 Found 791 hits with Last Name = 'peng' and Initial = 'w'
Found 791 hits with Last Name = 'peng' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at 5HT2A receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112214
BindingDB Entry DOI: 10.7270/Q2959N63 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged recombinant Aurora 1 (62 to 344) (unknown origin) expressed in baculovirus expression system by radiometric ass... |
Eur J Med Chem 78: 65-71 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.027
BindingDB Entry DOI: 10.7270/Q2Z60QKP |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at D2 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112214
BindingDB Entry DOI: 10.7270/Q2959N63 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM248056
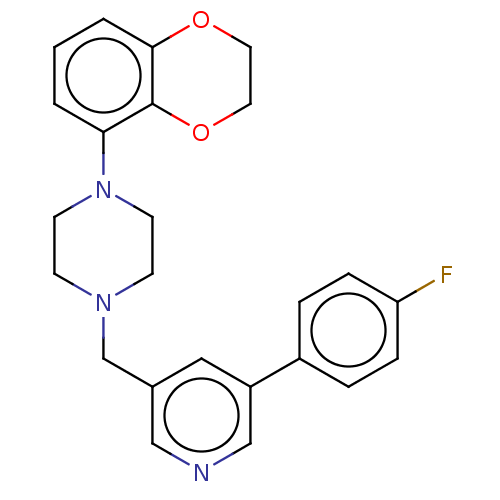
(1-(2,3-dihydro-1,4-benzodioxin-5-yl)-4-[[5-(4-fluo...)Show SMILES Fc1ccc(cc1)-c1cncc(CN2CCN(CC2)c2cccc3OCCOc23)c1 Show InChI InChI=1S/C24H24FN3O2/c25-21-6-4-19(5-7-21)20-14-18(15-26-16-20)17-27-8-10-28(11-9-27)22-2-1-3-23-24(22)30-13-12-29-23/h1-7,14-16H,8-13,17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]8-OH-DPAT from recombinant human 5HT1A receptor expressed in CHO cell membrane measured after 60 mins by scintillation counting m... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112214
BindingDB Entry DOI: 10.7270/Q2959N63 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at D2 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112214
BindingDB Entry DOI: 10.7270/Q2959N63 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM248056

(1-(2,3-dihydro-1,4-benzodioxin-5-yl)-4-[[5-(4-fluo...)Show SMILES Fc1ccc(cc1)-c1cncc(CN2CCN(CC2)c2cccc3OCCOc23)c1 Show InChI InChI=1S/C24H24FN3O2/c25-21-6-4-19(5-7-21)20-14-18(15-26-16-20)17-27-8-10-28(11-9-27)22-2-1-3-23-24(22)30-13-12-29-23/h1-7,14-16H,8-13,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]spiperone from recombinant human D2S receptor expressed in CHO cell membrane measured after 60 mins by scintillation counting met... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112214
