 Found 777 hits with Last Name = 'singh' and Initial = 'sk'
Found 777 hits with Last Name = 'singh' and Initial = 'sk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50402408
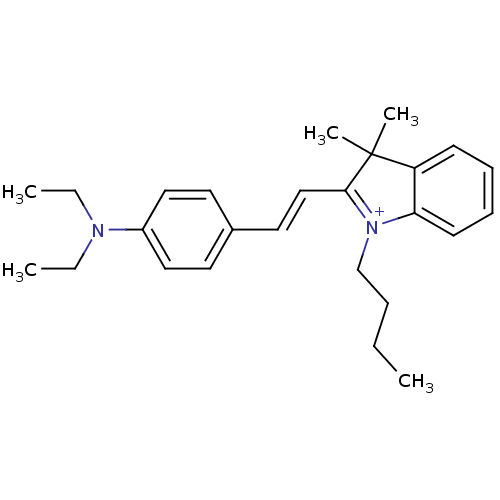
(CHEMBL2203332)Show SMILES CCCC[N+]1=C(\C=C\c2ccc(cc2)N(CC)CC)C(C)(C)c2ccccc12 |c:4| Show InChI InChI=1S/C26H35N2/c1-6-9-20-28-24-13-11-10-12-23(24)26(4,5)25(28)19-16-21-14-17-22(18-15-21)27(7-2)8-3/h10-19H,6-9,20H2,1-5H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01619
BindingDB Entry DOI: 10.7270/Q2X92GCK |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50018235

(5-Amino-2-{4-[(2,4-diamino-5-chloro-quinazolin-6-y...)Show SMILES NCCCC(NC(=O)c1ccc(NCc2ccc3nc(N)nc(N)c3c2Cl)cc1)C(O)=O Show InChI InChI=1S/C21H24ClN7O3/c22-17-12(5-8-14-16(17)18(24)29-21(25)28-14)10-26-13-6-3-11(4-7-13)19(30)27-15(20(31)32)2-1-9-23/h3-8,15,26H,1-2,9-10,23H2,(H,27,30)(H,31,32)(H4,24,25,28,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase |
J Med Chem 32: 1559-65 (1989)
BindingDB Entry DOI: 10.7270/Q22806MJ |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50139758
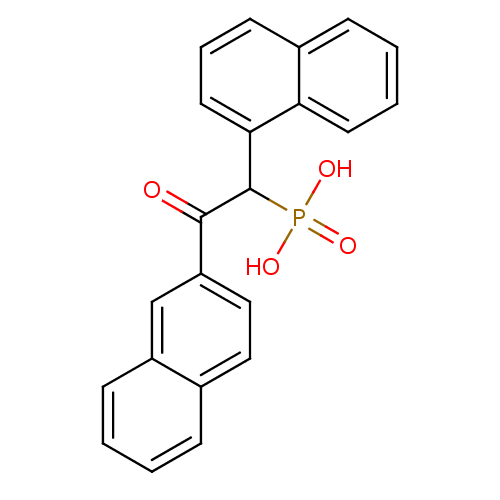
((2-Naphthalen-2-yl-1-naphthalen-1-yl-2-oxo-ethyl)-...)Show SMILES OP(O)(=O)C(C(=O)c1ccc2ccccc2c1)c1cccc2ccccc12 Show InChI InChI=1S/C22H17O4P/c23-21(18-13-12-15-6-1-2-8-17(15)14-18)22(27(24,25)26)20-11-5-9-16-7-3-4-10-19(16)20/h1-14,22H,(H2,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of chymase in human mast cells using Suc-Ala-Ala-Pro-Phe-(p-nitroanilide) as substrate for 15 mins by spectrophotometric method |
Eur J Med Chem 161: 252-276 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.018
BindingDB Entry DOI: 10.7270/Q25M690C |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50254908
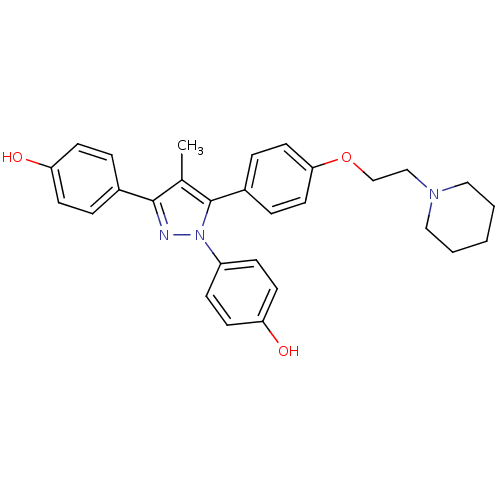
(4-[1-(4-hydroxyphenyl)-4-methyl-5-{4-[2-(piperidin...)Show SMILES Cc1c(nn(c1-c1ccc(OCCN2CCCCC2)cc1)-c1ccc(O)cc1)-c1ccc(O)cc1 Show InChI InChI=1S/C29H31N3O3/c1-21-28(22-5-11-25(33)12-6-22)30-32(24-9-13-26(34)14-10-24)29(21)23-7-15-27(16-8-23)35-20-19-31-17-3-2-4-18-31/h5-16,33-34H,2-4,17-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of ERalpha in human MCF7 cells |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50603512
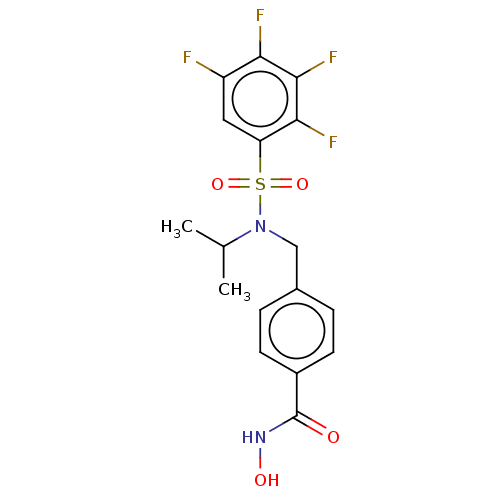
(CHEMBL5177475)Show SMILES CC(C)N(Cc1ccc(cc1)C(=O)NO)S(=O)(=O)c1cc(F)c(F)c(F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50488953
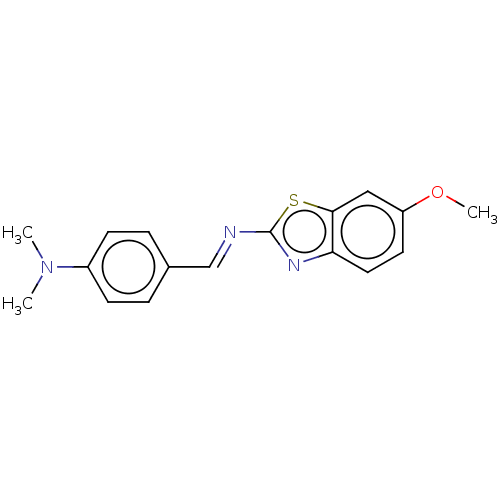
(CHEMBL2296467)Show InChI InChI=1S/C17H17N3OS/c1-20(2)13-6-4-12(5-7-13)11-18-17-19-15-9-8-14(21-3)10-16(15)22-17/h4-11H,1-3H3/b18-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01619
BindingDB Entry DOI: 10.7270/Q2X92GCK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50524365

