 Found 510 hits with Last Name = 'chan' and Initial = 'ty'
Found 510 hits with Last Name = 'chan' and Initial = 'ty' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Arg8-vasopressin from human vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter |
Bioorg Med Chem Lett 19: 6018-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.050
BindingDB Entry DOI: 10.7270/Q2J9679W |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A kinase |
Bioorg Med Chem Lett 18: 1623-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.068
BindingDB Entry DOI: 10.7270/Q261115V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vasopressin V1b receptor
(RAT) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter |
Bioorg Med Chem Lett 19: 6018-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.050
BindingDB Entry DOI: 10.7270/Q2J9679W |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase |
Bioorg Med Chem Lett 18: 1623-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.068
BindingDB Entry DOI: 10.7270/Q261115V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50386351
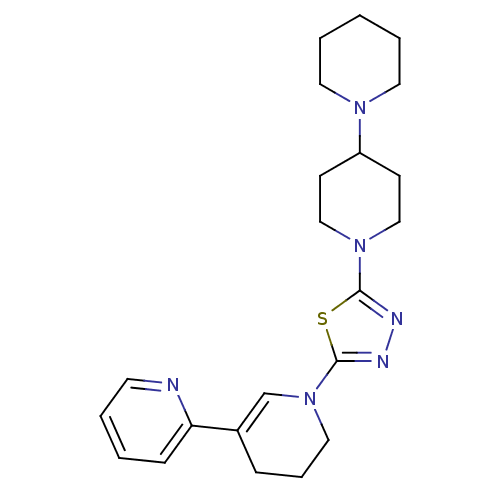
(CHEMBL2048589)Show SMILES C1CCN(CC1)C1CCN(CC1)c1nnc(s1)N1CCCC(=C1)c1ccccn1 |c:24| Show InChI InChI=1S/C22H30N6S/c1-4-12-26(13-5-1)19-9-15-27(16-10-19)21-24-25-22(29-21)28-14-6-7-18(17-28)20-8-2-3-11-23-20/h2-3,8,11,17,19H,1,4-7,9-10,12-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50386346
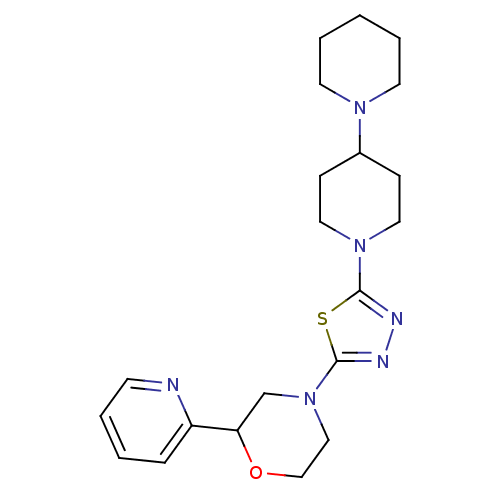
(CHEMBL2048594)Show SMILES C1CCN(CC1)C1CCN(CC1)c1nnc(s1)N1CCOC(C1)c1ccccn1 Show InChI InChI=1S/C21H30N6OS/c1-4-10-25(11-5-1)17-7-12-26(13-8-17)20-23-24-21(29-20)27-14-15-28-19(16-27)18-6-2-3-9-22-18/h2-3,6,9,17,19H,1,4-5,7-8,10-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50386346

(CHEMBL2048594)Show SMILES C1CCN(CC1)C1CCN(CC1)c1nnc(s1)N1CCOC(C1)c1ccccn1 Show InChI InChI=1S/C21H30N6OS/c1-4-10-25(11-5-1)17-7-12-26(13-8-17)20-23-24-21(29-20)27-14-15-28-19(16-27)18-6-2-3-9-22-18/h2-3,6,9,17,19H,1,4-5,7-8,10-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Mus musculus) | BDBM50386346

(CHEMBL2048594)Show SMILES C1CCN(CC1)C1CCN(CC1)c1nnc(s1)N1CCOC(C1)c1ccccn1 Show InChI InChI=1S/C21H30N6OS/c1-4-10-25(11-5-1)17-7-12-26(13-8-17)20-23-24-21(29-20)27-14-15-28-19(16-27)18-6-2-3-9-22-18/h2-3,6,9,17,19H,1,4-5,7-8,10-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50386364
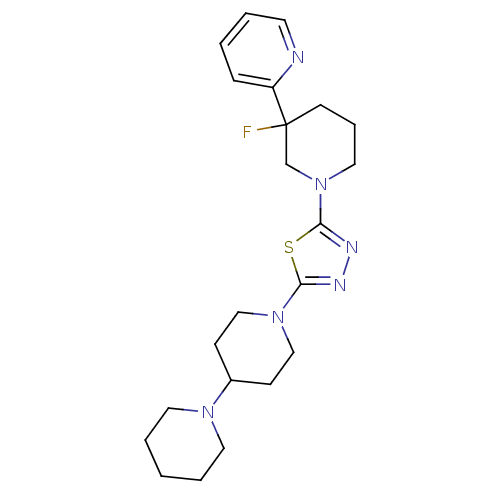
(CHEMBL2048592)Show SMILES FC1(CCCN(C1)c1nnc(s1)N1CCC(CC1)N1CCCCC1)c1ccccn1 Show InChI InChI=1S/C22H31FN6S/c23-22(19-7-2-3-11-24-19)10-6-14-29(17-22)21-26-25-20(30-21)28-15-8-18(9-16-28)27-12-4-1-5-13-27/h2-3,7,11,18H,1,4-6,8-10,12-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Mus musculus) | BDBM50386346