BindingDB Entry DOI: 10.7270/Q2959N63 |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged recombinant Aurora 3 (1 to 309) (unknown origin) expressed in baculovirus expression system by radiometric assa... |
Eur J Med Chem 78: 65-71 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.027
BindingDB Entry DOI: 10.7270/Q2Z60QKP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at 5HT2A receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112214
BindingDB Entry DOI: 10.7270/Q2959N63 |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493380
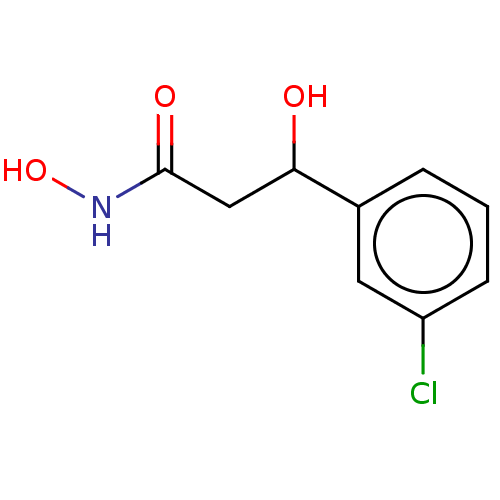
(CHEMBL2425469)Show InChI InChI=1S/C9H10ClNO3/c10-7-3-1-2-6(4-7)8(12)5-9(13)11-14/h1-4,8,12,14H,5H2,(H,11,13) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Mixed-type competitive inhibition of Helicobacter pylori ATCC 43504 urease using urea as substrate by Lineweaver-burk plot analysis |
Eur J Med Chem 68: 212-21 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.047
BindingDB Entry DOI: 10.7270/Q28G8PNR |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged recombinant Aurora 2 (1 to 403) (unknown origin) expressed in baculovirus expression system by radiometric assa... |
Eur J Med Chem 78: 65-71 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.027
BindingDB Entry DOI: 10.7270/Q2Z60QKP |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493373
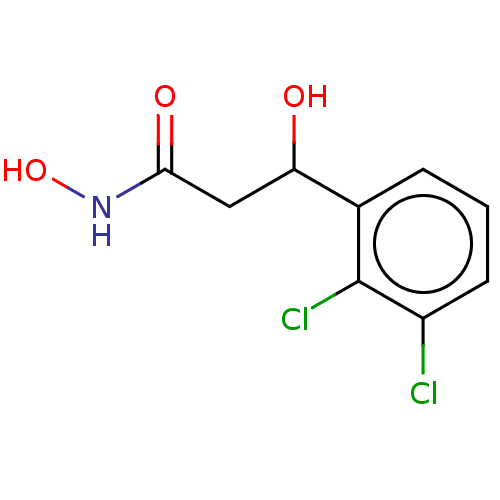
(CHEMBL2425478)Show InChI InChI=1S/C9H9Cl2NO3/c10-6-3-1-2-5(9(6)11)7(13)4-8(14)12-15/h1-3,7,13,15H,4H2,(H,12,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Mixed-type competitive inhibition of Helicobacter pylori ATCC 43504 urease using urea as substrate by Lineweaver-burk plot analysis |
Eur J Med Chem 68: 212-21 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.047
BindingDB Entry DOI: 10.7270/Q28G8PNR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50551638
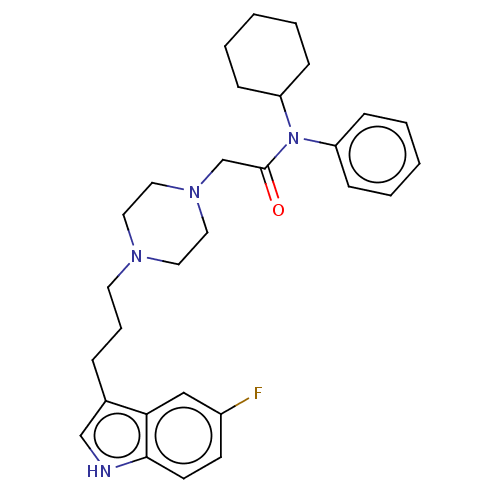
(CHEMBL4790303)Show SMILES Fc1ccc2[nH]cc(CCCN3CCN(CC(=O)N(C4CCCCC4)c4ccccc4)CC3)c2c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]5-HT from recombinant 5HT1A receptor (unknown origin) expressed in CHO cell membrane measured after 50 mins by microbeta scintill... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112214