(CHEMBL4579211)Show SMILES C(CN1CCCCC1)Oc1ccc(Nc2nccc(NCc3ccccc3)n2)cc1 Show InChI InChI=1S/C24H29N5O/c1-3-7-20(8-4-1)19-26-23-13-14-25-24(28-23)27-21-9-11-22(12-10-21)30-18-17-29-15-5-2-6-16-29/h1,3-4,7-14H,2,5-6,15-19H2,(H2,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Scientific and Innovative Research (AcSIR)
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from recombinant human H3 receptor expressed in HEK293T cells measured after 90 mins by liquid scintillat... |
J Med Chem 62: 4638-4655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00241
BindingDB Entry DOI: 10.7270/Q23T9MN6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50524374

(CHEMBL4453810)Show InChI InChI=1S/C18H20N4OS/c1-12-3-4-15(19-11-12)18(23)6-8-22(9-7-18)17-16-14(5-10-24-16)20-13(2)21-17/h3-5,10-11,23H,6-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Scientific and Innovative Research (AcSIR)
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from recombinant human H3 receptor expressed in HEK293T cells measured after 90 mins by liquid scintillat... |
J Med Chem 62: 4638-4655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00241
BindingDB Entry DOI: 10.7270/Q23T9MN6 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50528756
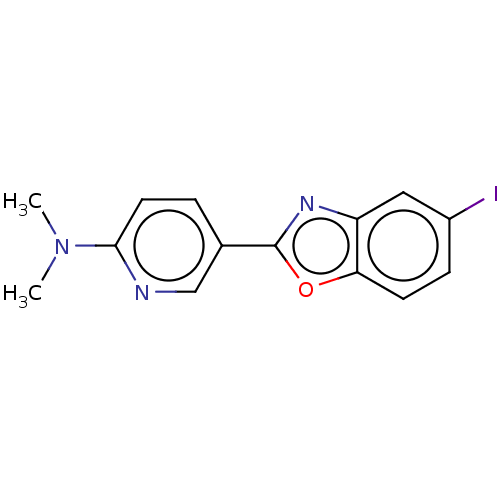
(CHEMBL4470128)Show InChI InChI=1S/C14H12IN3O/c1-18(2)13-6-3-9(8-16-13)14-17-11-7-10(15)4-5-12(11)19-14/h3-8H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University)
Curated by ChEMBL
| Assay Description
Displacement of [125I]IMPY from human amyloid beta(1-42) aggregates incubated for 3 hrs by gamma counting analysis |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111613
BindingDB Entry DOI: 10.7270/Q2GQ726C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50458333
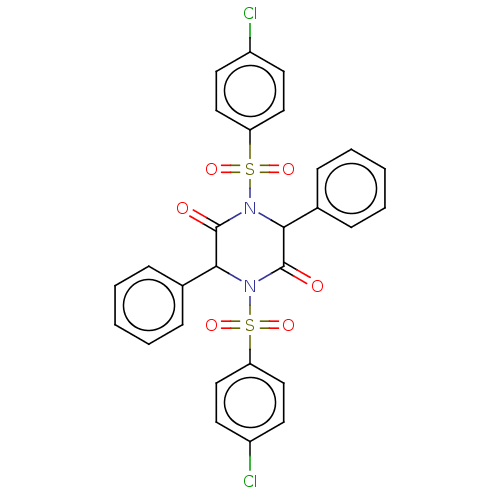
(CHEMBL4210820)Show SMILES Clc1ccc(cc1)S(=O)(=O)N1C(C(=O)N(C(C1=O)c1ccccc1)S(=O)(=O)c1ccc(Cl)cc1)c1ccccc1 Show InChI InChI=1S/C28H20Cl2N2O6S2/c29-21-11-15-23(16-12-21)39(35,36)31-25(19-7-3-1-4-8-19)27(33)32(26(28(31)34)20-9-5-2-6-10-20)40(37,38)24-17-13-22(30)14-18-24/h1-18,25-26H | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University)
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 30 mins followed by substrate addition b... |
Eur J Med Chem 150: 87-101 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.078
BindingDB Entry DOI: 10.7270/Q2SJ1P72 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM8465

((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C18H23N3O5S/c1-13(2)17(18(22)20-23)21(12-14-5-4-10-19-11-14)27(24,25)16-8-6-15(26-3)7-9-16/h4-11,13,17,23H,12H2,1-3H3,(H,20,22)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP9 |
Eur J Med Chem 60: 89-100 (2013)
Article DOI: 10.1016/j.ejmech.2012.10.016
BindingDB Entry DOI: 10.7270/Q2MC91CR |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50002472
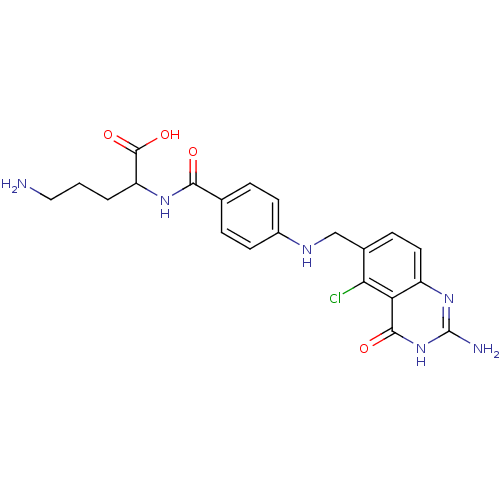
(5-Amino-2-{4-[(2-amino-5-chloro-4-oxo-3,4-dihydro-...)Show SMILES NCCCC(NC(=O)c1ccc(NCc2ccc3nc(N)[nH]c(=O)c3c2Cl)cc1)C(O)=O Show InChI InChI=1S/C21H23ClN6O4/c22-17-12(5-8-14-16(17)19(30)28-21(24)27-14)10-25-13-6-3-11(4-7-13)18(29)26-15(20(31)32)2-1-9-23/h3-8,15,25H,1-2,9-10,23H2,(H,26,29)(H,31,32)(H3,24,27,28,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against hog liver Folyl polyglutamate synthetase (FPGS) |
J Med Chem 35: 4078-85 (1992)
BindingDB Entry DOI: 10.7270/Q26M35S6 |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50002472

