

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50181840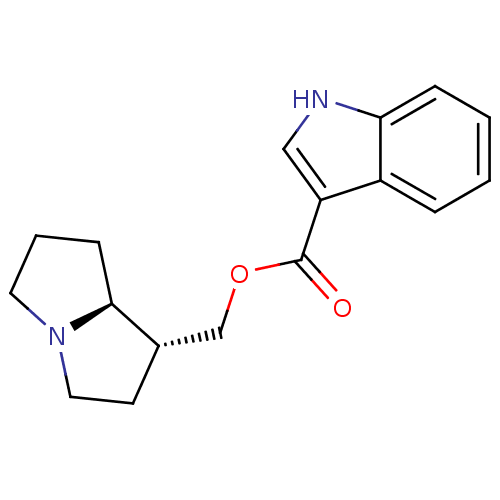 ((1R,7aS)-hexahydro-1H-pyrrolizin-1-ylmethyl 1-Meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50181844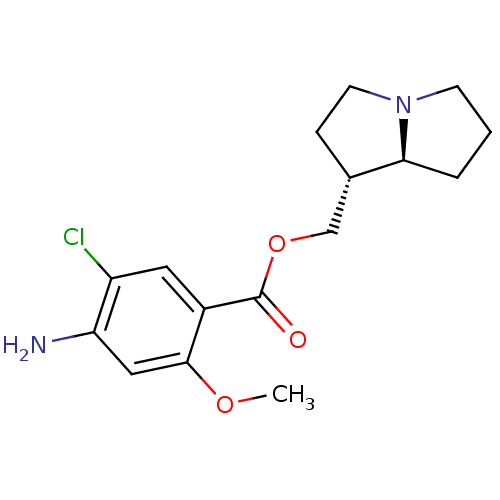 ((1R,7aS)-hexahydro-1H-pyrrolizin-1-ylmethyl 4-amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Agonist activity at recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 2 hrs by [35S]GTP-gammaS binding assay | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A (RAT) | BDBM50056419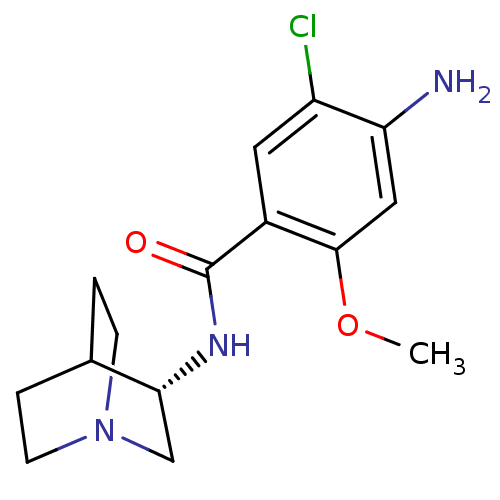 (4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl)-5-chloro-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vivo for the antagonistic activity towards 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 2: 1613-1618 (1992) Article DOI: 10.1016/S0960-894X(00)80441-2 BindingDB Entry DOI: 10.7270/Q2J67HFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50368604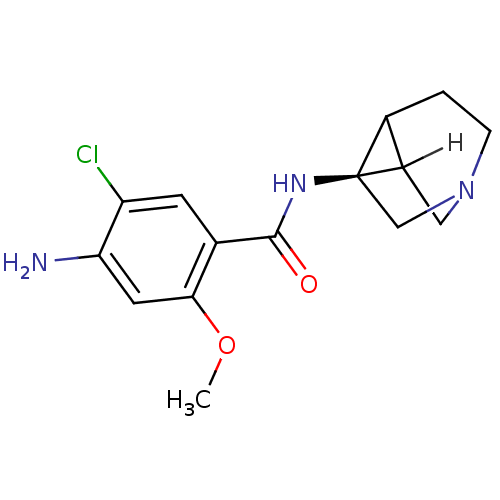 (CHEMBL1907770) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]GR-65630 binding to 5-hydroxytryptamine 3 receptor | J Med Chem 35: 1486-9 (1992) BindingDB Entry DOI: 10.7270/Q2CC11B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50181845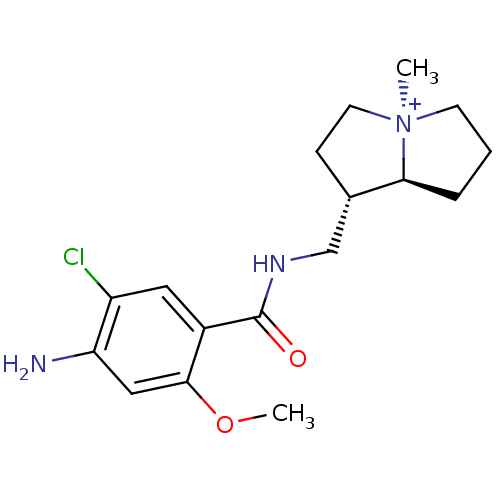 ((1S,7aS)-1-{[(4-amino-5-chloro-2-methoxybenzoyl)am...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50181837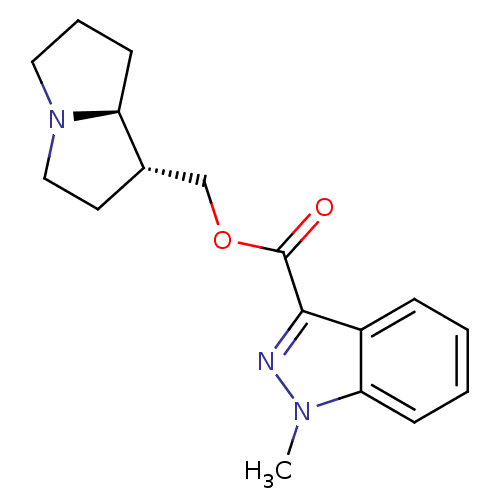 ((1S,7aR)-hexahydro-1H-pyrrolizin-1-ylmethyl 1-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50181842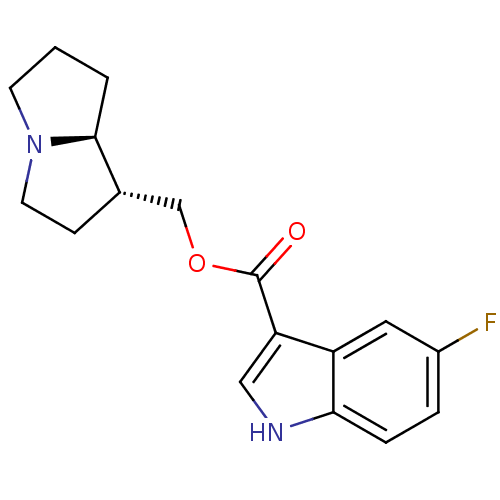 ((1R,7aS)-hexahydro-1H-pyrrolizin-1-ylmethyl 5-Fluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Agonist activity at recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 2 hrs by [35S]GTP-gammaS binding assay | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50181841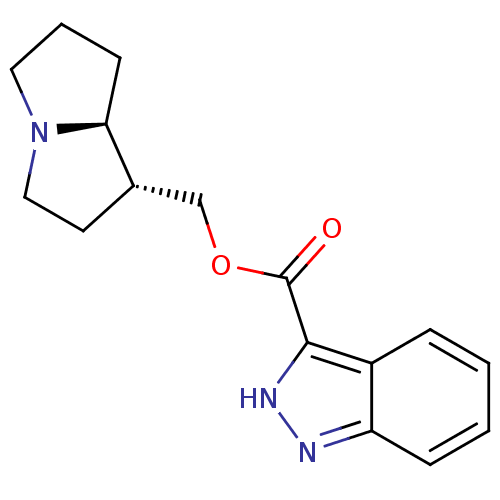 ((1S,7aR)-hexahydro-1H-pyrrolizin-1-ylmethyl 1H-ind...