BindingDB Entry DOI: 10.7270/Q2959N63 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50251187
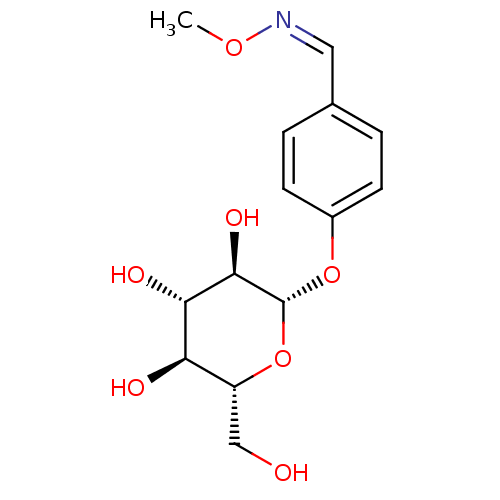
(1-(4-(O-beta-D-Glucopyranosyl) benzylidene) methox...)Show SMILES CO\N=C/c1ccc(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C14H19NO7/c1-20-15-6-8-2-4-9(5-3-8)21-14-13(19)12(18)11(17)10(7-16)22-14/h2-6,10-14,16-19H,7H2,1H3/b15-6-/t10-,11-,12+,13-,14-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Eur J Med Chem 43: 166-73 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.018
BindingDB Entry DOI: 10.7270/Q2DF6S3Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at D2 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112214
BindingDB Entry DOI: 10.7270/Q2959N63 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50551638

(CHEMBL4790303)Show SMILES Fc1ccc2[nH]cc(CCCN3CCN(CC(=O)N(C4CCCCC4)c4ccccc4)CC3)c2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 207 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human 5HT2A receptor expressed in CHO-K1 cells measured after 15 mins by FLIPR assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112214
BindingDB Entry DOI: 10.7270/Q2959N63 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Eur J Med Chem 43: 166-73 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.018
BindingDB Entry DOI: 10.7270/Q2DF6S3Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50245887

(4-((2S,3R,4R,5R)-3,4,5-trihydroxy-tetrahydro-2H-py...)Show SMILES O[C@@H]1CO[C@@H](Oc2ccc(C=O)cc2)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C12H14O6/c13-5-7-1-3-8(4-2-7)18-12-11(16)10(15)9(14)6-17-12/h1-5,9-12,14-16H,6H2/t9-,10-,11-,12+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Eur J Med Chem 43: 166-73 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.018
BindingDB Entry DOI: 10.7270/Q2DF6S3Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50245886

(4-((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymet...)Show SMILES OC[C@H]1O[C@@H](Oc2ccc(C=O)cc2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C13H16O7/c14-5-7-1-3-8(4-2-7)19-13-12(18)11(17)10(16)9(6-15)20-13/h1-5,9-13,15-18H,6H2/t9-,10-,11+,12-,13-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Eur J Med Chem 43: 166-73 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.018