(5-Amino-2-{4-[(2-amino-5-chloro-4-oxo-3,4-dihydro-...)Show SMILES NCCCC(NC(=O)c1ccc(NCc2ccc3nc(N)[nH]c(=O)c3c2Cl)cc1)C(O)=O Show InChI InChI=1S/C21H23ClN6O4/c22-17-12(5-8-14-16(17)19(30)28-21(24)27-14)10-25-13-6-3-11(4-7-13)18(29)26-15(20(31)32)2-1-9-23/h3-8,15,25H,1-2,9-10,23H2,(H,26,29)(H,31,32)(H3,24,27,28,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase |
J Med Chem 32: 1559-65 (1989)
BindingDB Entry DOI: 10.7270/Q22806MJ |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50003467
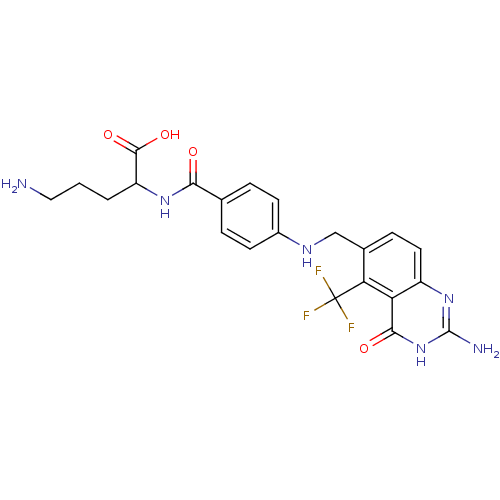
(5-Amino-2-{4-[(2-amino-4-oxo-5-trifluoromethyl-3,4...)Show SMILES NCCCC(NC(=O)c1ccc(NCc2ccc3nc(N)[nH]c(=O)c3c2C(F)(F)F)cc1)C(O)=O Show InChI InChI=1S/C22H23F3N6O4/c23-22(24,25)17-12(5-8-14-16(17)19(33)31-21(27)30-14)10-28-13-6-3-11(4-7-13)18(32)29-15(20(34)35)2-1-9-26/h3-8,15,28H,1-2,9-10,26H2,(H,29,32)(H,34,35)(H3,27,30,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against hog liver Folyl polyglutamate synthetase (FPGS) |
J Med Chem 35: 4078-85 (1992)
BindingDB Entry DOI: 10.7270/Q26M35S6 |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50003471

(5-Amino-2-{4-[(2-amino-4-oxo-5-trifluoromethyl-3,4...)Show SMILES NCCCC(NC(=O)c1ccc(CNc2ccc3nc(N)[nH]c(=O)c3c2C(F)(F)F)cc1)C(O)=O Show InChI InChI=1S/C22H23F3N6O4/c23-22(24,25)17-14(8-7-13-16(17)19(33)31-21(27)30-13)28-10-11-3-5-12(6-4-11)18(32)29-15(20(34)35)2-1-9-26/h3-8,15,28H,1-2,9-10,26H2,(H,29,32)(H,34,35)(H3,27,30,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against hog liver Folyl polyglutamate synthetase (FPGS) |
J Med Chem 35: 4078-85 (1992)
BindingDB Entry DOI: 10.7270/Q26M35S6 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50598566
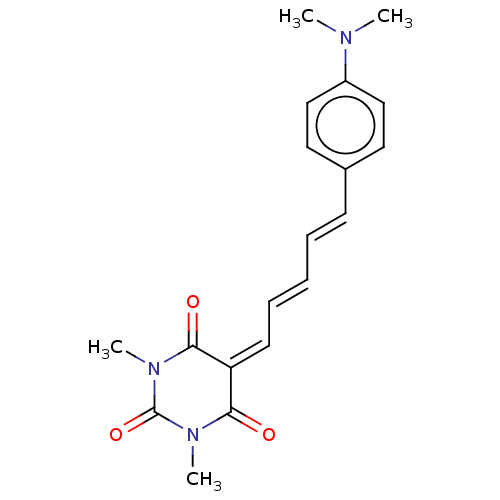
(CHEMBL5192359)Show SMILES [#6]-[#7](-[#6])-c1ccc(\[#6]=[#6]\[#6]=[#6]\[#6]=[#6]-2/[#6](=O)-[#7](-[#6])-[#6](=O)-[#7](-[#6])-[#6]-2=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01619
BindingDB Entry DOI: 10.7270/Q2X92GCK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50595960

(CHEMBL5192865)Show SMILES ONC(=O)c1ccc(CN(C2CC2)S(=O)(=O)c2cc(F)c(F)c(F)c2F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50109051
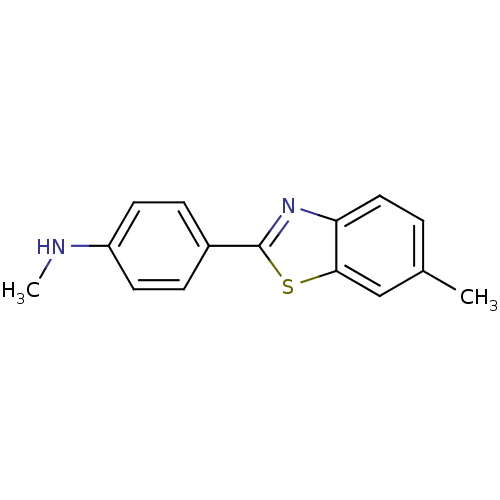
(CHEMBL330529 | Methyl-[4-(6-methyl-benzothiazol-2-...)Show InChI InChI=1S/C15H14N2S/c1-10-3-8-13-14(9-10)18-15(17-13)11-4-6-12(16-2)7-5-11/h3-9,16H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01619
BindingDB Entry DOI: 10.7270/Q2X92GCK |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM8465

((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C18H23N3O5S/c1-13(2)17(18(22)20-23)21(12-14-5-4-10-19-11-14)27(24,25)16-8-6-15(26-3)7-9-16/h4-11,13,17,23H,12H2,1-3H3,(H,20,22)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP2 |
Eur J Med Chem 60: 89-100 (2013)
Article DOI: 10.1016/j.ejmech.2012.10.016
BindingDB Entry DOI: 10.7270/Q2MC91CR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50458333