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50205990 (CHEMBL395429 | OXYTOCIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Agonist activity at human oxytocin receptor expressed in HEK293 cells assessed as increase in IP1 accumulation preincubated for 1 hr followed by addi... | Eur J Med Chem 108: 730-40 (2016) Article DOI: 10.1016/j.ejmech.2015.11.050 BindingDB Entry DOI: 10.7270/Q2XW4MNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50181836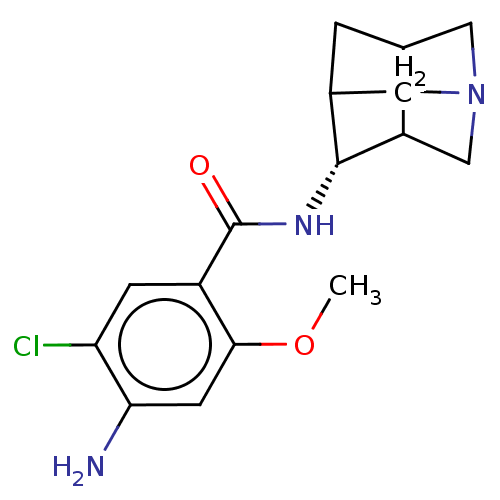 (4-Amino-N-(1-aza-tricyclo[3.3.1.0*3,7*]non-4-yl)-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR65630 from 5HT3 receptor in brain cortex from Wistar rat | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (RAT) | BDBM50181836 (4-Amino-N-(1-aza-tricyclo[3.3.1.0*3,7*]non-4-yl)-5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vivo for the antagonistic activity towards 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 2: 1613-1618 (1992) Article DOI: 10.1016/S0960-894X(00)80441-2 BindingDB Entry DOI: 10.7270/Q2J67HFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50181839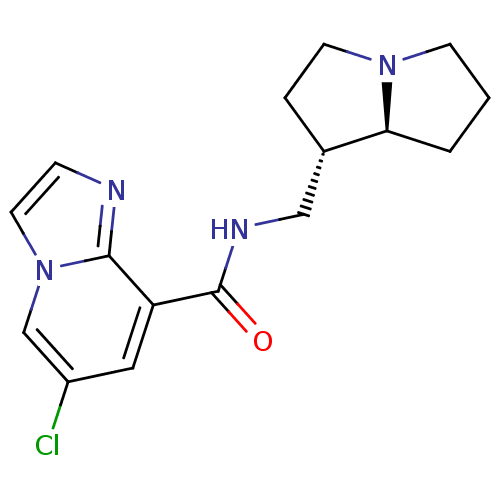 (CHEMBL202434 | N-exo-((4S,7alphaS)-tetrahydro-1H-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (RAT) | BDBM50449570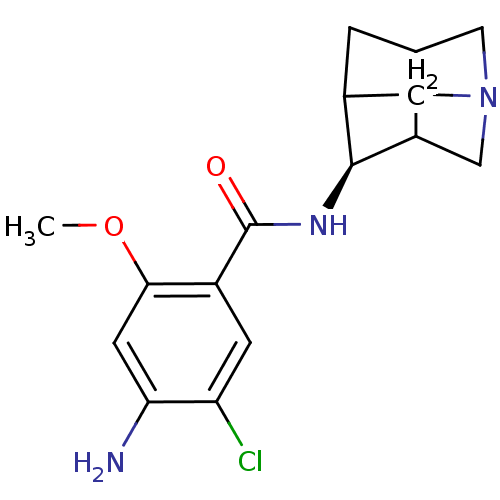 (CHEMBL2448137) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vivo for the antagonistic activity towards 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 2: 1613-1618 (1992) Article DOI: 10.1016/S0960-894X(00)80441-2 BindingDB Entry DOI: 10.7270/Q2J67HFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50181840 ((1R,7aS)-hexahydro-1H-pyrrolizin-1-ylmethyl 1-Meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR65630 from 5HT3 receptor in brain cortex from Wistar rat | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50005833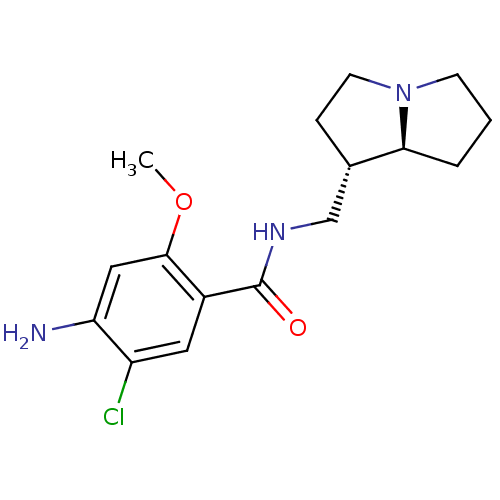 ((exo)4-Amino-5-chloro-N-(hexahydro-pyrrolizin-1-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (RAT) | BDBM50212465 (Renzapride) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the agonistic activity towards 5-hydroxytryptamine 4 receptor using the rat tunica muscularis mucosae (TMM) esophagus stri... | Bioorg Med Chem Lett 2: 1613-1618 (1992) Article DOI: 10.1016/S0960-894X(00)80441-2 BindingDB Entry DOI: 10.7270/Q2J67HFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50005836 (4-Amino-5-chloro-N-{1-[3-(4-fluoro-phenoxy)-propyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]GR-65630 binding to 5-hydroxytryptamine 3 receptor | J Med Chem 35: 1486-9 (1992) BindingDB Entry DOI: 10.7270/Q2CC11B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50181843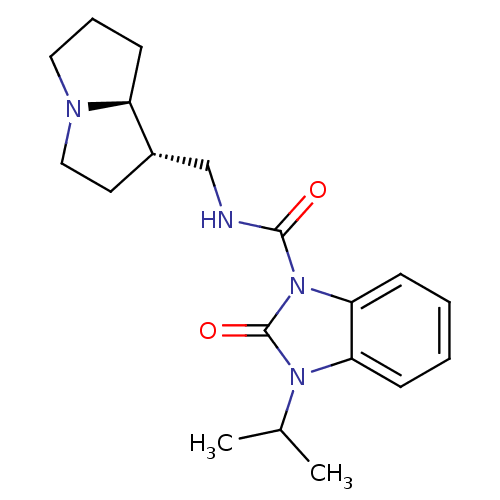 (CHEMBL201414 | cis-N-[(hexahydro-1H-pyrrolizin-l-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50031475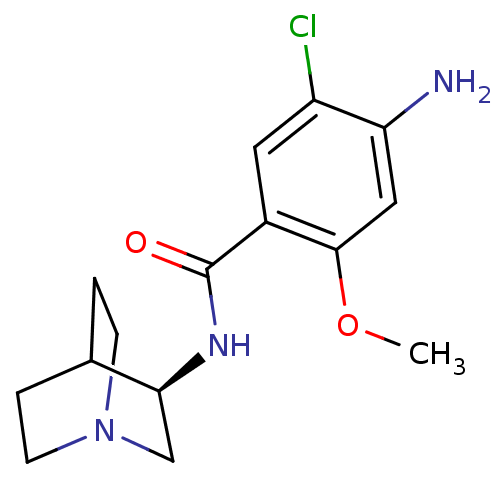 (4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl)-5-chloro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]GR-65630 binding to 5-hydroxytryptamine 3 receptor | J Med Chem 35: 1486-9 (1992) BindingDB Entry DOI: 10.7270/Q2CC11B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50464596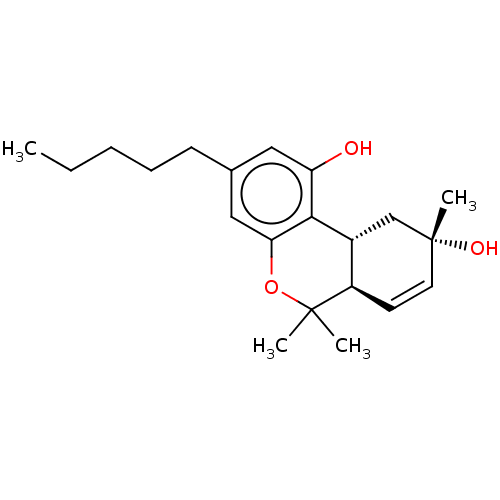 (CHEMBL4282822) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50181842 ((1R,7aS)-hexahydro-1H-pyrrolizin-1-ylmethyl 5-Fluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR65630 from 5HT3 receptor in brain cortex from Wistar rat | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50005840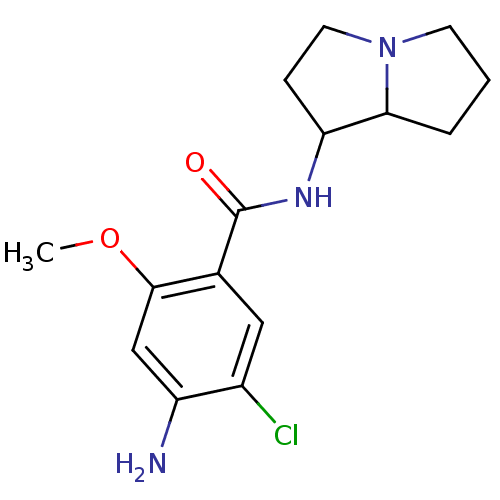 ((endo) 4-Amino-5-chloro-N-(hexahydro-pyrrolizin-1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]GR-65630 binding to 5-hydroxytryptamine 3 receptor | J Med Chem 35: 1486-9 (1992) BindingDB Entry DOI: 10.7270/Q2CC11B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50005840 ((endo) 4-Amino-5-chloro-N-(hexahydro-pyrrolizin-1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]GR-65630 binding to 5-hydroxytryptamine 3 receptor | J Med Chem 35: 1486-9 (1992) BindingDB Entry DOI: 10.7270/Q2CC11B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50005836 (4-Amino-5-chloro-N-{1-[3-(4-fluoro-phenoxy)-propyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50181843 (CHEMBL201414 | cis-N-[(hexahydro-1H-pyrrolizin-l-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR65630 from 5HT3 receptor in brain cortex from Wistar rat | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50181837 ((1S,7aR)-hexahydro-1H-pyrrolizin-1-ylmethyl 1-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR65630 from 5HT3 receptor in brain cortex from Wistar rat | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50005838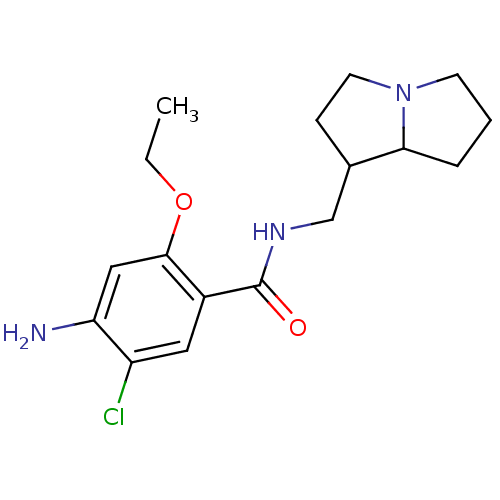 ((exo) 4-Amino-5-chloro-2-ethoxy-N-(hexahydro-pyrro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]GR-65630 binding to 5-hydroxytryptamine 3 receptor | J Med Chem 35: 1486-9 (1992) BindingDB Entry DOI: 10.7270/Q2CC11B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50181838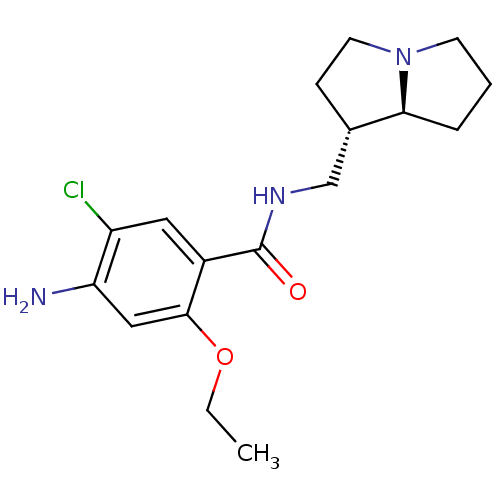 (4-amino-5-chloro-2-ethoxy-N-[(1S,7aS)-hexahydro-1H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR65630 from 5HT3 receptor in brain cortex from Wistar rat | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50139178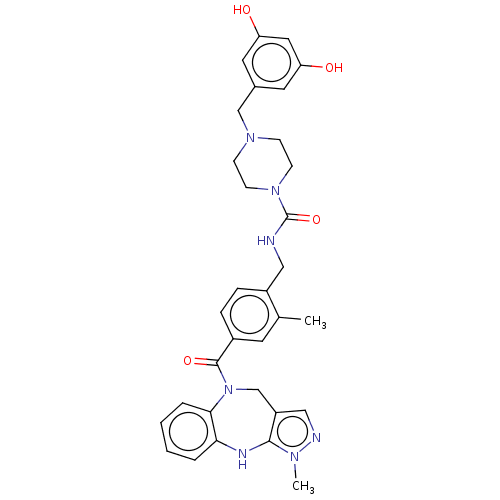 (CHEMBL3763342) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Agonist activity at human oxytocin receptor expressed in HEK293 cells assessed as increase in IP1 accumulation preincubated for 1 hr followed by addi... | Eur J Med Chem 108: 730-40 (2016) Article DOI: 10.1016/j.ejmech.2015.11.050 BindingDB Entry DOI: 10.7270/Q2XW4MNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50139178 (CHEMBL3763342) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from human vasopressin V1a receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay | Eur J Med Chem 143: 1644-1656 (2018) Article DOI: 10.1016/j.ejmech.2017.10.059 BindingDB Entry DOI: 10.7270/Q2MS3W92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50181836 (4-Amino-N-(1-aza-tricyclo[3.3.1.0*3,7*]non-4-yl)-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50005836 (4-Amino-5-chloro-N-{1-[3-(4-fluoro-phenoxy)-propyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description The ability to inhibit [3H]prazosin binding to Alpha-1 adrenergic receptor in rat whole brain | J Med Chem 35: 1486-9 (1992) BindingDB Entry DOI: 10.7270/Q2CC11B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50464598 (CHEMBL4281658) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Agonist activity at recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 2 hrs by [35S]GTP-gammaS binding assay | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50464598 (CHEMBL4281658) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50464593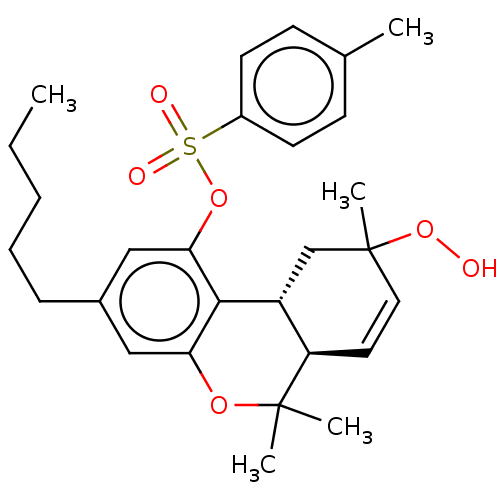 (CHEMBL4291438) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Agonist activity at recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 2 hrs by [35S]GTP-gammaS binding assay | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50181844 ((1R,7aS)-hexahydro-1H-pyrrolizin-1-ylmethyl 4-amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR65630 from 5HT3 receptor in brain cortex from Wistar rat | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50139178 (CHEMBL3763342) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from human vasopressin 1a receptor expressed in HEK293 cells after 90 mins by microbeta 2 microplate-reader method | Eur J Med Chem 108: 730-40 (2016) Article DOI: 10.1016/j.ejmech.2015.11.050 BindingDB Entry DOI: 10.7270/Q2XW4MNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50139179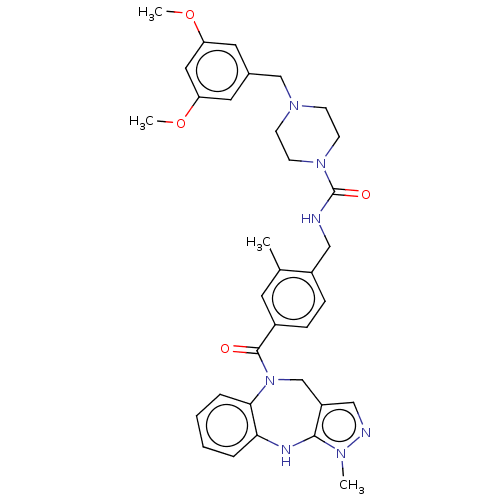 (CHEMBL3763951) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Agonist activity at human oxytocin receptor expressed in HEK293 cells assessed as increase in IP1 accumulation preincubated for 1 hr followed by addi... | Eur J Med Chem 108: 730-40 (2016) Article DOI: 10.1016/j.ejmech.2015.11.050 BindingDB Entry DOI: 10.7270/Q2XW4MNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50139179 (CHEMBL3763951) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from human vasopressin V1a receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay | Eur J Med Chem 143: 1644-1656 (2018) Article DOI: 10.1016/j.ejmech.2017.10.059 BindingDB Entry DOI: 10.7270/Q2MS3W92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50139373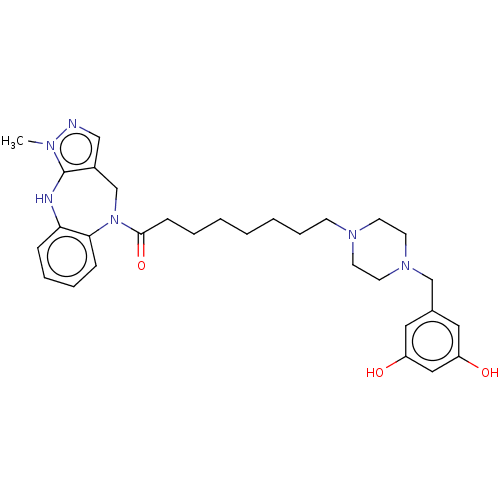 (CHEMBL3763823) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Antagonist activity at human vasopressin 1a receptor expressed in HEK293 cells assessed as inhibition of vasopressin induced IP1 accumulation pretrea... | Eur J Med Chem 108: 730-40 (2016) Article DOI: 10.1016/j.ejmech.2015.11.050 BindingDB Entry DOI: 10.7270/Q2XW4MNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50139373 (CHEMBL3763823) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from human vasopressin V1a receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay | Eur J Med Chem 143: 1644-1656 (2018) Article DOI: 10.1016/j.ejmech.2017.10.059 BindingDB Entry DOI: 10.7270/Q2MS3W92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50005841 ((exo) 4-Amino-5-chloro-N-(hexahydro-pyrrolizin-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]GR-65630 binding to 5-hydroxytryptamine 3 receptor | J Med Chem 35: 1486-9 (1992) BindingDB Entry DOI: 10.7270/Q2CC11B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50005841 ((exo) 4-Amino-5-chloro-N-(hexahydro-pyrrolizin-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR65630 from 5HT3 receptor in brain cortex from Wistar rat | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50005839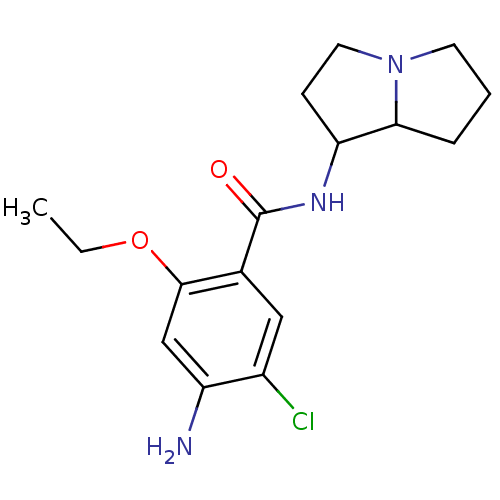 ((endo) 4-Amino-5-chloro-2-ethoxy-N-(hexahydro-pyrr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]GR-65630 binding to 5-hydroxytryptamine 3 receptor | J Med Chem 35: 1486-9 (1992) BindingDB Entry DOI: 10.7270/Q2CC11B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50005839 ((endo) 4-Amino-5-chloro-2-ethoxy-N-(hexahydro-pyrr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]GR-65630 binding to 5-hydroxytryptamine 3 receptor | J Med Chem 35: 1486-9 (1992) BindingDB Entry DOI: 10.7270/Q2CC11B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50005837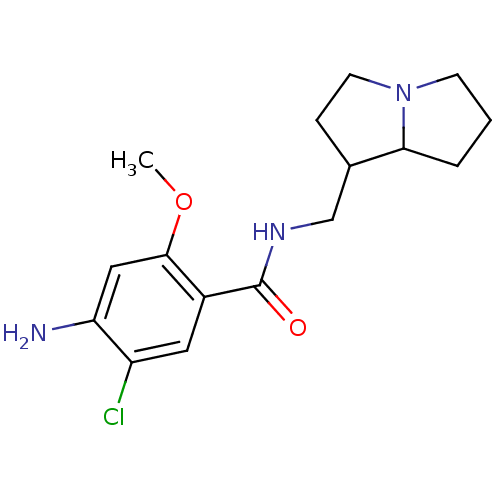 ((endo) 4-Amino-5-chloro-N-(hexahydro-pyrrolizin-1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]GR-65630 binding to 5-hydroxytryptamine 3 receptor | J Med Chem 35: 1486-9 (1992) BindingDB Entry DOI: 10.7270/Q2CC11B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 427 total ) | Next | Last >> |