(CHEMBL2048594)Show SMILES C1CCN(CC1)C1CCN(CC1)c1nnc(s1)N1CCOC(C1)c1ccccn1 Show InChI InChI=1S/C21H30N6OS/c1-4-10-25(11-5-1)17-7-12-26(13-8-17)20-23-24-21(29-20)27-14-15-28-19(16-27)18-6-2-3-9-22-18/h2-3,6,9,17,19H,1,4-5,7-8,10-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora C kinase |
Bioorg Med Chem Lett 18: 1623-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.068
BindingDB Entry DOI: 10.7270/Q261115V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(Mus musculus) | BDBM50386351

(CHEMBL2048589)Show SMILES C1CCN(CC1)C1CCN(CC1)c1nnc(s1)N1CCCC(=C1)c1ccccn1 |c:24| Show InChI InChI=1S/C22H30N6S/c1-4-12-26(13-5-1)19-9-15-27(16-10-19)21-24-25-22(29-21)28-14-6-7-18(17-28)20-8-2-3-11-23-20/h2-3,8,11,17,19H,1,4-7,9-10,12-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Mus musculus) | BDBM50386364

(CHEMBL2048592)Show SMILES FC1(CCCN(C1)c1nnc(s1)N1CCC(CC1)N1CCCCC1)c1ccccn1 Show InChI InChI=1S/C22H31FN6S/c23-22(19-7-2-3-11-24-19)10-6-14-29(17-22)21-26-25-20(30-21)28-15-8-18(9-16-28)27-12-4-1-5-13-27/h2-3,7,11,18H,1,4-6,8-10,12-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50309158
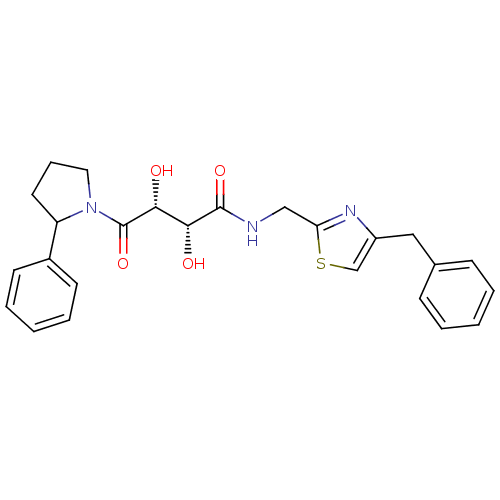
((2R,3R)-N-((4-benzylthiazol-2-yl)methyl)-2,3-dihyd...)Show SMILES O[C@H]([C@@H](O)C(=O)N1CCCC1c1ccccc1)C(=O)NCc1nc(Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C25H27N3O4S/c29-22(23(30)25(32)28-13-7-12-20(28)18-10-5-2-6-11-18)24(31)26-15-21-27-19(16-33-21)14-17-8-3-1-4-9-17/h1-6,8-11,16,20,22-23,29-30H,7,12-15H2,(H,26,31)/t20?,22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 1189-93 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.004
BindingDB Entry DOI: 10.7270/Q2BK1CFB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50386347
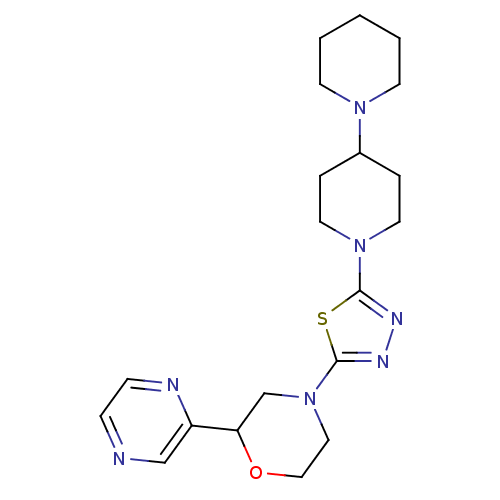
(CHEMBL2048595)Show SMILES C1CCN(CC1)C1CCN(CC1)c1nnc(s1)N1CCOC(C1)c1cnccn1 Show InChI InChI=1S/C20H29N7OS/c1-2-8-25(9-3-1)16-4-10-26(11-5-16)19-23-24-20(29-19)27-12-13-28-18(15-27)17-14-21-6-7-22-17/h6-7,14,16,18H,1-5,8-13,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50309147