(CHEMBL4210820)Show SMILES Clc1ccc(cc1)S(=O)(=O)N1C(C(=O)N(C(C1=O)c1ccccc1)S(=O)(=O)c1ccc(Cl)cc1)c1ccccc1 Show InChI InChI=1S/C28H20Cl2N2O6S2/c29-21-11-15-23(16-12-21)39(35,36)31-25(19-7-3-1-4-8-19)27(33)32(26(28(31)34)20-9-5-2-6-10-20)40(37,38)24-17-13-22(30)14-18-24/h1-18,25-26H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University)
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH as substrate preincubated for 60 mins followed by substrate addition by... |
Eur J Med Chem 150: 87-101 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.078
BindingDB Entry DOI: 10.7270/Q2SJ1P72 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50528740
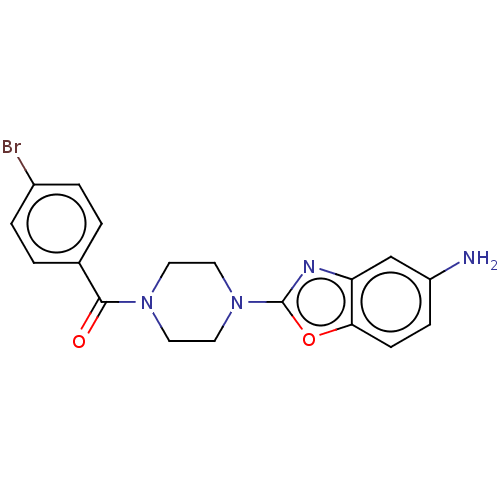
(CHEMBL4536953)Show SMILES Nc1ccc2oc(nc2c1)N1CCN(CC1)C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C18H17BrN4O2/c19-13-3-1-12(2-4-13)17(24)22-7-9-23(10-8-22)18-21-15-11-14(20)5-6-16(15)25-18/h1-6,11H,7-10,20H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University)
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of electric eel AChE using varying levels of acetylthiocholine iodide as substrate measured at 2 mins interval for 10 mins... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111613
BindingDB Entry DOI: 10.7270/Q2GQ726C |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM8465

((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C18H23N3O5S/c1-13(2)17(18(22)20-23)21(12-14-5-4-10-19-11-14)27(24,25)16-8-6-15(26-3)7-9-16/h4-11,13,17,23H,12H2,1-3H3,(H,20,22)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP1 |
Eur J Med Chem 60: 89-100 (2013)
Article DOI: 10.1016/j.ejmech.2012.10.016
BindingDB Entry DOI: 10.7270/Q2MC91CR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50598556
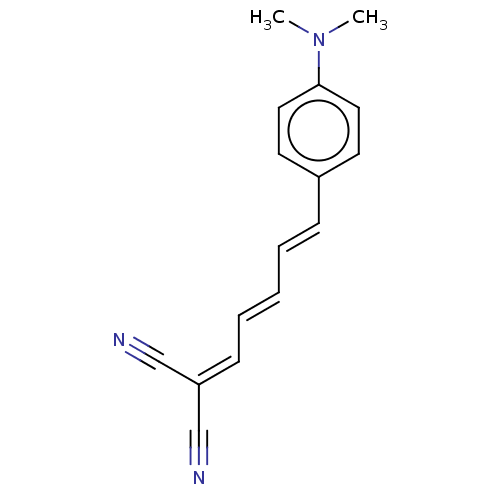
(CHEMBL5174620)Show SMILES [#6]-[#7](-[#6])-c1ccc(\[#6]=[#6]\[#6]=[#6]\[#6]=[#6](\C#N)C#N)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01619
BindingDB Entry DOI: 10.7270/Q2X92GCK |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50506770

(CHEMBL4472052)Show SMILES CN(C1CCN(CC1)C(=O)c1ccc2ccccc2c1)C(=O)c1c(ccc2ccccc12)C(=O)C(c1cccc2ccccc12)P(O)(O)=O Show InChI InChI=1S/C40H35N2O6P/c1-41(31-21-23-42(24-22-31)39(44)30-18-17-26-9-2-3-12-29(26)25-30)40(45)36-33-15-7-5-11-28(33)19-20-35(36)37(43)38(49(46,47)48)34-16-8-13-27-10-4-6-14-32(27)34/h2-20,25,31,38H,21-24H2,1H3,(H2,46,47,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin G in human neutrophils using Suc-Ala-Ala-Pro-Phe-(p-nitroanilide) as substrate for 15 mins by spectrophotometric method |
Eur J Med Chem 161: 252-276 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.018
BindingDB Entry DOI: 10.7270/Q25M690C |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM8465

((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C18H23N3O5S/c1-13(2)17(18(22)20-23)21(12-14-5-4-10-19-11-14)27(24,25)16-8-6-15(26-3)7-9-16/h4-11,13,17,23H,12H2,1-3H3,(H,20,22)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP3 |
Eur J Med Chem 60: 89-100 (2013)
Article DOI: 10.1016/j.ejmech.2012.10.016
BindingDB Entry DOI: 10.7270/Q2MC91CR |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50525255
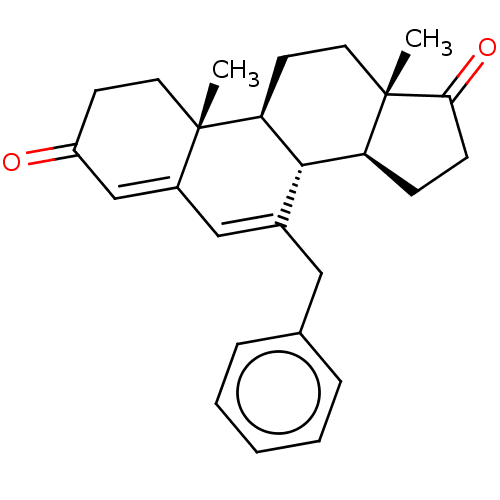
(CHEMBL3754505)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C(Cc2ccccc2)=CC2=CC(=O)CC[C@]12C |r,c:24,t:26| Show InChI InChI=1S/C26H30O2/c1-25-12-10-20(27)16-19(25)15-18(14-17-6-4-3-5-7-17)24-21-8-9-23(28)26(21,2)13-11-22(24)25/h3-7,15-16,21-22,24H,8-14H2,1-2H3/t21-,22-,24-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50002471
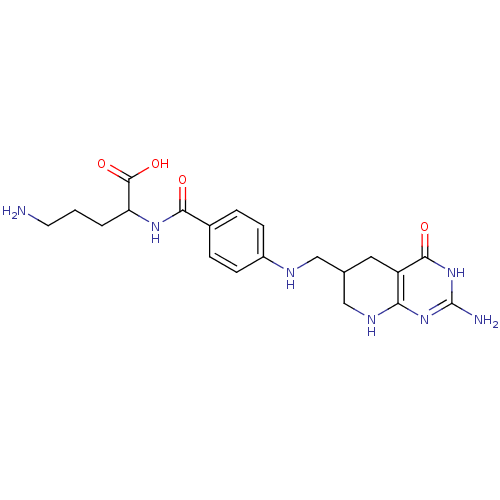
(5-Amino-2-{4-[(2-amino-4-oxo-2,3,4,4a,5,6,7,8-octa...)Show SMILES NCCCC(NC(=O)c1ccc(NCC2CNc3nc(N)[nH]c(=O)c3C2)cc1)C(O)=O Show InChI InChI=1S/C20H27N7O4/c21-7-1-2-15(19(30)31)25-17(28)12-3-5-13(6-4-12)23-9-11-8-14-16(24-10-11)26-20(22)27-18(14)29/h3-6,11,15,23H,1-2,7-10,21H2,(H,25,28)(H,30,31)(H4,22,24,26,27,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Potent inhibitor of Folyl-polyglutamate synthase obtained from porcine |
J Med Chem 35: 2002-6 (1992)
BindingDB Entry DOI: 10.7270/Q2X9297Q |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50598555
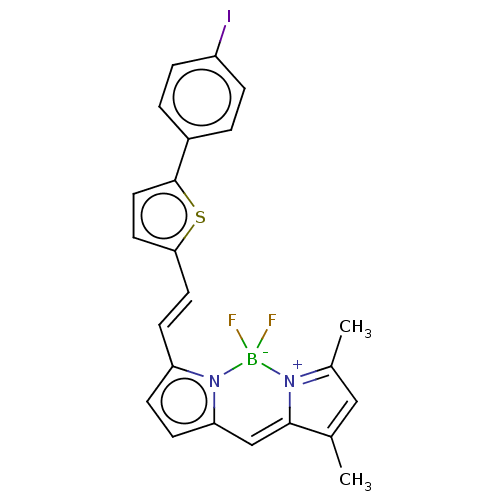
(CHEMBL1171026 | [125I]BODIPY7)Show SMILES CC1=CC(C)=[N+]2C1=Cc1ccc(\C=C\c3ccc(s3)-c3ccc([125I])cc3)n1[B-]2(F)F |c:4,t:1,7| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 108 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01619
BindingDB Entry DOI: 10.7270/Q2X92GCK |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50129790

