)N1CCC1 Show InChI InChI=1S/C25H24Cl2N6O2/c1-32(2)23(28)15-4-7-18(21(12-15)33-10-3-11-33)24(34)30-20-8-5-16(26)13-19(20)25(35)31-22-9-6-17(27)14-29-22/h4-9,12-14,28H,3,10-11H2,1-2H3,(H,30,34)(H,29,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50117334
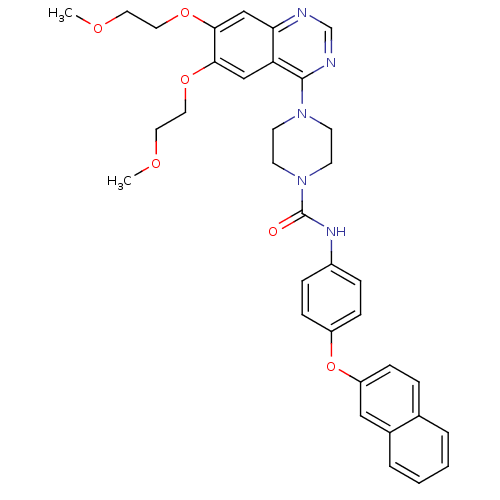
(4-[6,7-Bis-(2-methoxy-ethoxy)-quinazolin-4-yl]-pip...)Show SMILES COCCOc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(Oc4ccc5ccccc5c4)cc3)c2cc1OCCOC Show InChI InChI=1S/C35H37N5O6/c1-42-17-19-44-32-22-30-31(23-33(32)45-20-18-43-2)36-24-37-34(30)39-13-15-40(16-14-39)35(41)38-27-8-11-28(12-9-27)46-29-10-7-25-5-3-4-6-26(25)21-29/h3-12,21-24H,13-20H2,1-2H3,(H,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma |
J Med Chem 45: 3772-93 (2002)
BindingDB Entry DOI: 10.7270/Q2WW7JD9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142111

(1-{4'-[4-Chloro-2-(5-chloro-pyridin-2-ylcarbamoyl)...)Show SMILES OC(=O)C1CCN(CC1)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C31H26Cl2N4O4/c32-22-9-11-26(25(17-22)30(39)36-28-12-10-23(33)18-34-28)35-29(38)20-7-5-19(6-8-20)24-3-1-2-4-27(24)37-15-13-21(14-16-37)31(40)41/h1-12,17-18,21H,13-16H2,(H,35,38)(H,40,41)(H,34,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against coagulation factor Xa. |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50117342
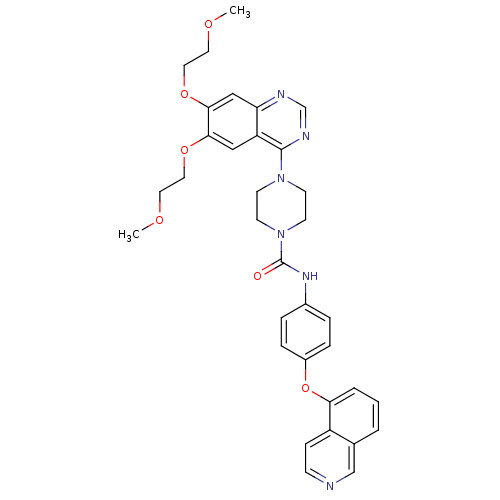
(4-[6,7-Bis-(2-methoxy-ethoxy)-quinazolin-4-yl]-pip...)Show SMILES COCCOc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(Oc4cccc5cnccc45)cc3)c2cc1OCCOC Show InChI InChI=1S/C34H36N6O6/c1-42-16-18-44-31-20-28-29(21-32(31)45-19-17-43-2)36-23-37-33(28)39-12-14-40(15-13-39)34(41)38-25-6-8-26(9-7-25)46-30-5-3-4-24-22-35-11-10-27(24)30/h3-11,20-23H,12-19H2,1-2H3,(H,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma |
J Med Chem 45: 3772-93 (2002)
BindingDB Entry DOI: 10.7270/Q2WW7JD9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50193861

(5-chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N-dimet...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H19Cl2N5O2/c1-29(2)20(25)13-3-5-14(6-4-13)21(30)27-18-9-7-15(23)11-17(18)22(31)28-19-10-8-16(24)12-26-19/h3-12,25H,1-2H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249165

(CHEMBL515254 | N-(4-chloro-2-(5-chloropyridin-2-yl...)Show SMILES COCCN(C)c1cc(ccc1C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1)C(=N)N(C)C Show InChI InChI=1S/C26H28Cl2N6O3/c1-33(2)24(29)16-5-8-19(22(13-16)34(3)11-12-37-4)25(35)31-21-9-6-17(27)14-20(21)26(36)32-23-10-7-18(28)15-30-23/h5-10,13-15,29H,11-12H2,1-4H3,(H,31,35)(H,30,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249121
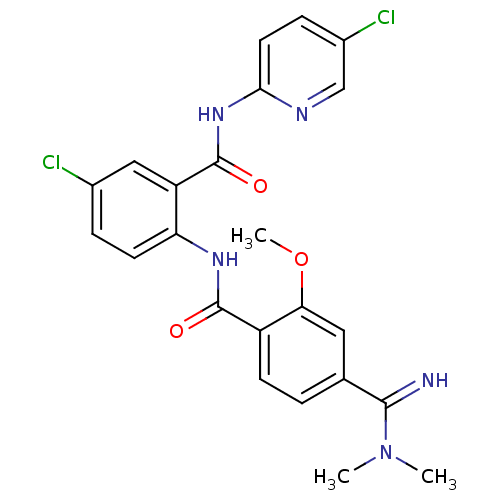
(CHEMBL472778 | N-(4-chloro-2-(5-chloropyridin-2-yl...)Show SMILES COc1cc(ccc1C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1)C(=N)N(C)C Show InChI InChI=1S/C23H21Cl2N5O3/c1-30(2)21(26)13-4-7-16(19(10-13)33-3)22(31)28-18-8-5-14(24)11-17(18)23(32)29-20-9-6-15(25)12-27-20/h4-12,26H,1-3H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data