((2R,3R)-4-(2-(3-chlorophenyl)pyrrolidin-1-yl)-2,3-...)Show SMILES O[C@H]([C@@H](O)C(=O)N1CCCC1c1cccc(Cl)c1)C(=O)NCCc1cccs1 |r| Show InChI InChI=1S/C20H23ClN2O4S/c21-14-5-1-4-13(12-14)16-7-2-10-23(16)20(27)18(25)17(24)19(26)22-9-8-15-6-3-11-28-15/h1,3-6,11-12,16-18,24-25H,2,7-10H2,(H,22,26)/t16?,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 1189-93 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.004
BindingDB Entry DOI: 10.7270/Q2BK1CFB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50386350
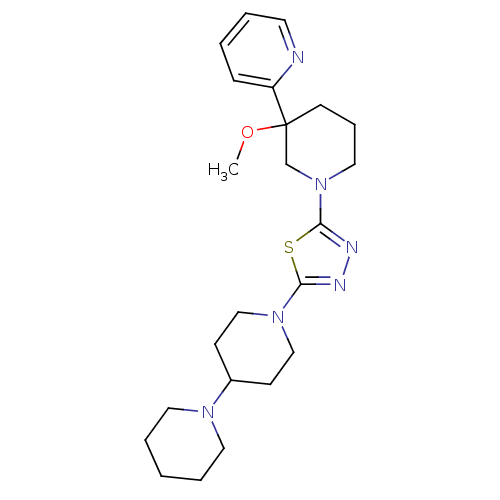
(CHEMBL2048590)Show SMILES COC1(CCCN(C1)c1nnc(s1)N1CCC(CC1)N1CCCCC1)c1ccccn1 Show InChI InChI=1S/C23H34N6OS/c1-30-23(20-8-3-4-12-24-20)11-7-15-29(18-23)22-26-25-21(31-22)28-16-9-19(10-17-28)27-13-5-2-6-14-27/h3-4,8,12,19H,2,5-7,9-11,13-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50386353
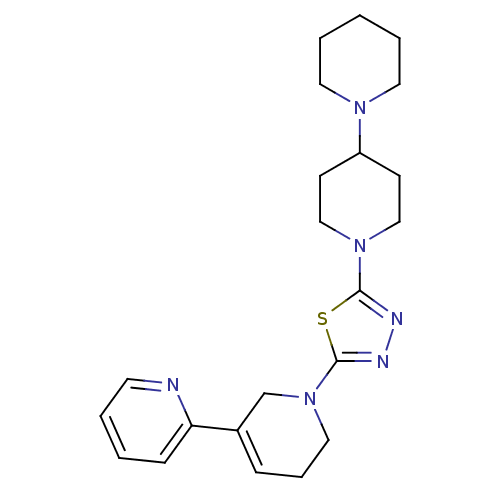
(CHEMBL2048587)Show SMILES C1CCN(CC1)C1CCN(CC1)c1nnc(s1)N1CCC=C(C1)c1ccccn1 |c:23| Show InChI InChI=1S/C22H30N6S/c1-4-12-26(13-5-1)19-9-15-27(16-10-19)21-24-25-22(29-21)28-14-6-7-18(17-28)20-8-2-3-11-23-20/h2-3,7-8,11,19H,1,4-6,9-10,12-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50309149