BindingDB Entry DOI: 10.7270/Q2DF6S3Z |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241354
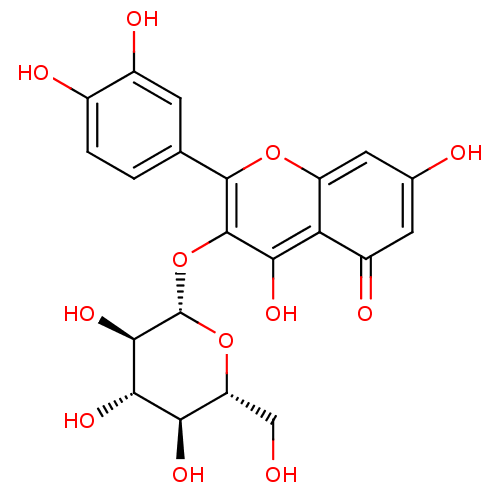
(2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-3-(3,4,5-tr...)Show SMILES OC[C@H]1O[C@@H](Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)c(O)c2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C21H20O12/c22-6-13-15(27)17(29)18(30)21(32-13)33-20-16(28)14-11(26)4-8(23)5-12(14)31-19(20)7-1-2-9(24)10(25)3-7/h1-5,13,15,17-18,21-25,27-30H,6H2/t13-,15-,17+,18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of LSD1 (unknown origin) using H3Kme2 as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 27: 370-374 (2019)
Article DOI: 10.1016/j.bmc.2018.12.013
BindingDB Entry DOI: 10.7270/Q2JW8J40 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50551638

(CHEMBL4790303)Show SMILES Fc1ccc2[nH]cc(CCCN3CCN(CC(=O)N(C4CCCCC4)c4ccccc4)CC3)c2c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human D2 receptor expressed in HEK293 cells incubated for 60 mins by Eu-cAMP tracer based LANCE ultra cAMP assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112214
BindingDB Entry DOI: 10.7270/Q2959N63 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50300123
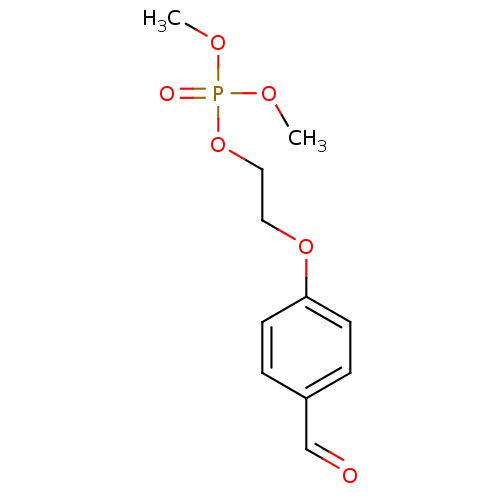
(2-(4-Formylphenoxy)ethyl dimethyl phosphate | CHEM...)Show InChI InChI=1S/C11H15O6P/c1-14-18(13,15-2)17-8-7-16-11-5-3-10(9-12)4-6-11/h3-6,9H,7-8H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase assessed as oxidation of L-DOPA by Lineweaver-Burke plot analysis |
Eur J Med Chem 45: 639-46 (2010)
Article DOI: 10.1016/j.ejmech.2009.11.007
BindingDB Entry DOI: 10.7270/Q2348M9F |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50206197
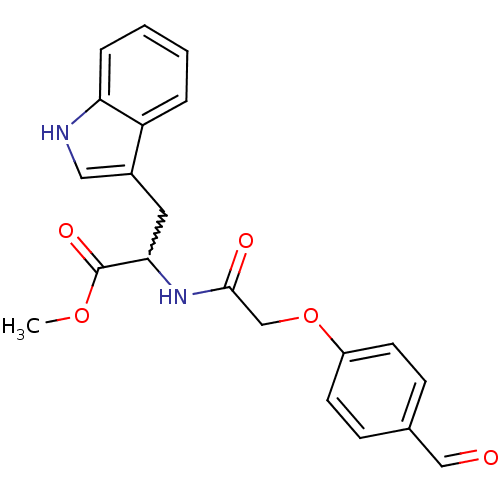
(CHEMBL235565 | methyl 2-(2-(4-formylphenoxy)acetam...)Show SMILES COC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)COc1ccc(C=O)cc1 |w:4.4| Show InChI InChI=1S/C21H20N2O5/c1-27-21(26)19(10-15-11-22-18-5-3-2-4-17(15)18)23-20(25)13-28-16-8-6-14(12-24)7-9-16/h2-9,11-12,19,22H,10,13H2,1H3,(H,23,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase assessed as oxidation of L-DOPA by Lineweaver-Burke plot analysis |