(CHEMBL329640 | Dimethyl-[4-(6-methyl-benzothiazol-...)Show InChI InChI=1S/C16H16N2S/c1-11-4-9-14-15(10-11)19-16(17-14)12-5-7-13(8-6-12)18(2)3/h4-10H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01619
BindingDB Entry DOI: 10.7270/Q2X92GCK |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50003470

(5-Amino-2-{4-[(5-fluoro-2-methyl-4-oxo-3,4-dihydro...)Show SMILES Cc1nc2ccc(NCc3ccc(cc3)C(=O)NC(CCCN)C(O)=O)c(F)c2c(=O)[nH]1 Show InChI InChI=1S/C22H24FN5O4/c1-12-26-15-8-9-16(19(23)18(15)21(30)27-12)25-11-13-4-6-14(7-5-13)20(29)28-17(22(31)32)3-2-10-24/h4-9,17,25H,2-3,10-11,24H2,1H3,(H,28,29)(H,31,32)(H,26,27,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against hog liver Folyl polyglutamate synthetase (FPGS) |
J Med Chem 35: 4078-85 (1992)
BindingDB Entry DOI: 10.7270/Q26M35S6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50525268
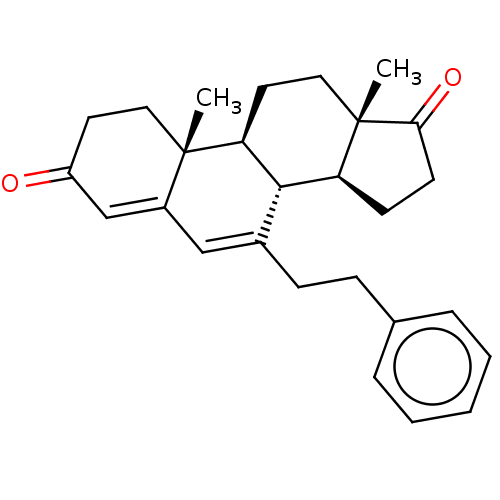
(CHEMBL3753089)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C(CCc2ccccc2)=CC2=CC(=O)CC[C@]12C |r,c:25,t:27| Show InChI InChI=1S/C27H32O2/c1-26-14-12-21(28)17-20(26)16-19(9-8-18-6-4-3-5-7-18)25-22-10-11-24(29)27(22,2)15-13-23(25)26/h3-7,16-17,22-23,25H,8-15H2,1-2H3/t22-,23-,25-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9454
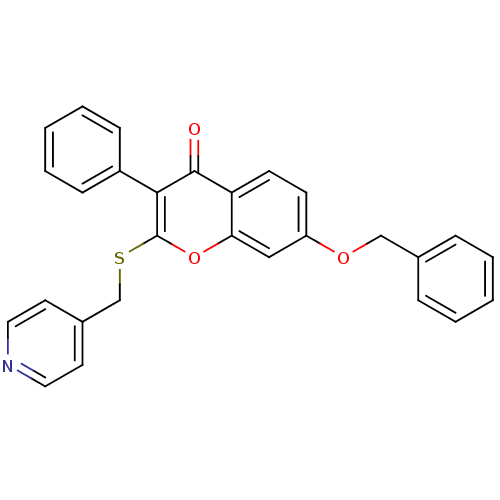
(7-(benzyloxy)-3-phenyl-2-[(pyridin-4-ylmethyl)sulf...)Show SMILES O=c1c(-c2ccccc2)c(SCc2ccncc2)oc2cc(OCc3ccccc3)ccc12 Show InChI InChI=1S/C28H21NO3S/c30-27-24-12-11-23(31-18-20-7-3-1-4-8-20)17-25(24)32-28(26(27)22-9-5-2-6-10-22)33-19-21-13-15-29-16-14-21/h1-17H,18-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome aromatase using [1beta[3H]]androst-4-ene-3,17-dione as substrate after 15 mins in presence of NADPH by liquid... |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50525262
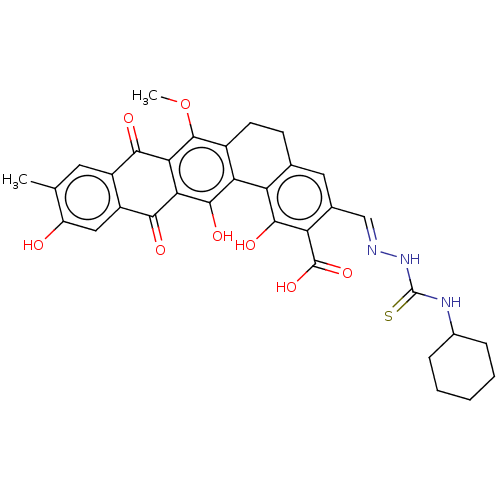
(CHEMBL4444347)Show SMILES COc1c2CCc3cc(\C=N\NC(=S)NC4CCCCC4)c(C(O)=O)c(O)c3-c2c(O)c2C(=O)c3cc(O)c(C)cc3C(=O)c12 Show InChI InChI=1S/C33H31N3O8S/c1-14-10-19-20(12-21(14)37)27(38)25-26(28(19)39)31(44-2)18-9-8-15-11-16(13-34-36-33(45)35-17-6-4-3-5-7-17)23(32(42)43)29(40)22(15)24(18)30(25)41/h10-13,17,37,40-41H,3-9H2,1-2H3,(H,42,43)(H2,35,36,45)/b34-13+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of sulfatase (unknown origin) using [3H]E1S as substrate after 20 mins by scintillation counting method |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50598557