((2R,3R)-2,3-dihydroxy-N-methyl-4-oxo-N-(2-(thiophe...)Show SMILES CN(CCc1cccs1)C(=O)[C@H](O)[C@@H](O)C(=O)N1CCCC1c1cccc(C)c1 |r| Show InChI InChI=1S/C22H28N2O4S/c1-15-6-3-7-16(14-15)18-9-4-11-24(18)22(28)20(26)19(25)21(27)23(2)12-10-17-8-5-13-29-17/h3,5-8,13-14,18-20,25-26H,4,9-12H2,1-2H3/t18?,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 1189-93 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.004
BindingDB Entry DOI: 10.7270/Q2BK1CFB |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12985
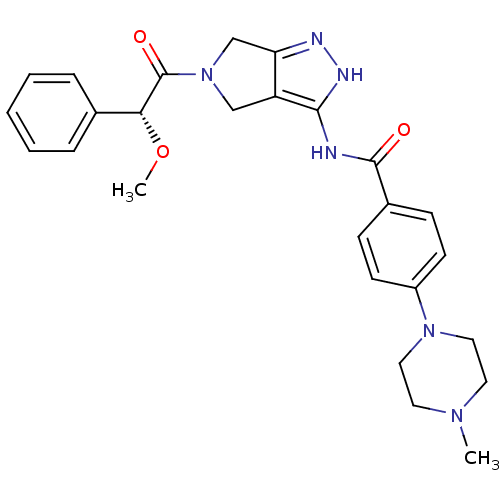
(5-Amido-pyrrolopyrazole 9d | CHEMBL402548 | N-{5-[...)Show SMILES CO[C@@H](C(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1)c1ccccc1 |r| Show InChI InChI=1S/C26H30N6O3/c1-30-12-14-31(15-13-30)20-10-8-19(9-11-20)25(33)27-24-21-16-32(17-22(21)28-29-24)26(34)23(35-2)18-6-4-3-5-7-18/h3-11,23H,12-17H2,1-2H3,(H2,27,28,29,33)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A kinase |
Bioorg Med Chem Lett 18: 1623-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.068
BindingDB Entry DOI: 10.7270/Q261115V |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50309161
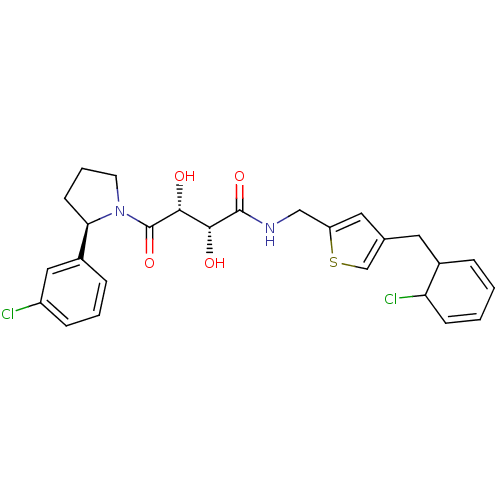
((2R,3R)-N-((4-((6-chlorocyclohexa-2,4-dienyl)methy...)Show SMILES O[C@H]([C@@H](O)C(=O)N1CCC[C@@H]1c1cccc(Cl)c1)C(=O)NCc1cc(CC2C=CC=CC2Cl)cs1 |r,c:29,31| Show InChI InChI=1S/C26H28Cl2N2O4S/c27-19-7-3-6-18(13-19)22-9-4-10-30(22)26(34)24(32)23(31)25(33)29-14-20-12-16(15-35-20)11-17-5-1-2-8-21(17)28/h1-3,5-8,12-13,15,17,21-24,31-32H,4,9-11,14H2,(H,29,33)/t17?,21?,22-,23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 1189-93 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.004
BindingDB Entry DOI: 10.7270/Q2BK1CFB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50386352
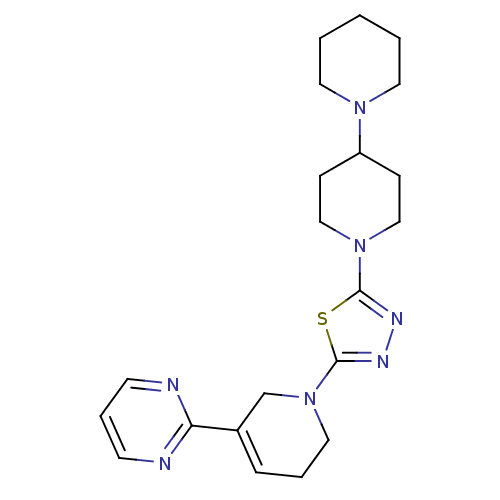
(CHEMBL2048588)Show SMILES C1CCN(CC1)C1CCN(CC1)c1nnc(s1)N1CCC=C(C1)c1ncccn1 |c:23| Show InChI InChI=1S/C21H29N7S/c1-2-11-26(12-3-1)18-7-14-27(15-8-18)20-24-25-21(29-20)28-13-4-6-17(16-28)19-22-9-5-10-23-19/h5-6,9-10,18H,1-4,7-8,11-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50386359
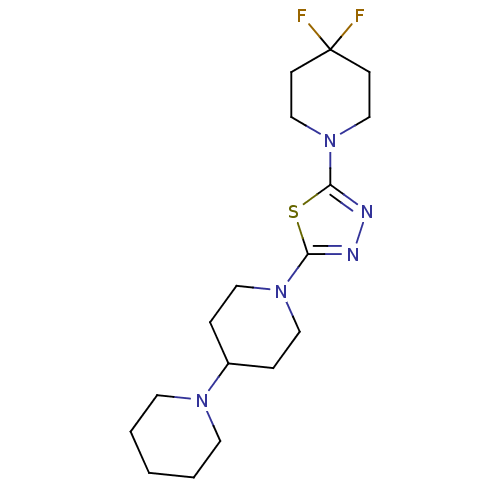
(CHEMBL2048581)Show InChI InChI=1S/C17H27F2N5S/c18-17(19)6-12-24(13-7-17)16-21-20-15(25-16)23-10-4-14(5-11-23)22-8-2-1-3-9-22/h14H,1-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Mus musculus) | BDBM50386347

(CHEMBL2048595)Show SMILES C1CCN(CC1)C1CCN(CC1)c1nnc(s1)N1CCOC(C1)c1cnccn1 Show InChI InChI=1S/C20H29N7OS/c1-2-8-25(9-3-1)16-4-10-26(11-5-16)19-23-24-20(29-19)27-12-13-28-18(15-27)17-14-21-6-7-22-17/h6-7,14,16,18H,1-5,8-13,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50299342

(CHEMBL1204126 | CHEMBL1204403 | CHEMBL572709 | N-(...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(CCS(=O)(=O)N2CCC(CC2)NCC2CCCCC2)CCc2ccccc12 Show InChI InChI=1S/C31H45N3O6S2/c1-39-28-14-15-31(30(22-28)40-2)42(37,38)34-27(13-12-25-10-6-7-11-29(25)34)18-21-41(35,36)33-19-16-26(17-20-33)32-23-24-8-4-3-5-9-24/h6-7,10-11,14-15,22,24,26-27,32H,3-5,8-9,12-13,16-21,23H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter |
Bioorg Med Chem Lett 19: 6018-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.050
BindingDB Entry DOI: 10.7270/Q2J9679W |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Mus musculus) | BDBM50386359

