

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50146559 (But-3-enoic acid (R)-1-acetoxymethyl-1-hydroxymeth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Binding affinity for protein kinase C alpha | Bioorg Med Chem Lett 14: 2963-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.097 BindingDB Entry DOI: 10.7270/Q2GB23GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50146555 (Acetic acid (S)-4-decyloxy-1-hydroxymethyl-3-oxo-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Binding affinity for protein kinase C alpha | Bioorg Med Chem Lett 14: 2963-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.097 BindingDB Entry DOI: 10.7270/Q2GB23GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50146558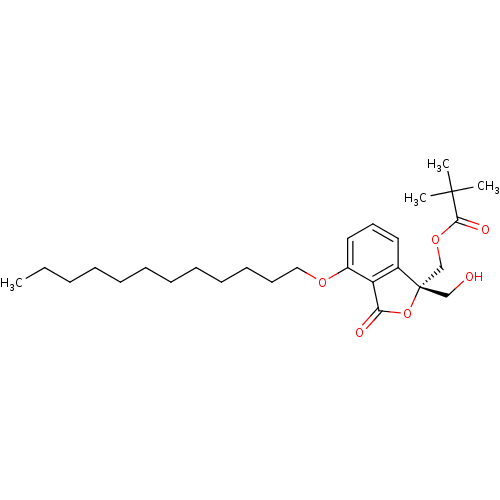 (2,2-Dimethyl-propionic acid (S)-4-dodecyloxy-1-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Binding affinity for protein kinase C alpha | Bioorg Med Chem Lett 14: 2963-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.097 BindingDB Entry DOI: 10.7270/Q2GB23GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50146556 (Acetic acid (R)-4-decyloxy-1-hydroxymethyl-3-oxo-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Binding affinity for protein kinase C alpha | Bioorg Med Chem Lett 14: 2963-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.097 BindingDB Entry DOI: 10.7270/Q2GB23GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50146554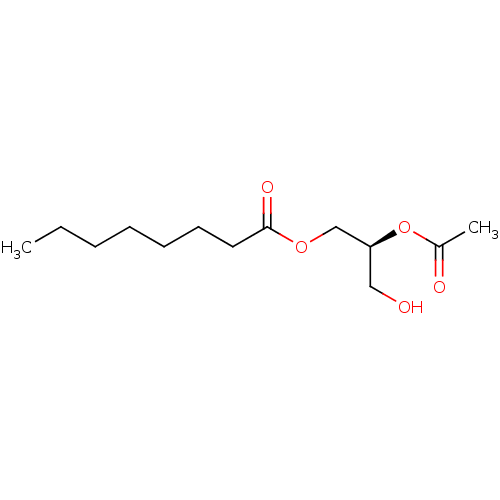 (CHEMBL102042 | Octanoic acid (S)-2-acetoxy-3-hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Binding affinity for protein kinase C alpha | Bioorg Med Chem Lett 14: 2963-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.097 BindingDB Entry DOI: 10.7270/Q2GB23GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50146561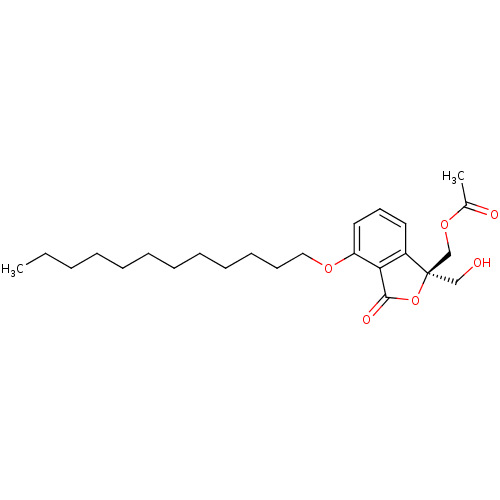 (Acetic acid (R)-4-dodecyloxy-1-hydroxymethyl-3-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Binding affinity for protein kinase C alpha | Bioorg Med Chem Lett 14: 2963-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.097 BindingDB Entry DOI: 10.7270/Q2GB23GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50146560 (Acetic acid (S)-4-dodecyloxy-1-hydroxymethyl-3-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Binding affinity for protein kinase C alpha | Bioorg Med Chem Lett 14: 2963-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.097 BindingDB Entry DOI: 10.7270/Q2GB23GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50146557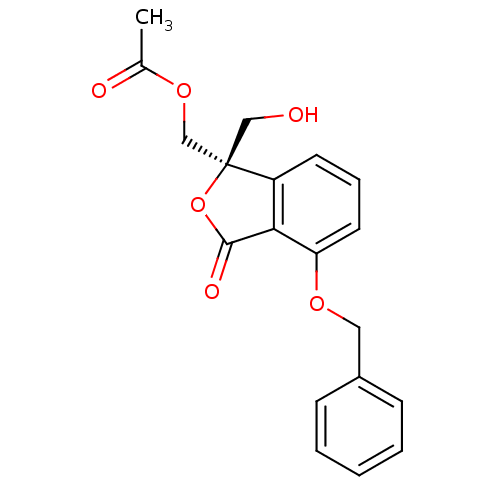 (Acetic acid (R)-4-benzyloxy-1-hydroxymethyl-3-oxo-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Binding affinity for protein kinase C alpha | Bioorg Med Chem Lett 14: 2963-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.097 BindingDB Entry DOI: 10.7270/Q2GB23GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Rattus norvegicus) | BDBM50272596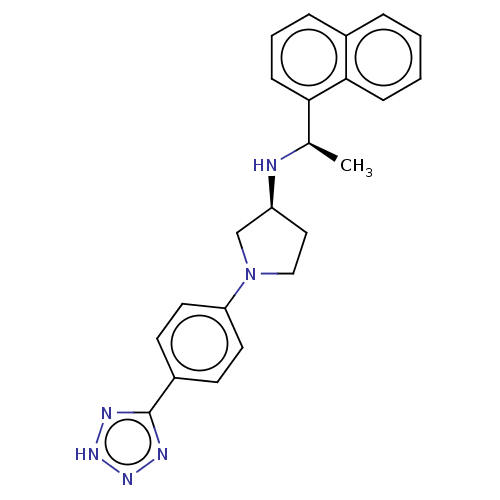 (CHEMBL4126450) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA | Bioorg Med Chem Lett 28: 2055-2060 (2018) Article DOI: 10.1016/j.bmcl.2018.04.055 BindingDB Entry DOI: 10.7270/Q2DF6TP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Rattus norvegicus) | BDBM50272602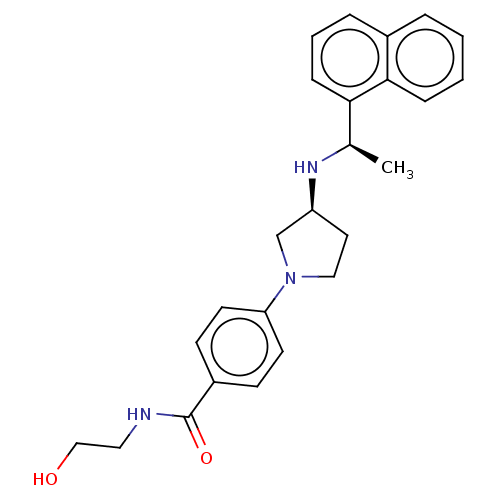 (CHEMBL4126877) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA | Bioorg Med Chem Lett 28: 2055-2060 (2018) Article DOI: 10.1016/j.bmcl.2018.04.055 BindingDB Entry DOI: 10.7270/Q2DF6TP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Rattus norvegicus) | BDBM50272605 (CHEMBL4128542) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA | Bioorg Med Chem Lett 28: 2055-2060 (2018) Article DOI: 10.1016/j.bmcl.2018.04.055 BindingDB Entry DOI: 10.7270/Q2DF6TP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Rattus norvegicus) | BDBM50272606 (CHEMBL4125917) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA | Bioorg Med Chem Lett 28: 2055-2060 (2018) Article DOI: 10.1016/j.bmcl.2018.04.055 BindingDB Entry DOI: 10.7270/Q2DF6TP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Rattus norvegicus) | BDBM50272607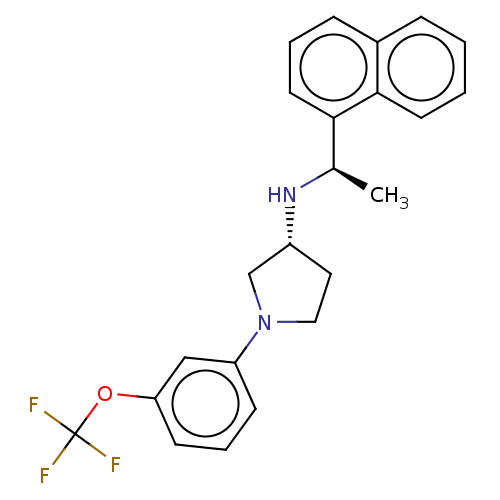 (CHEMBL4126057) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA | Bioorg Med Chem Lett 28: 2055-2060 (2018) Article DOI: 10.1016/j.bmcl.2018.04.055 BindingDB Entry DOI: 10.7270/Q2DF6TP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50128065 (CHEMBL304858 | CHEMBL324162 | N-allyl-6-(4-(4-brom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged OSC expressed in Pichia pastoris GS115 using 2,3-oxidosqualene as substrate after 90 mins by 1H NMR method | J Med Chem 61: 5047-5053 (2018) Article DOI: 10.1021/acs.jmedchem.8b00484 BindingDB Entry DOI: 10.7270/Q2NK3HN7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Extracellular calcium-sensing receptor (Rattus norvegicus) | BDBM50272604 (CHEMBL4129011) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA | Bioorg Med Chem Lett 28: 2055-2060 (2018) Article DOI: 10.1016/j.bmcl.2018.04.055 BindingDB Entry DOI: 10.7270/Q2DF6TP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Rattus norvegicus) | BDBM50261663 (CHEMBL4079960) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA | Bioorg Med Chem Lett 28: 2055-2060 (2018) Article DOI: 10.1016/j.bmcl.2018.04.055 BindingDB Entry DOI: 10.7270/Q2DF6TP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Rattus norvegicus) | BDBM50272608 (CHEMBL4125688) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA | Bioorg Med Chem Lett 28: 2055-2060 (2018) Article DOI: 10.1016/j.bmcl.2018.04.055 BindingDB Entry DOI: 10.7270/Q2DF6TP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Rattus norvegicus) | BDBM50272592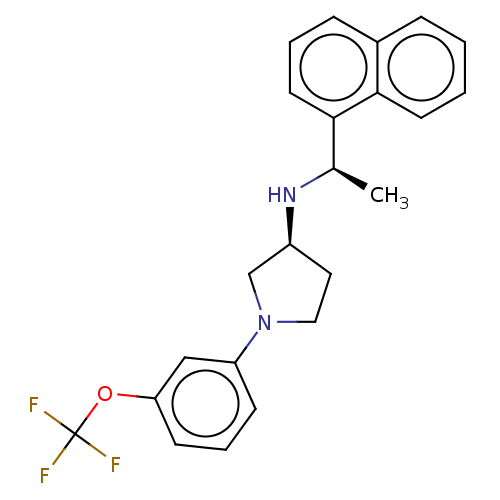 (CHEMBL4125724) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA | Bioorg Med Chem Lett 28: 2055-2060 (2018) Article DOI: 10.1016/j.bmcl.2018.04.055 BindingDB Entry DOI: 10.7270/Q2DF6TP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Rattus norvegicus) | BDBM50272595 (CHEMBL4127153) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA | Bioorg Med Chem Lett 28: 2055-2060 (2018) Article DOI: 10.1016/j.bmcl.2018.04.055 BindingDB Entry DOI: 10.7270/Q2DF6TP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50456340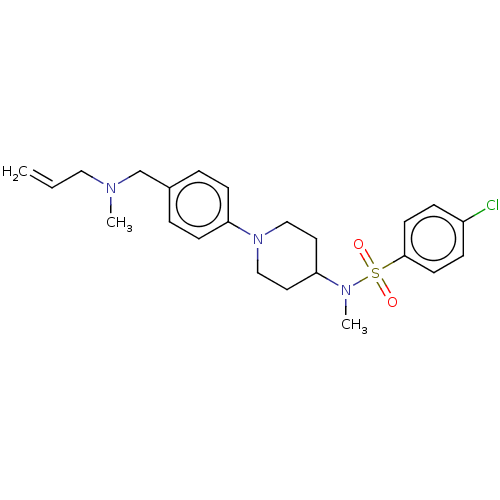 (CHEMBL4207987) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged OSC expressed in Pichia pastoris GS115 using 2,3-oxidosqualene as substrate after 90 mins by 1H NMR method | J Med Chem 61: 5047-5053 (2018) Article DOI: 10.1021/acs.jmedchem.8b00484 BindingDB Entry DOI: 