Eur J Med Chem 45: 639-46 (2010)
Article DOI: 10.1016/j.ejmech.2009.11.007
BindingDB Entry DOI: 10.7270/Q2348M9F |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50206195

(CHEMBL232590 | methyl 2-(2-(4-formylphenoxy)acetam...)Show InChI InChI=1S/C12H13NO5/c1-17-12(16)6-13-11(15)8-18-10-4-2-9(7-14)3-5-10/h2-5,7H,6,8H2,1H3,(H,13,15) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase assessed as oxidation of L-DOPA by Lineweaver-Burke plot analysis |
Eur J Med Chem 45: 639-46 (2010)
Article DOI: 10.1016/j.ejmech.2009.11.007
BindingDB Entry DOI: 10.7270/Q2348M9F |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50206194

(CHEMBL394901 | methyl 2-(2-(4-formylphenoxy)acetam...)Show InChI InChI=1S/C13H15NO5/c1-9(13(17)18-2)14-12(16)8-19-11-5-3-10(7-15)4-6-11/h3-7,9H,8H2,1-2H3,(H,14,16) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase assessed as oxidation of L-DOPA by Lineweaver-Burke plot analysis |
Eur J Med Chem 45: 639-46 (2010)
Article DOI: 10.1016/j.ejmech.2009.11.007
BindingDB Entry DOI: 10.7270/Q2348M9F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425862
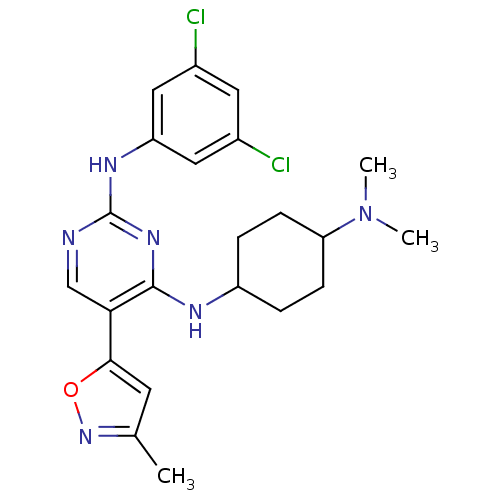
(CHEMBL2312654)Show SMILES CN(C)C1CCC(CC1)Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1-c1cc(C)no1 |(9.04,-43.08,;10.39,-42.35,;11.7,-43.16,;10.44,-40.81,;9.13,-40,;9.18,-38.46,;10.53,-37.74,;11.85,-38.53,;11.8,-40.08,;10.57,-36.2,;9.81,-34.87,;8.27,-34.86,;7.51,-33.53,;5.96,-33.52,;5.2,-32.19,;5.97,-30.85,;5.21,-29.52,;5.98,-28.19,;3.67,-29.52,;2.89,-30.86,;1.35,-30.86,;3.66,-32.19,;8.27,-32.2,;9.8,-32.19,;10.58,-33.53,;12.11,-33.53,;12.6,-34.99,;14.14,-34.99,;15.06,-36.23,;14.61,-33.52,;13.35,-32.62,)| Show InChI InChI=1S/C22H26Cl2N6O/c1-13-8-20(31-29-13)19-12-25-22(27-17-10-14(23)9-15(24)11-17)28-21(19)26-16-4-6-18(7-5-16)30(2)3/h8-12,16,18H,4-7H2,1-3H3,(H2,25,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50142796
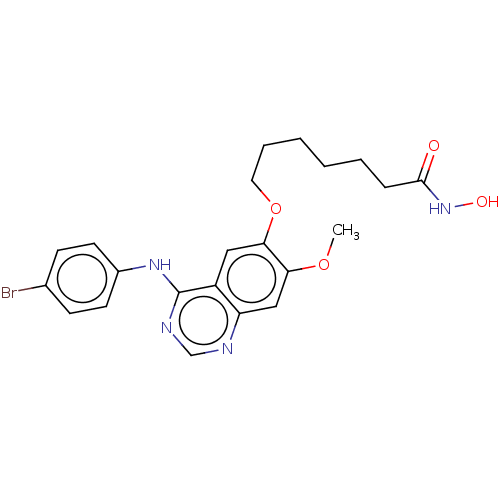