(CHEMBL2048581)Show InChI InChI=1S/C17H27F2N5S/c18-17(19)6-12-24(13-7-17)16-21-20-15(25-16)23-10-4-14(5-11-23)22-8-2-1-3-9-22/h14H,1-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter |
Bioorg Med Chem Lett 19: 6018-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.050
BindingDB Entry DOI: 10.7270/Q2J9679W |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50309167

((2R,3R)-2,3-dihydroxy-4-oxo-4-((R)-2-phenylpyrroli...)Show SMILES O[C@H]([C@@H](O)C(=O)N1CCC[C@@H]1c1ccccc1)C(=O)NCCc1cccs1 |r| Show InChI InChI=1S/C20H24N2O4S/c23-17(19(25)21-11-10-15-8-5-13-27-15)18(24)20(26)22-12-4-9-16(22)14-6-2-1-3-7-14/h1-3,5-8,13,16-18,23-24H,4,9-12H2,(H,21,25)/t16-,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 1189-93 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.004
BindingDB Entry DOI: 10.7270/Q2BK1CFB |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50299342

(CHEMBL1204126 | CHEMBL1204403 | CHEMBL572709 | N-(...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(CCS(=O)(=O)N2CCC(CC2)NCC2CCCCC2)CCc2ccccc12 Show InChI InChI=1S/C31H45N3O6S2/c1-39-28-14-15-31(30(22-28)40-2)42(37,38)34-27(13-12-25-10-6-7-11-29(25)34)18-21-41(35,36)33-19-16-26(17-20-33)32-23-24-8-4-3-5-9-24/h6-7,10-11,14-15,22,24,26-27,32H,3-5,8-9,12-13,16-21,23H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter |
Bioorg Med Chem Lett 19: 6018-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.050
BindingDB Entry DOI: 10.7270/Q2J9679W |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Arg8-vasopressin from human vasopressin V1a receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter |
Bioorg Med Chem Lett 19: 6018-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.050
BindingDB Entry DOI: 10.7270/Q2J9679W |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50309160

((2R,3R)-N-((4-benzyloxazol-2-yl)methyl)-2,3-dihydr...)Show SMILES O[C@H]([C@@H](O)C(=O)N1CCCC1c1ccccc1)C(=O)NCc1nc(Cc2ccccc2)co1 |r| Show InChI InChI=1S/C25H27N3O5/c29-22(23(30)25(32)28-13-7-12-20(28)18-10-5-2-6-11-18)24(31)26-15-21-27-19(16-33-21)14-17-8-3-1-4-9-17/h1-6,8-11,16,20,22-23,29-30H,7,12-15H2,(H,26,31)/t20?,22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 1189-93 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.004
BindingDB Entry DOI: 10.7270/Q2BK1CFB |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50299349

(1-(2-(1-(2,4-dimethoxyphenylsulfonyl)-1,2,3,4-tetr...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(CCS(=O)(=O)N2CCC(CC2)NCc2cccs2)CCc2ccccc12 Show InChI InChI=1S/C29H37N3O6S3/c1-37-25-11-12-29(28(20-25)38-2)41(35,36)32-24(10-9-22-6-3-4-8-27(22)32)15-19-40(33,34)31-16-13-23(14-17-31)30-21-26-7-5-18-39-26/h3-8,11-12,18,20,23-24,30H,9-10,13-17,19,21H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter |
Bioorg Med Chem Lett 19: 6018-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.050
BindingDB Entry DOI: 10.7270/Q2J9679W |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50299358
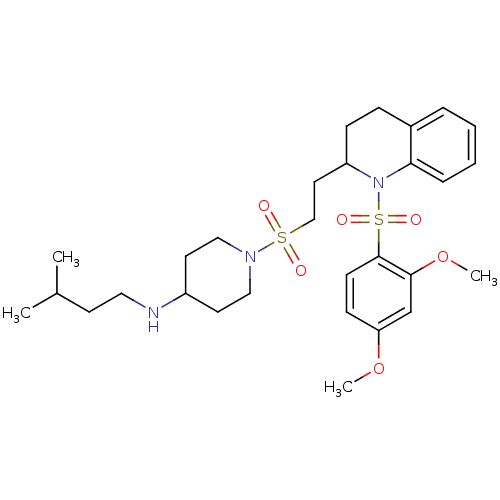
(1-(2-(1-(2,4-dimethoxyphenylsulfonyl)-1,2,3,4-tetr...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(CCS(=O)(=O)N2CCC(CC2)NCCC(C)C)CCc2ccccc12 Show InChI InChI=1S/C29H43N3O6S2/c1-22(2)13-17-30-24-14-18-31(19-15-24)39(33,34)20-16-25-10-9-23-7-5-6-8-27(23)32(25)40(35,36)29-12-11-26(37-3)21-28(29)38-4/h5-8,11-12,21-22,24-25,30H,9-10,13-20H2,1-4H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter |
Bioorg Med Chem Lett 19: 6018-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.050
BindingDB Entry DOI: 10.7270/Q2J9679W |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 10
(Homo sapiens (Human)) | BDBM50309147