10.7270/Q2NK3HN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Rattus norvegicus) | BDBM50272591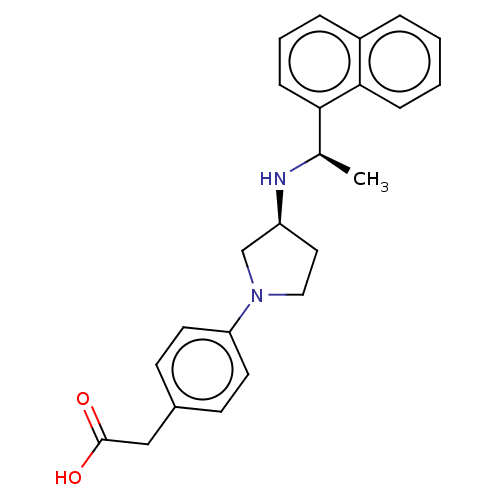 (CHEMBL4128835) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA | Bioorg Med Chem Lett 28: 2055-2060 (2018) Article DOI: 10.1016/j.bmcl.2018.04.055 BindingDB Entry DOI: 10.7270/Q2DF6TP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Rattus norvegicus) | BDBM50272594 (CHEMBL4130093) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA | Bioorg Med Chem Lett 28: 2055-2060 (2018) Article DOI: 10.1016/j.bmcl.2018.04.055 BindingDB Entry DOI: 10.7270/Q2DF6TP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50416875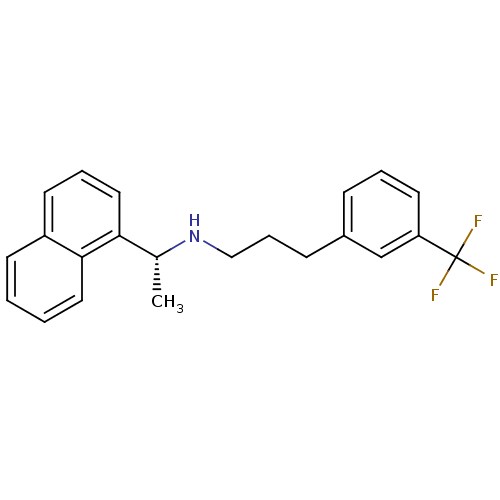 (AMG-073 | AMG073 HCL | CINACALCET | CINACALCET HYD...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP2D6 expressed in bacterial membranes using AMMC as substrate preincubated for 10 mins followed by NADPH addition m... | Bioorg Med Chem Lett 28: 2055-2060 (2018) Article DOI: 10.1016/j.bmcl.2018.04.055 BindingDB Entry DOI: 10.7270/Q2DF6TP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Rattus norvegicus) | BDBM50272603 (CHEMBL4130141) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA | Bioorg Med Chem Lett 28: 2055-2060 (2018) Article DOI: 10.1016/j.bmcl.2018.04.055 BindingDB Entry DOI: 10.7270/Q2DF6TP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Rattus norvegicus) | BDBM50272593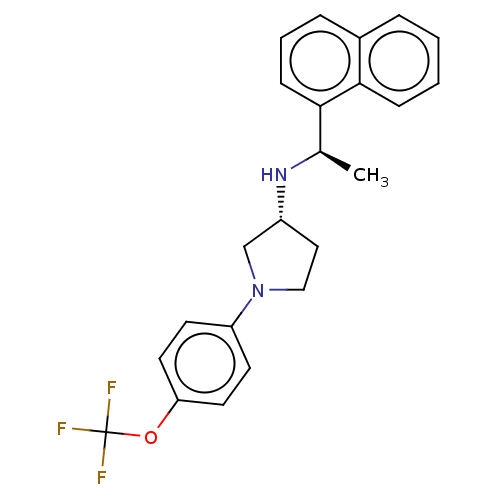 (CHEMBL4129371) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA | Bioorg Med Chem Lett 28: 2055-2060 (2018) Article DOI: 10.1016/j.bmcl.2018.04.055 BindingDB Entry DOI: 10.7270/Q2DF6TP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50456341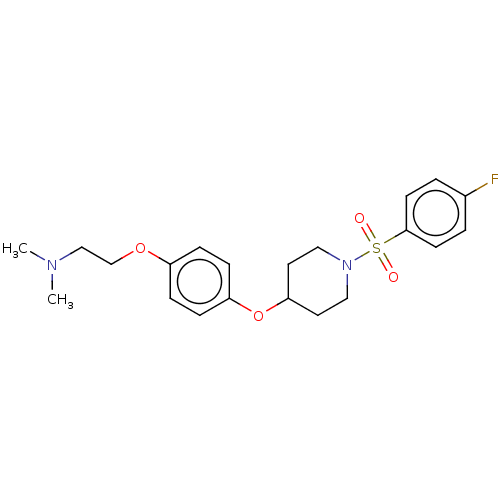 (CHEMBL4212932) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged OSC expressed in Pichia pastoris GS115 using 2,3-oxidosqualene as substrate after 90 mins by 1H NMR method | J Med Chem 61: 5047-5053 (2018) Article DOI: 10.1021/acs.jmedchem.8b00484 BindingDB Entry DOI: 10.7270/Q2NK3HN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50264003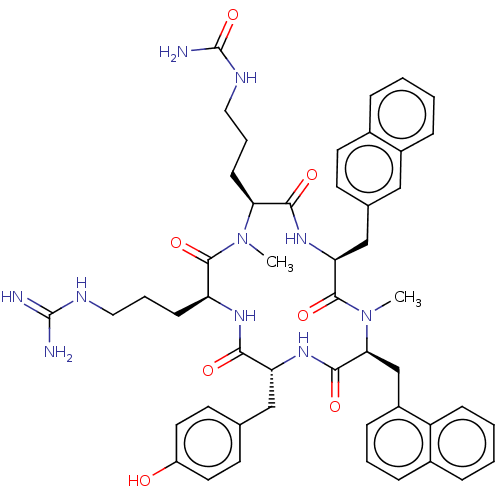 (CHEMBL4093348) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from