(CHEMBL3759186)Show SMILES COc1cc2ncnc(Nc3ccc(Br)cc3)c2cc1OCCCCCCC(=O)NO Show InChI InChI=1S/C22H25BrN4O4/c1-30-19-13-18-17(12-20(19)31-11-5-3-2-4-6-21(28)27-29)22(25-14-24-18)26-16-9-7-15(23)8-10-16/h7-10,12-14,29H,2-6,11H2,1H3,(H,27,28)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) preincubated for 15 mins followed by addition of Fluor de Lys as substrate for 1 hr by fluorometric assay |
Eur J Med Chem 109: 1-12 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.033
BindingDB Entry DOI: 10.7270/Q2377BJX |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50551625
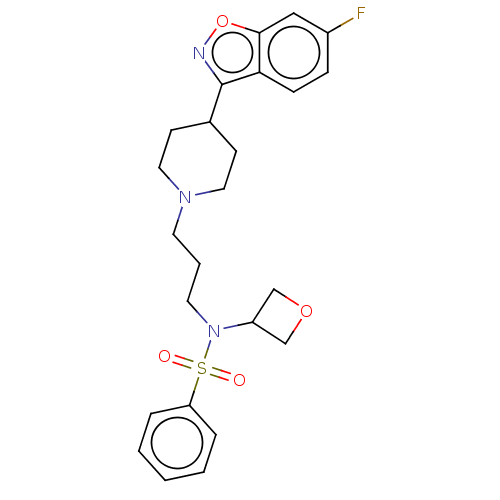
(CHEMBL4776840)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCN(C2COC2)S(=O)(=O)c2ccccc2)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human D2 receptor expressed in HEK293 cells incubated for 60 mins by Eu-cAMP tracer based LANCE ultra cAMP assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112214
BindingDB Entry DOI: 10.7270/Q2959N63 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425864
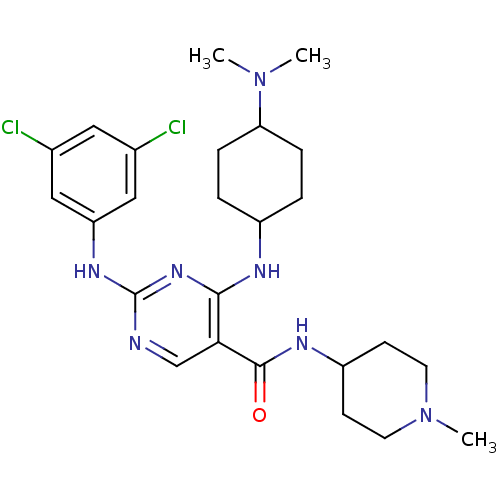
(CHEMBL2312649)Show SMILES CN(C)C1CCC(CC1)Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1C(=O)NC1CCN(C)CC1 |(47.36,-51.36,;48.9,-51.37,;49.67,-52.7,;49.68,-50.04,;48.92,-48.7,;49.7,-47.37,;51.23,-47.39,;52.01,-48.71,;51.22,-50.05,;52,-46.06,;51.24,-44.72,;49.7,-44.72,;48.94,-43.38,;47.4,-43.38,;46.63,-42.04,;47.41,-40.71,;46.64,-39.37,;47.41,-38.04,;45.1,-39.37,;44.33,-40.71,;42.79,-40.71,;45.1,-42.04,;49.7,-42.05,;51.24,-42.05,;52.01,-43.39,;53.55,-43.39,;54.32,-44.72,;54.32,-42.06,;55.86,-42.06,;56.62,-43.39,;58.16,-43.4,;58.94,-42.07,;60.48,-42.08,;58.17,-40.73,;56.62,-40.72,)| Show InChI InChI=1S/C25H35Cl2N7O/c1-33(2)21-6-4-18(5-7-21)29-23-22(24(35)30-19-8-10-34(3)11-9-19)15-28-25(32-23)31-20-13-16(26)12-17(27)14-20/h12-15,18-19,21H,4-11H2,1-3H3,(H,30,35)(H2,28,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50551623

(CHEMBL4759817)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCN(C2COC2)S(=O)(=O)c2ccc(Cl)cc2)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human D2 receptor expressed in HEK293 cells incubated for 60 mins by Eu-cAMP tracer based LANCE ultra cAMP assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112214