((2R,3R)-4-(2-(3-chlorophenyl)pyrrolidin-1-yl)-2,3-...)Show SMILES O[C@H]([C@@H](O)C(=O)N1CCCC1c1cccc(Cl)c1)C(=O)NCCc1cccs1 |r| Show InChI InChI=1S/C20H23ClN2O4S/c21-14-5-1-4-13(12-14)16-7-2-10-23(16)20(27)18(25)17(24)19(26)22-9-8-15-6-3-11-28-15/h1,3-6,11-12,16-18,24-25H,2,7-10H2,(H,22,26)/t16?,17-,18-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of ADAM10 |
Bioorg Med Chem Lett 20: 1189-93 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.004
BindingDB Entry DOI: 10.7270/Q2BK1CFB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50386362
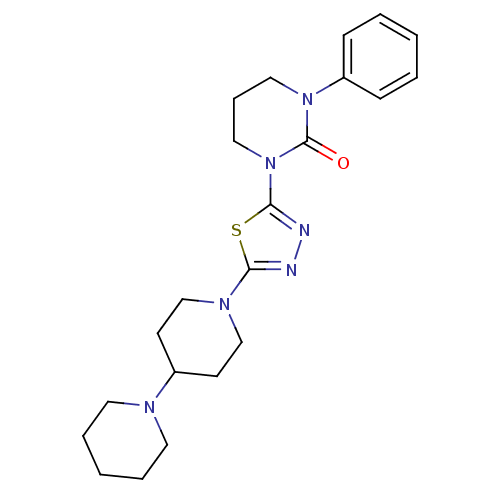
(CHEMBL2048412)Show SMILES O=C1N(CCCN1c1ccccc1)c1nnc(s1)N1CCC(CC1)N1CCCCC1 Show InChI InChI=1S/C22H30N6OS/c29-22-27(19-8-3-1-4-9-19)14-7-15-28(22)21-24-23-20(30-21)26-16-10-18(11-17-26)25-12-5-2-6-13-25/h1,3-4,8-9,18H,2,5-7,10-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Mus musculus) | BDBM50386362

(CHEMBL2048412)Show SMILES O=C1N(CCCN1c1ccccc1)c1nnc(s1)N1CCC(CC1)N1CCCCC1 Show InChI InChI=1S/C22H30N6OS/c29-22-27(19-8-3-1-4-9-19)14-7-15-28(22)21-24-23-20(30-21)26-16-10-18(11-17-26)25-12-5-2-6-13-25/h1,3-4,8-9,18H,2,5-7,10-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50299350

(1-(2-(1-(2,4-dimethoxyphenylsulfonyl)-1,2,3,4-tetr...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(CCS(=O)(=O)N2CCC(CC2)NCc2ccsc2)CCc2ccccc12 Show InChI InChI=1S/C29H37N3O6S3/c1-37-26-9-10-29(28(19-26)38-2)41(35,36)32-25(8-7-23-5-3-4-6-27(23)32)14-18-40(33,34)31-15-11-24(12-16-31)30-20-22-13-17-39-21-22/h3-6,9-10,13,17,19,21,24-25,30H,7-8,11-12,14-16,18,20H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Arg8-vasopressin from human vasopressin V1a receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter |
Bioorg Med Chem Lett 19: 6018-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.050
BindingDB Entry DOI: 10.7270/Q2J9679W |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50309150

((2R,3R)-2,3-dihydroxy-4-(2-(3-methoxyphenyl)pyrrol...)Show SMILES COc1cccc(c1)C1CCCN1C(=O)[C@H](O)[C@@H](O)C(=O)N(C)CCc1cccs1 |r| Show InChI InChI=1S/C22H28N2O5S/c1-23(12-10-17-8-5-13-30-17)21(27)19(25)20(26)22(28)24-11-4-9-18(24)15-6-3-7-16(14-15)29-2/h3,5-8,13-14,18-20,25-26H,4,9-12H2,1-2H3/t18?,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 1189-93 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.004
BindingDB Entry DOI: 10.7270/Q2BK1CFB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50386363
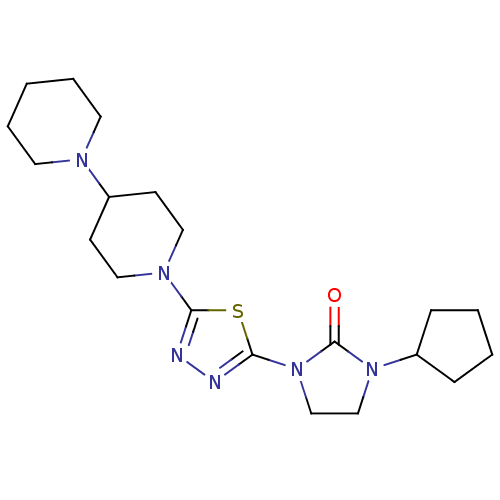
(CHEMBL2048411)Show SMILES O=C1N(CCN1c1nnc(s1)N1CCC(CC1)N1CCCCC1)C1CCCC1 Show InChI InChI=1S/C20H32N6OS/c27-20-25(17-6-2-3-7-17)14-15-26(20)19-22-21-18(28-19)24-12-8-16(9-13-24)23-10-4-1-5-11-23/h16-17H,1-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50386346