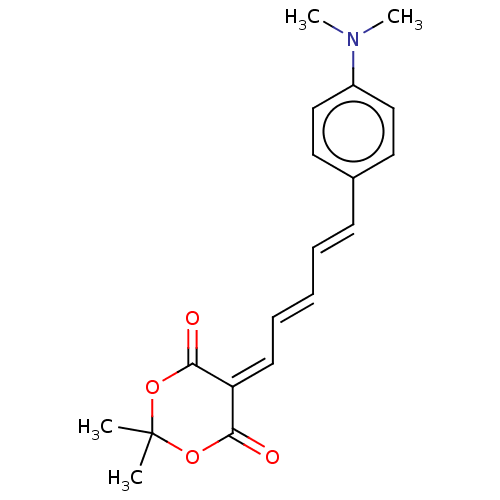
(CHEMBL5207309)Show SMILES [#6]-[#7](-[#6])-c1ccc(\[#6]=[#6]\[#6]=[#6]\[#6]=[#6]-2\[#6](=O)-[#8]C([#6])([#6])[#8]-[#6]-2=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 354 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01619
BindingDB Entry DOI: 10.7270/Q2X92GCK |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50018236
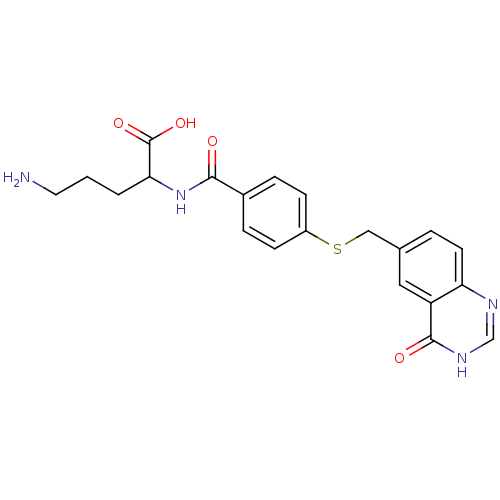
(5-Amino-2-[4-(4-oxo-3,4-dihydro-quinazolin-6-ylmet...)Show SMILES NCCCC(NC(=O)c1ccc(SCc2ccc3nc[nH]c(=O)c3c2)cc1)C(O)=O Show InChI InChI=1S/C21H22N4O4S/c22-9-1-2-18(21(28)29)25-19(26)14-4-6-15(7-5-14)30-11-13-3-8-17-16(10-13)20(27)24-12-23-17/h3-8,10,12,18H,1-2,9,11,22H2,(H,25,26)(H,28,29)(H,23,24,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase |
J Med Chem 32: 1559-65 (1989)
BindingDB Entry DOI: 10.7270/Q22806MJ |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50003468
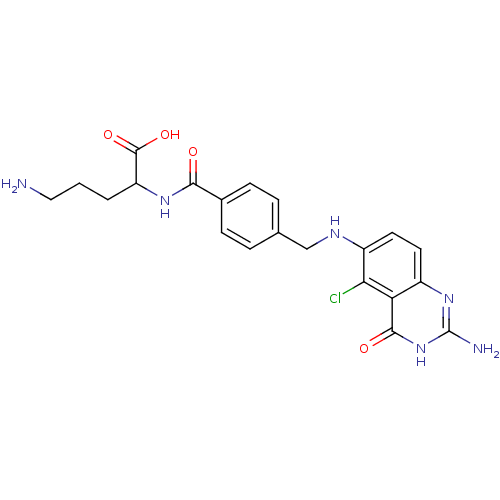
(5-Amino-2-{4-[(2-amino-5-chloro-4-oxo-3,4-dihydro-...)Show SMILES NCCCC(NC(=O)c1ccc(CNc2ccc3nc(N)[nH]c(=O)c3c2Cl)cc1)C(O)=O Show InChI InChI=1S/C21H23ClN6O4/c22-17-14(8-7-13-16(17)19(30)28-21(24)27-13)25-10-11-3-5-12(6-4-11)18(29)26-15(20(31)32)2-1-9-23/h3-8,15,25H,1-2,9-10,23H2,(H,26,29)(H,31,32)(H3,24,27,28,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against hog liver Folyl polyglutamate synthetase (FPGS) |
J Med Chem 35: 4078-85 (1992)
BindingDB Entry DOI: 10.7270/Q26M35S6 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50598558
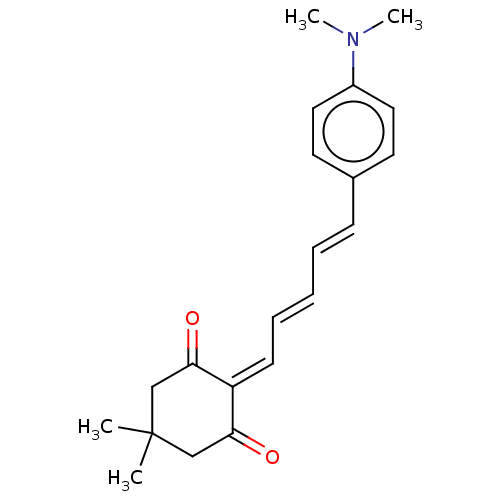
(CHEMBL5182168)Show SMILES [#6]-[#7](-[#6])-c1ccc(\[#6]=[#6]\[#6]=[#6]\[#6]=[#6]-2\[#6](=O)-[#6]C([#6])([#6])[#6]-[#6]-2=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 645 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01619
BindingDB Entry DOI: 10.7270/Q2X92GCK |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50598565
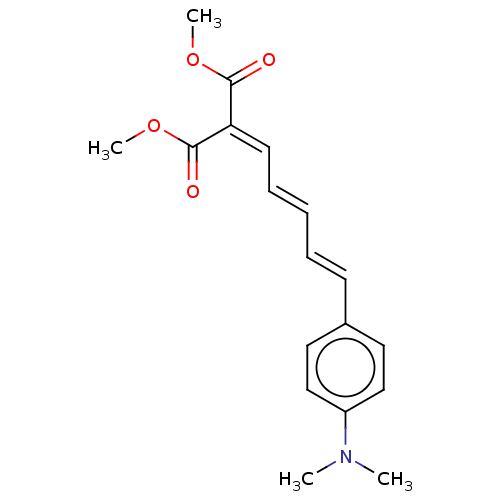
(CHEMBL5170177)Show SMILES [#6]-[#8]-[#6](=O)-[#6](=[#6]\[#6]=[#6]\[#6]=[#6]\c1ccc(cc1)-[#7](-[#6])-[#6])\[#6](=O)-[#8]-[#6] | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 652 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01619
BindingDB Entry DOI: 10.7270/Q2X92GCK |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50528740