BindingDB Entry DOI: 10.7270/Q2959N63 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50142796

(CHEMBL3759186)Show SMILES COc1cc2ncnc(Nc3ccc(Br)cc3)c2cc1OCCCCCCC(=O)NO Show InChI InChI=1S/C22H25BrN4O4/c1-30-19-13-18-17(12-20(19)31-11-5-3-2-4-6-21(28)27-29)22(25-14-24-18)26-16-9-7-15(23)8-10-16/h7-10,12-14,29H,2-6,11H2,1H3,(H,27,28)(H,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extracts preincubated for 15 mins followed by addition of Fluor de Lys as substrate for 1 hr by fluorom... |
Eur J Med Chem 109: 1-12 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.033
BindingDB Entry DOI: 10.7270/Q2377BJX |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50551622
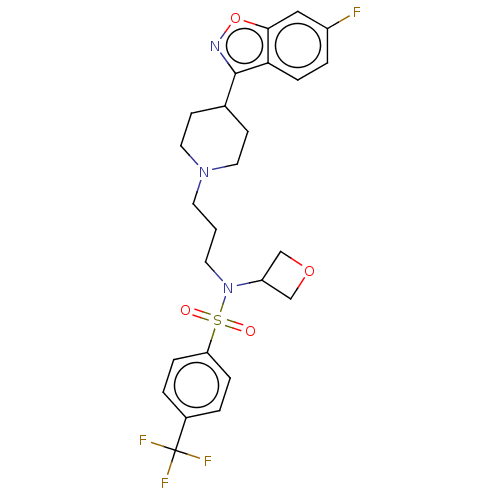
(CHEMBL4746981)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCN(C2COC2)S(=O)(=O)c2ccc(cc2)C(F)(F)F)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human D2 receptor expressed in HEK293 cells incubated for 60 mins by Eu-cAMP tracer based LANCE ultra cAMP assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112214
BindingDB Entry DOI: 10.7270/Q2959N63 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50132484
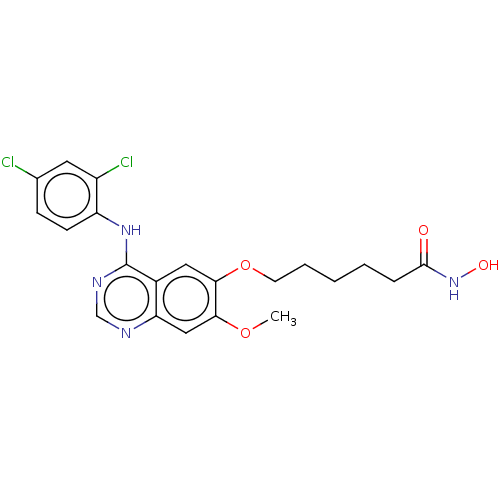
(CHEMBL3633769)Show SMILES COc1cc2ncnc(Nc3ccc(Cl)cc3Cl)c2cc1OCCCCCC(=O)NO Show InChI InChI=1S/C21H22Cl2N4O4/c1-30-18-11-17-14(10-19(18)31-8-4-2-3-5-20(28)27-29)21(25-12-24-17)26-16-7-6-13(22)9-15(16)23/h6-7,9-12,29H,2-5,8H2,1H3,(H,27,28)(H,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Beta Glucosidase from Almonds |
Bioorg Med Chem Lett 25: 5137-41 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.006
BindingDB Entry DOI: 10.7270/Q2CN75QQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425870
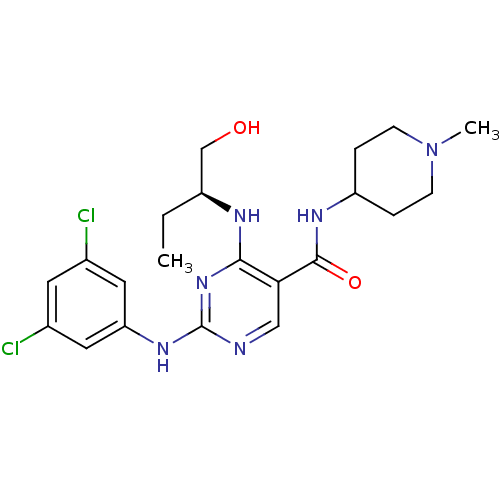
(CHEMBL2311550)Show SMILES CC[C@@H](CO)Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1C(=O)NC1CCN(C)CC1 |r| Show InChI InChI=1S/C21H28Cl2N6O2/c1-3-15(12-30)25-19-18(20(31)26-16-4-6-29(2)7-5-16)11-24-21(28-19)27-17-9-13(22)8-14(23)10-17/h8-11,15-16,30H,3-7,12H2,1-2H3,(H,26,31)(H2,24,25,27,28)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K delta by scintillation proximity radiometric assay |