ACKR3 (unknown origin) expressed in CHO cells after 1 hr by scintillation counting method | J Med Chem 61: 3745-3751 (2018) Article DOI: 10.1021/acs.jmedchem.8b00336 BindingDB Entry DOI: 10.7270/Q23B62M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50263987 (CHEMBL4086307) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from ACKR3 (unknown origin) expressed in CHO cells after 1 hr by scintillation counting method | J Med Chem 61: 3745-3751 (2018) Article DOI: 10.1021/acs.jmedchem.8b00336 BindingDB Entry DOI: 10.7270/Q23B62M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50456342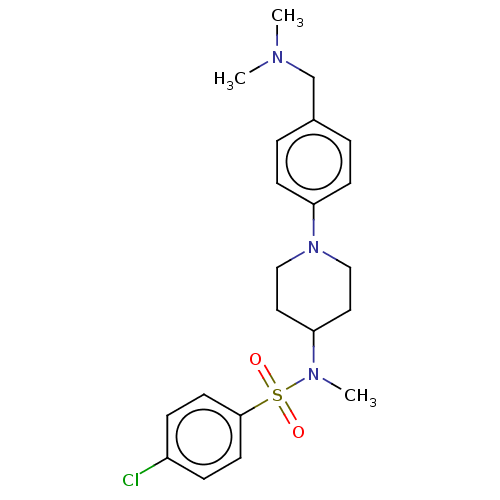 (CHEMBL4210513) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged OSC expressed in Pichia pastoris GS115 using 2,3-oxidosqualene as substrate after 90 mins by 1H NMR method | J Med Chem 61: 5047-5053 (2018) Article DOI: 10.1021/acs.jmedchem.8b00484 BindingDB Entry DOI: 10.7270/Q2NK3HN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50263973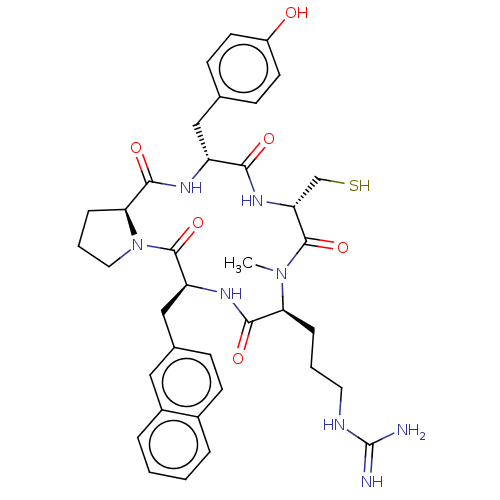 (CHEMBL4088071) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from ACKR3 (unknown origin) expressed in CHO cells after 1 hr by scintillation counting method | J Med Chem 61: 3745-3751 (2018) Article DOI: 10.1021/acs.jmedchem.8b00336 BindingDB Entry DOI: 10.7270/Q23B62M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50263986 (CHEMBL4102699) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from ACKR3 (unknown origin) expressed in CHO cells after 1 hr by scintillation counting method | J Med Chem 61: 3745-3751 (2018) Article DOI: 10.1021/acs.jmedchem.8b00336 BindingDB Entry DOI: 10.7270/Q23B62M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50264057 (CHEMBL4083207) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from ACKR3 (unknown origin) expressed in CHO cells after 1 hr by scintillation counting method | J Med Chem 61: 3745-3751 (2018) Article DOI: 10.1021/acs.jmedchem.8b00336 BindingDB Entry DOI: 10.7270/Q23B62M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50263941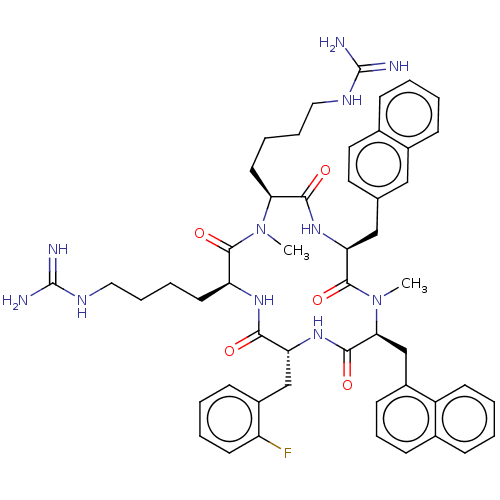 (CHEMBL4092022) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from ACKR3 (unknown origin) expressed in CHO cells