(CHEMBL4536953)Show SMILES Nc1ccc2oc(nc2c1)N1CCN(CC1)C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C18H17BrN4O2/c19-13-3-1-12(2-4-13)17(24)22-7-9-23(10-8-22)18-21-15-11-14(20)5-6-16(15)25-18/h1-6,11H,7-10,20H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University)
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of equine serum BuChE using varying levels of butyrylthiocholine iodide as substrate measured at 2 mins interval for 10 mi... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111613
BindingDB Entry DOI: 10.7270/Q2GQ726C |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50100134

(2-(4-Dimethylamino-phenyl)-3,6-dimethyl-benzothiaz...)Show InChI InChI=1S/C17H19N2S/c1-12-5-10-15-16(11-12)20-17(19(15)4)13-6-8-14(9-7-13)18(2)3/h5-11H,1-4H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01619
BindingDB Entry DOI: 10.7270/Q2X92GCK |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50003469
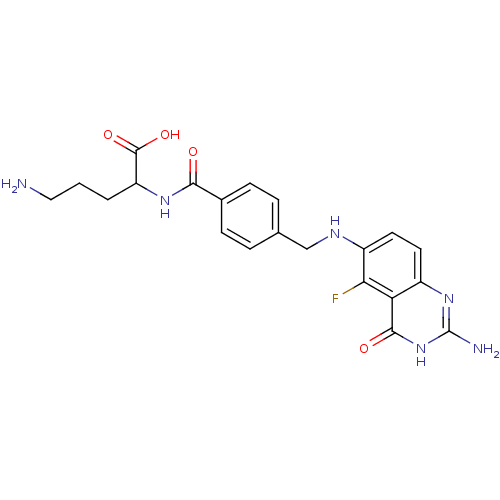
(5-Amino-2-{4-[(2-amino-5-fluoro-4-oxo-3,4-dihydro-...)Show SMILES NCCCC(NC(=O)c1ccc(CNc2ccc3nc(N)[nH]c(=O)c3c2F)cc1)C(O)=O Show InChI InChI=1S/C21H23FN6O4/c22-17-14(8-7-13-16(17)19(30)28-21(24)27-13)25-10-11-3-5-12(6-4-11)18(29)26-15(20(31)32)2-1-9-23/h3-8,15,25H,1-2,9-10,23H2,(H,26,29)(H,31,32)(H3,24,27,28,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against hog liver Folyl polyglutamate synthetase (FPGS) |
J Med Chem 35: 4078-85 (1992)
BindingDB Entry DOI: 10.7270/Q26M35S6 |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50018232
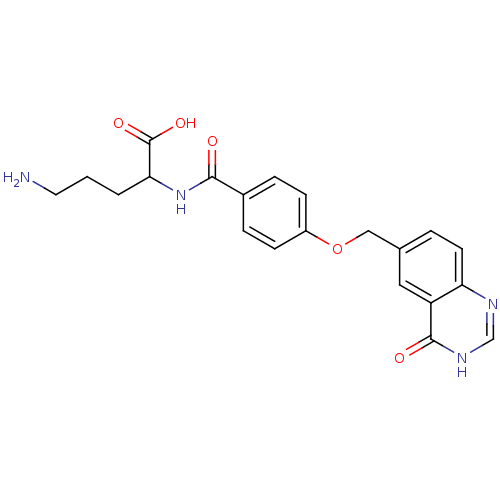
(5-Amino-2-[4-(4-oxo-3,4-dihydro-quinazolin-6-ylmet...)Show SMILES NCCCC(NC(=O)c1ccc(OCc2ccc3nc[nH]c(=O)c3c2)cc1)C(O)=O Show InChI InChI=1S/C21H22N4O5/c22-9-1-2-18(21(28)29)25-19(26)14-4-6-15(7-5-14)30-11-13-3-8-17-16(10-13)20(27)24-12-23-17/h3-8,10,12,18H,1-2,9,11,22H2,(H,25,26)(H,28,29)(H,23,24,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase |
J Med Chem 32: 1559-65 (1989)
BindingDB Entry DOI: 10.7270/Q22806MJ |
More data for this
Ligand-Target Pair | |
Trifunctional purine biosynthetic protein adenosine-3
(Mus musculus) | BDBM50002471

(5-Amino-2-{4-[(2-amino-4-oxo-2,3,4,4a,5,6,7,8-octa...)Show SMILES NCCCC(NC(=O)c1ccc(NCC2CNc3nc(N)[nH]c(=O)c3C2)cc1)C(O)=O Show InChI InChI=1S/C20H27N7O4/c21-7-1-2-15(19(30)31)25-17(28)12-3-5-13(6-4-12)23-9-11-8-14-16(24-10-11)26-20(22)27-18(14)29/h3-6,11,15,23H,1-2,7-10,21H2,(H,25,28)(H,30,31)(H4,22,24,26,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
potent inhibitor of GAR Tfase obtained from porcine |
J Med Chem 35: 2002-6 (1992)
BindingDB Entry DOI: 10.7270/Q2X9297Q |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50254908