(CHEMBL2048594)Show SMILES C1CCN(CC1)C1CCN(CC1)c1nnc(s1)N1CCOC(C1)c1ccccn1 Show InChI InChI=1S/C21H30N6OS/c1-4-10-25(11-5-1)17-7-12-26(13-8-17)20-23-24-21(29-20)27-14-15-28-19(16-27)18-6-2-3-9-22-18/h2-3,6,9,17,19H,1,4-5,7-8,10-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Mus musculus) | BDBM50386354
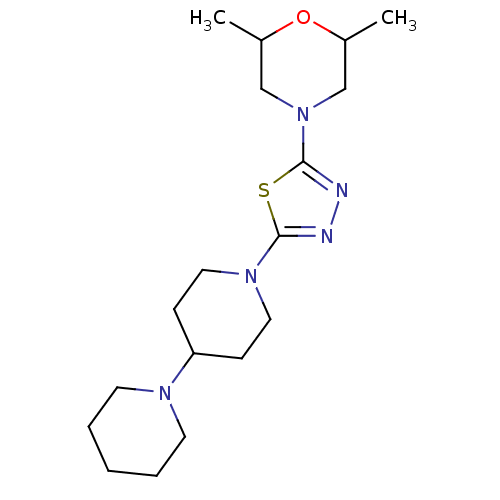
(CHEMBL2048586)Show InChI InChI=1S/C18H31N5OS/c1-14-12-23(13-15(2)24-14)18-20-19-17(25-18)22-10-6-16(7-11-22)21-8-4-3-5-9-21/h14-16H,3-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50299358

(1-(2-(1-(2,4-dimethoxyphenylsulfonyl)-1,2,3,4-tetr...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(CCS(=O)(=O)N2CCC(CC2)NCCC(C)C)CCc2ccccc12 Show InChI InChI=1S/C29H43N3O6S2/c1-22(2)13-17-30-24-14-18-31(19-15-24)39(33,34)20-16-25-10-9-23-7-5-6-8-27(23)32(25)40(35,36)29-12-11-26(37-3)21-28(29)38-4/h5-8,11-12,21-22,24-25,30H,9-10,13-20H2,1-4H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Arg8-vasopressin from human vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter |
Bioorg Med Chem Lett 19: 6018-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.050
BindingDB Entry DOI: 10.7270/Q2J9679W |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50386357
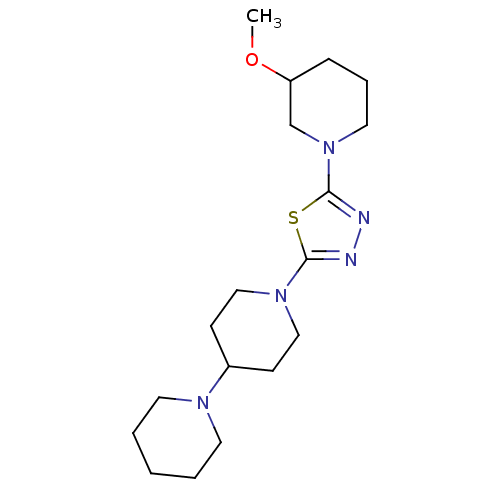
(CHEMBL2048583)Show InChI InChI=1S/C18H31N5OS/c1-24-16-6-5-11-23(14-16)18-20-19-17(25-18)22-12-7-15(8-13-22)21-9-3-2-4-10-21/h15-16H,2-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50309151

((2R,3R)-4-(2-(3-(dimethylamino)phenyl)pyrrolidin-1...)Show SMILES CN(C)c1cccc(c1)C1CCCN1C(=O)[C@H](O)[C@@H](O)C(=O)NCCc1cccs1 |r| Show InChI InChI=1S/C22H29N3O4S/c1-24(2)16-7-3-6-15(14-16)18-9-4-12-25(18)22(29)20(27)19(26)21(28)23-11-10-17-8-5-13-30-17/h3,5-8,13-14,18-20,26-27H,4,9-12H2,1-2H3,(H,23,28)/t18?,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 1189-93 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.004
BindingDB Entry DOI: 10.7270/Q2BK1CFB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Mus musculus) | BDBM50386352

(CHEMBL2048588)Show SMILES C1CCN(CC1)C1CCN(CC1)c1nnc(s1)N1CCC=C(C1)c1ncccn1 |c:23| Show InChI InChI=1S/C21H29N7S/c1-2-11-26(12-3-1)18-7-14-27(15-8-18)20-24-25-21(29-20)28-13-4-6-17(16-28)19-22-9-5-10-23-19/h5-6,9-10,18H,1-4,7-8,11-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50299342