after 1 hr by scintillation counting method | J Med Chem 61: 3745-3751 (2018) Article DOI: 10.1021/acs.jmedchem.8b00336 BindingDB Entry DOI: 10.7270/Q23B62M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50264098 (CHEMBL4101089) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from ACKR3 (unknown origin) expressed in CHO cells after 1 hr by scintillation counting method | J Med Chem 61: 3745-3751 (2018) Article DOI: 10.1021/acs.jmedchem.8b00336 BindingDB Entry DOI: 10.7270/Q23B62M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50263985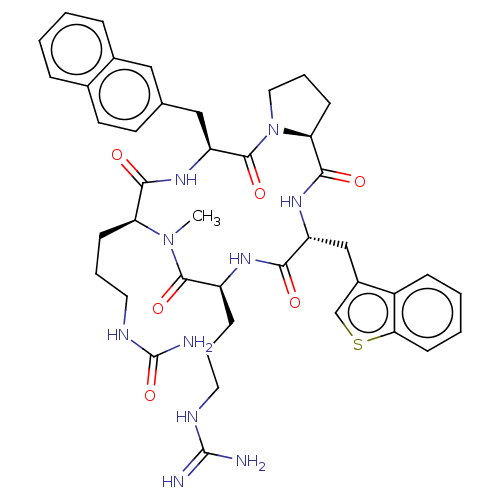 (CHEMBL4078445) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from ACKR3 (unknown origin) expressed in CHO cells after 1 hr by scintillation counting method | J Med Chem 61: 3745-3751 (2018) Article DOI: 10.1021/acs.jmedchem.8b00336 BindingDB Entry DOI: 10.7270/Q23B62M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50263977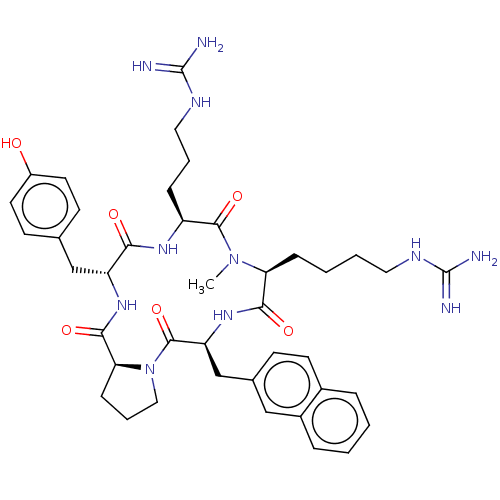 (CHEMBL4071724) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from ACKR3 (unknown origin) expressed in CHO cells after 1 hr by scintillation counting method | J Med Chem 61: 3745-3751 (2018) Article DOI: 10.1021/acs.jmedchem.8b00336 BindingDB Entry DOI: 10.7270/Q23B62M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50263992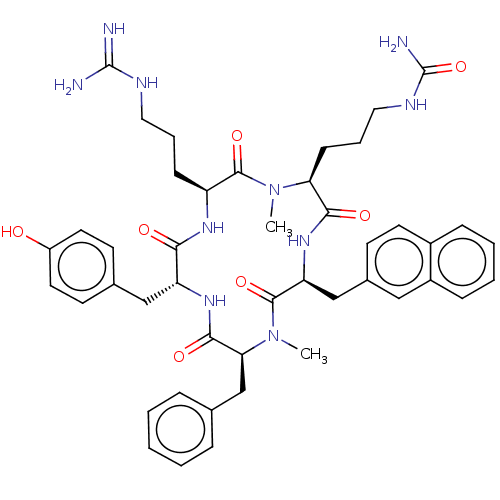 (CHEMBL4077750) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from ACKR3 (unknown origin) expressed in CHO cells after 1 hr by scintillation counting method | J Med Chem 61: 3745-3751 (2018) Article DOI: 10.1021/acs.jmedchem.8b00336 BindingDB Entry DOI: 10.7270/Q23B62M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50263984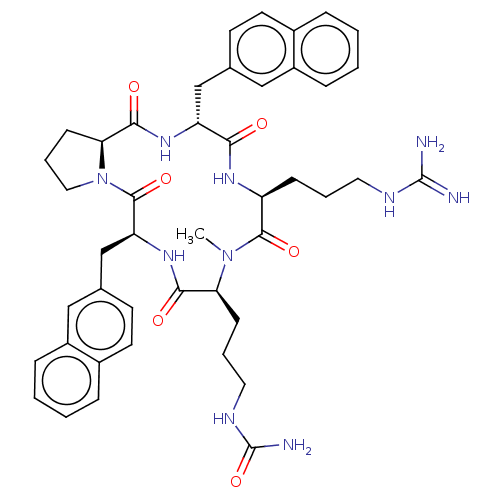 (CHEMBL4067542) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from ACKR3 (unknown origin) expressed in CHO cells after 1 hr by scintillation counting method | J Med Chem 61: 3745-3751 (2018) Article DOI: 10.1021/acs.jmedchem.8b00336 BindingDB Entry DOI: 10.7270/Q23B62M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50263982 (CHEMBL4102492) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from ACKR3 (unknown origin) expressed in CHO cells after 1 hr by