(4-[1-(4-hydroxyphenyl)-4-methyl-5-{4-[2-(piperidin...)Show SMILES Cc1c(nn(c1-c1ccc(OCCN2CCCCC2)cc1)-c1ccc(O)cc1)-c1ccc(O)cc1 Show InChI InChI=1S/C29H31N3O3/c1-21-28(22-5-11-25(33)12-6-22)30-32(24-9-13-26(34)14-10-24)29(21)23-7-15-27(16-8-23)35-20-19-31-17-3-2-4-18-31/h5-16,33-34H,2-4,17-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of ERbeta in human MCF7 cells |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50018234
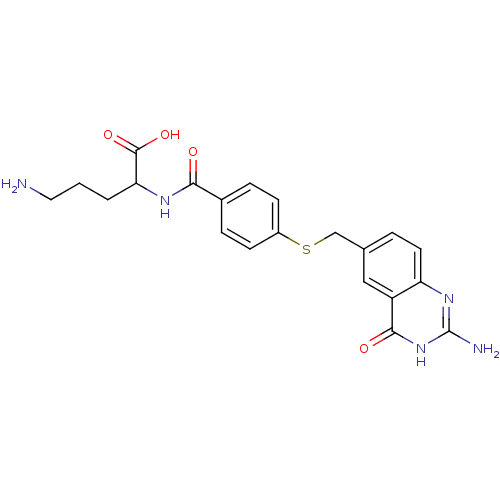
(5-Amino-2-[4-(2-amino-4-oxo-3,4-dihydro-quinazolin...)Show SMILES NCCCC(NC(=O)c1ccc(SCc2ccc3nc(N)[nH]c(=O)c3c2)cc1)C(O)=O Show InChI InChI=1S/C21H23N5O4S/c22-9-1-2-17(20(29)30)24-18(27)13-4-6-14(7-5-13)31-11-12-3-8-16-15(10-12)19(28)26-21(23)25-16/h3-8,10,17H,1-2,9,11,22H2,(H,24,27)(H,29,30)(H3,23,25,26,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase |
J Med Chem 32: 1559-65 (1989)
BindingDB Entry DOI: 10.7270/Q22806MJ |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50002473
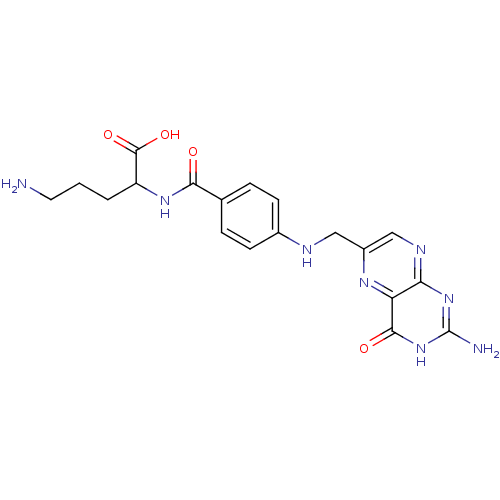
(5-Amino-2-{4-[(2-amino-4-oxo-3,4-dihydro-pteridin-...)Show SMILES NCCCC(NC(=O)c1ccc(NCc2cnc3nc(N)[nH]c(=O)c3n2)cc1)C(O)=O Show InChI InChI=1S/C19H22N8O4/c20-7-1-2-13(18(30)31)25-16(28)10-3-5-11(6-4-10)22-8-12-9-23-15-14(24-12)17(29)27-19(21)26-15/h3-6,9,13,22H,1-2,7-8,20H2,(H,25,28)(H,30,31)(H3,21,23,26,27,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for hog liver Folyl-polyglutamate synthase was evaluated |
J Med Chem 32: 1559-65 (1989)
BindingDB Entry DOI: 10.7270/Q22806MJ |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50002473

(5-Amino-2-{4-[(2-amino-4-oxo-3,4-dihydro-pteridin-...)Show SMILES NCCCC(NC(=O)c1ccc(NCc2cnc3nc(N)[nH]c(=O)c3n2)cc1)C(O)=O Show InChI InChI=1S/C19H22N8O4/c20-7-1-2-13(18(30)31)25-16(28)10-3-5-11(6-4-10)22-8-12-9-23-15-14(24-12)17(29)27-19(21)26-15/h3-6,9,13,22H,1-2,7-8,20H2,(H,25,28)(H,30,31)(H3,21,23,26,27,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against hog liver Folyl polyglutamate synthetase (FPGS) |
J Med Chem 35: 4078-85 (1992)
BindingDB Entry DOI: 10.7270/Q26M35S6 |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
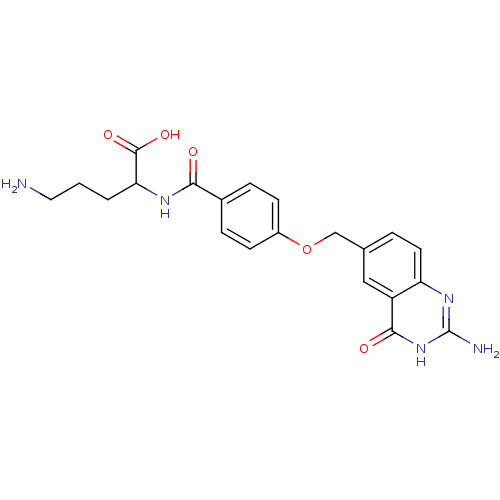
(Homo sapiens (Human)) | BDBM50018233
(5-Amino-2-[4-(2-amino-4-oxo-3,4-dihydro-quinazolin...)Show SMILES NCCCC(NC(=O)c1ccc(OCc2ccc3nc(N)[nH]c(=O)c3c2)cc1)C(O)=O Show InChI InChI=1S/C21H23N5O5/c22-9-1-2-17(20(29)30)24-18(27)13-4-6-14(7-5-13)31-11-12-3-8-16-15(10-12)19(28)26-21(23)25-16/h3-8,10,17H,1-2,9,11,22H2,(H,24,27)(H,29,30)(H3,23,25,26,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase |
J Med Chem 32: 1559-65 (1989)
BindingDB Entry DOI: 10.7270/Q22806MJ |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50002473

(5-Amino-2-{4-[(2-amino-4-oxo-3,4-dihydro-pteridin-...)Show SMILES NCCCC(NC(=O)c1ccc(NCc2cnc3nc(N)[nH]c(=O)c3n2)cc1)C(O)=O Show InChI InChI=1S/C19H22N8O4/c20-7-1-2-13(18(30)31)25-16(28)10-3-5-11(6-4-10)22-8-12-9-23-15-14(24-12)17(29)27-19(21)26-15/h3-6,9,13,22H,1-2,7-8,20H2,(H,25,28)(H,30,31)(H3,21,23,26,27,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase |
J Med Chem 35: 2002-6 (1992)
BindingDB Entry DOI: 10.7270/Q2X9297Q |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50002472

(5-Amino-2-{4-[(2-amino-5-chloro-4-oxo-3,4-dihydro-...)Show SMILES NCCCC(NC(=O)c1ccc(NCc2ccc3nc(N)[nH]c(=O)c3c2Cl)cc1)C(O)=O Show InChI InChI=1S/C21H23ClN6O4/c22-17-12(5-8-14-16(17)19(30)28-21(24)27-14)10-25-13-6-3-11(4-7-13)18(29)26-15(20(31)32)2-1-9-23/h3-8,15,25H,1-2,9-10,23H2,(H,26,29)(H,31,32)(H3,24,27,28,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against hog liver Folyl-polyglutamate synthase |
J Med Chem 35: 2002-6 (1992)
BindingDB Entry DOI: 10.7270/Q2X9297Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data