(CHEMBL1204126 | CHEMBL1204403 | CHEMBL572709 | N-(...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(CCS(=O)(=O)N2CCC(CC2)NCC2CCCCC2)CCc2ccccc12 Show InChI InChI=1S/C31H45N3O6S2/c1-39-28-14-15-31(30(22-28)40-2)42(37,38)34-27(13-12-25-10-6-7-11-29(25)34)18-21-41(35,36)33-19-16-26(17-20-33)32-23-24-8-4-3-5-9-24/h6-7,10-11,14-15,22,24,26-27,32H,3-5,8-9,12-13,16-21,23H2,1-2H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Arg8-vasopressin from human vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter |
Bioorg Med Chem Lett 19: 6018-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.050
BindingDB Entry DOI: 10.7270/Q2J9679W |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Mus musculus) | BDBM50386357

(CHEMBL2048583)Show InChI InChI=1S/C18H31N5OS/c1-24-16-6-5-11-23(14-16)18-20-19-17(25-18)22-12-7-15(8-13-22)21-9-3-2-4-10-21/h15-16H,2-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50386356
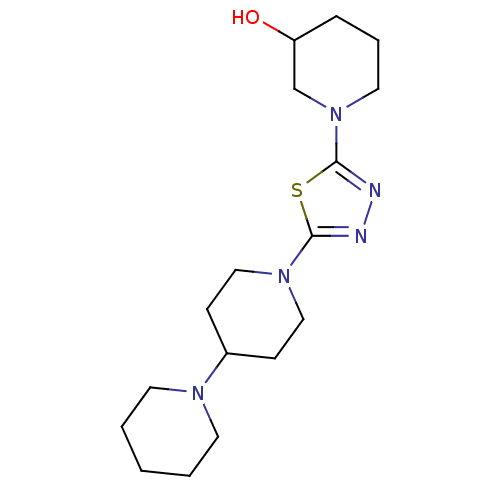
(CHEMBL2048584)Show InChI InChI=1S/C17H29N5OS/c23-15-5-4-10-22(13-15)17-19-18-16(24-17)21-11-6-14(7-12-21)20-8-2-1-3-9-20/h14-15,23H,1-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Mus musculus) | BDBM50333504
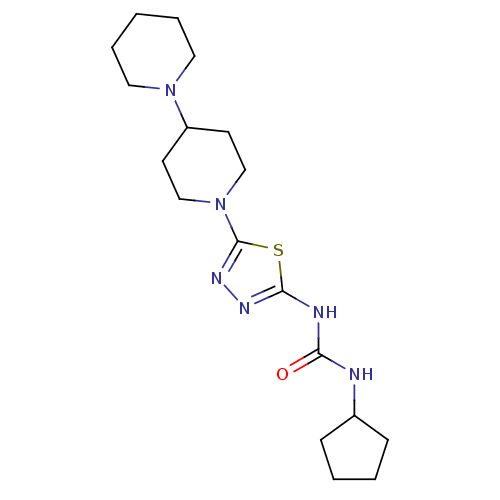
(1-(5-(1,4'-bipiperidin-1'-yl)-1,3,4-thiadiazol-2-y...)Show InChI InChI=1S/C18H30N6OS/c25-16(19-14-6-2-3-7-14)20-17-21-22-18(26-17)24-12-8-15(9-13-24)23-10-4-1-5-11-23/h14-15H,1-13H2,(H2,19,20,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting |
ACS Med Chem Lett 3: 198-202 (2012)
Article DOI: 10.1021/ml200250t
BindingDB Entry DOI: 10.7270/Q2736S0M |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50309157
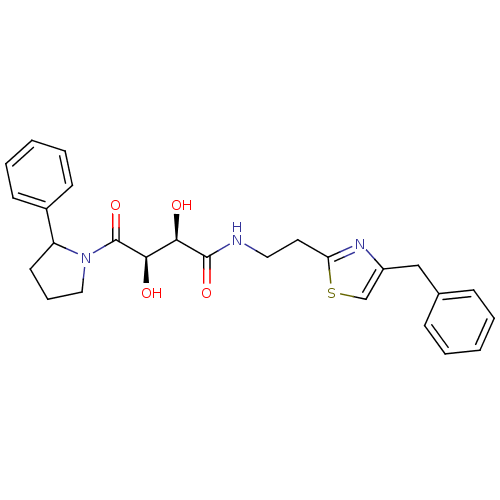
((2R,3R)-N-(2-(4-benzylthiazol-2-yl)ethyl)-2,3-dihy...)Show SMILES O[C@H]([C@@H](O)C(=O)N1CCCC1c1ccccc1)C(=O)NCCc1nc(Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C26H29N3O4S/c30-23(24(31)26(33)29-15-7-12-21(29)19-10-5-2-6-11-19)25(32)27-14-13-22-28-20(17-34-22)16-18-8-3-1-4-9-18/h1-6,8-11,17,21,23-24,30-31H,7,12-16H2,(H,27,32)/t21?,23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 1189-93 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.004
BindingDB Entry DOI: 10.7270/Q2BK1CFB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data