scintillation counting method | J Med Chem 61: 3745-3751 (2018) Article DOI: 10.1021/acs.jmedchem.8b00336 BindingDB Entry DOI: 10.7270/Q23B62M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50263983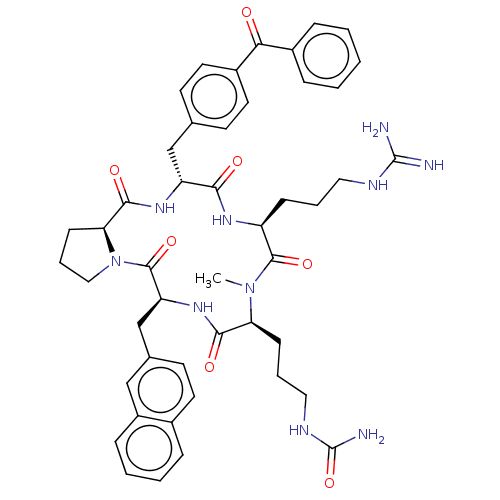 (CHEMBL4074806) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from ACKR3 (unknown origin) expressed in CHO cells after 1 hr by scintillation counting method | J Med Chem 61: 3745-3751 (2018) Article DOI: 10.1021/acs.jmedchem.8b00336 BindingDB Entry DOI: 10.7270/Q23B62M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50264062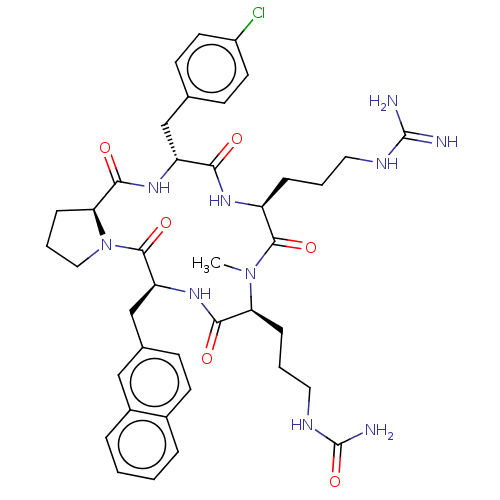 (CHEMBL4077860) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from ACKR3 (unknown origin) expressed in CHO cells after 1 hr by scintillation counting method | J Med Chem 61: 3745-3751 (2018) Article DOI: 10.1021/acs.jmedchem.8b00336 BindingDB Entry DOI: 10.7270/Q23B62M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50092206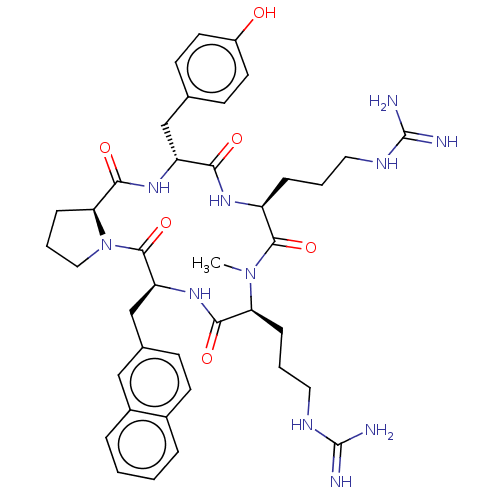 (CHEMBL3581276) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]SDF-1alpha binding to CXCR7 (unknown origin) expressed in CHO cell membranes incubated for 1 hr by radioligand displacement assay | J Med Chem 58: 5218-25 (2015) Article DOI: 10.1021/acs.jmedchem.5b00216 BindingDB Entry DOI: 10.7270/Q29P33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50263962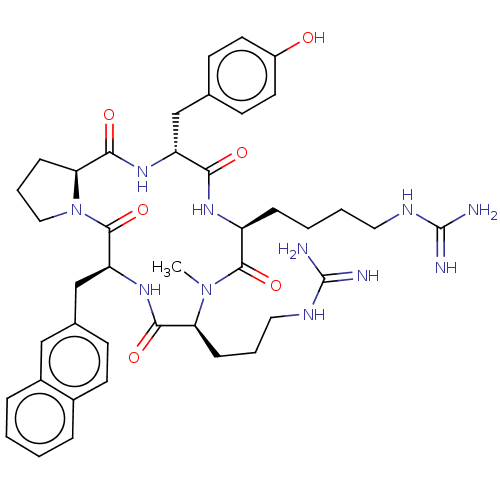 (CHEMBL4059795) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from ACKR3 (unknown origin) expressed in CHO cells after 1 hr by scintillation counting method | J Med Chem 61: 3745-3751 (2018) Article DOI: 10.1021/acs.jmedchem.8b00336 BindingDB Entry DOI: 10.7270/Q23B62M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50264006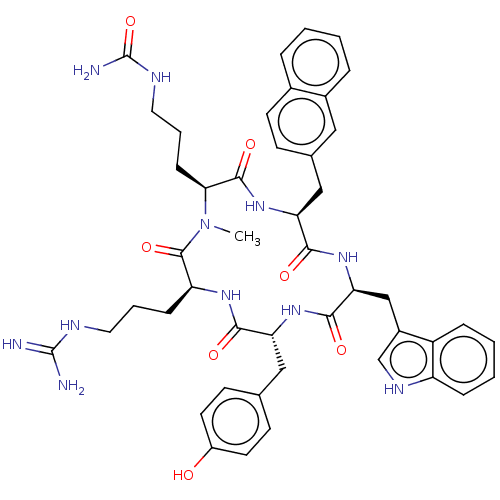 (CHEMBL4090573) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from ACKR3 (unknown origin) expressed in CHO cells after 1 hr by scintillation counting method | J Med Chem 61: 3745-3751 (2018) Article DOI: 10.1021/acs.jmedchem.8b00336 BindingDB Entry DOI: 10.7270/Q23B62M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50092206 (CHEMBL3581276) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from ACKR3 (unknown origin) expressed in CHO cells after 1 hr by scintillation counting method | J Med Chem 61: 3745-3751 (2018) Article DOI: 10.1021/acs.jmedchem.8b00336 BindingDB Entry DOI: 10.7270/Q23B62M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50264004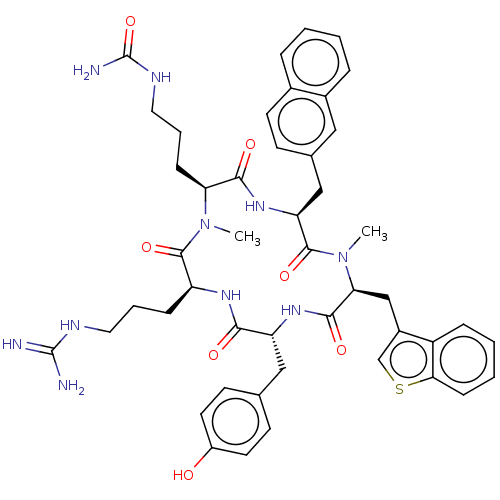 (CHEMBL4071592) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from ACKR3 (unknown origin) expressed in CHO cells after 1 hr by scintillation counting method | J Med Chem 61: 3745-3751 (2018) Article DOI: 10.1021/acs.jmedchem.8b00336 BindingDB Entry DOI: 10.7270/Q23B62M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50263978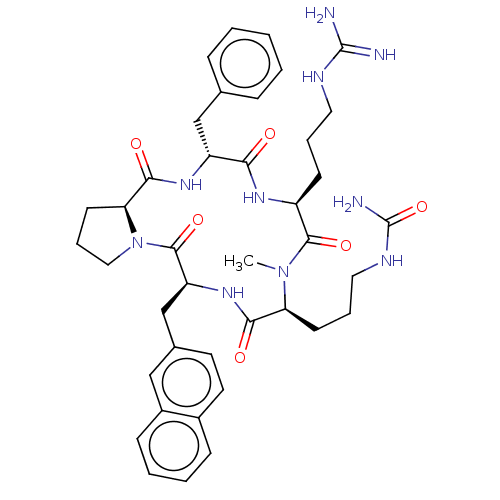 (CHEMBL4072745) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from ACKR3 (unknown origin) expressed in CHO cells after 1 hr by scintillation counting method | J Med Chem 61: 3745-3751 (2018) Article DOI: 10.1021/acs.jmedchem.8b00336 BindingDB Entry DOI: 10.7270/Q23B62M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50166106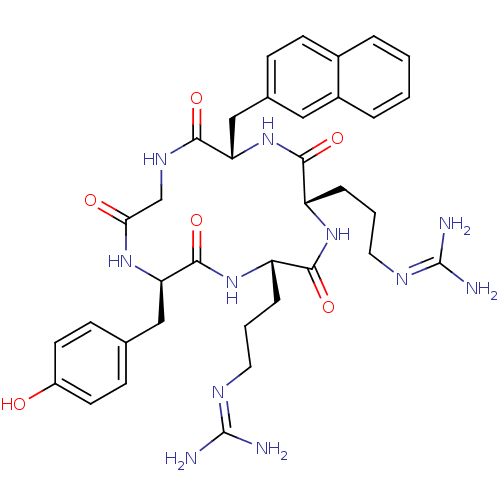 (CHEMBL436283 | N-{3-[(2S,5S,8S,14R)-5-(3-Guanidino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]SDF-1alpha binding to CXCR4 (unknown origin) expressed in HEK293 cell membranes incubated for 1 hr by radioligand displacement as... | J Med Chem 58: 5218-25 (2015) Article DOI: 10.1021/acs.jmedchem.5b00216 BindingDB Entry DOI: 10.7270/Q29P33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50263981 (CHEMBL4102116) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from ACKR3 (unknown origin) expressed in CHO cells after 1 hr by scintillation counting method | J Med Chem 61: 3745-3751 (2018) Article DOI: 10.1021/acs.jmedchem.8b00336 BindingDB Entry DOI: 10.7270/Q23B62M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50263976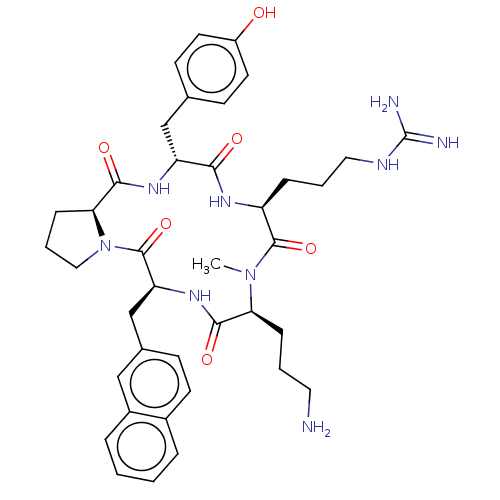 (CHEMBL4063107) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from ACKR3 (unknown origin) expressed in CHO cells after 1 hr by scintillation counting method | J Med Chem 61: 3745-3751 (2018) Article DOI: 10.1021/acs.jmedchem.8b00336 BindingDB Entry DOI: 10.7270/Q23B62M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 251 total ) | Next | Last >> |