Eur J Med Chem 108: 644-54 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.038
BindingDB Entry DOI: 10.7270/Q2C53NQ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K delta (unknown origin) using PIP2 as substrate after 1 hr by kinase-Glo luminescent assay |
Eur J Med Chem 108: 644-54 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.038
BindingDB Entry DOI: 10.7270/Q2C53NQ5 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50551624
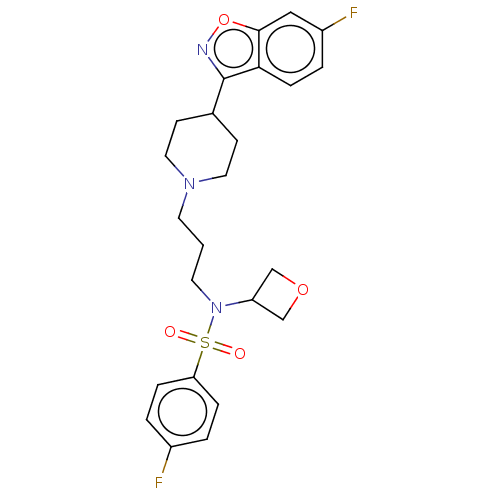
(CHEMBL4787495)Show SMILES Fc1ccc(cc1)S(=O)(=O)N(CCCN1CCC(CC1)c1noc2cc(F)ccc12)C1COC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human D2 receptor expressed in HEK293 cells incubated for 60 mins by Eu-cAMP tracer based LANCE ultra cAMP assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112214
BindingDB Entry DOI: 10.7270/Q2959N63 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50142857
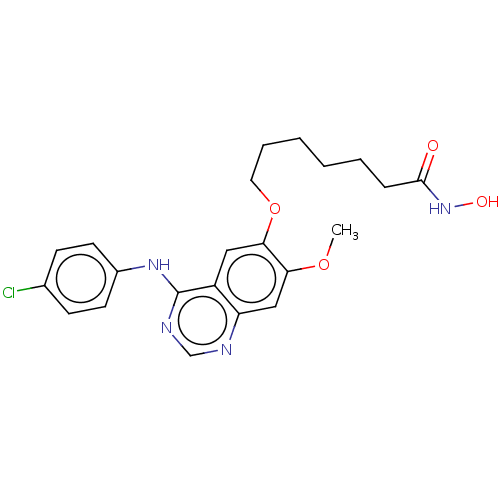
(CHEMBL3758839)Show SMILES COc1cc2ncnc(Nc3ccc(Cl)cc3)c2cc1OCCCCCCC(=O)NO Show InChI InChI=1S/C22H25ClN4O4/c1-30-19-13-18-17(12-20(19)31-11-5-3-2-4-6-21(28)27-29)22(25-14-24-18)26-16-9-7-15(23)8-10-16/h7-10,12-14,29H,2-6,11H2,1H3,(H,27,28)(H,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extracts preincubated for 15 mins followed by addition of Fluor de Lys as substrate for 1 hr by fluorom... |
Eur J Med Chem 109: 1-12 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.033
BindingDB Entry DOI: 10.7270/Q2377BJX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50142796

(CHEMBL3759186)Show SMILES COc1cc2ncnc(Nc3ccc(Br)cc3)c2cc1OCCCCCCC(=O)NO Show InChI InChI=1S/C22H25BrN4O4/c1-30-19-13-18-17(12-20(19)31-11-5-3-2-4-6-21(28)27-29)22(25-14-24-18)26-16-9-7-15(23)8-10-16/h7-10,12-14,29H,2-6,11H2,1H3,(H,27,28)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) preincubated for 15 mins followed by addition of Fluor de Lys as substrate for 1 hr by fluorometric assay |
Eur J Med Chem 109: 1-12 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.033
BindingDB Entry DOI: 10.7270/Q2377BJX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50132484

(CHEMBL3633769)Show SMILES COc1cc2ncnc(Nc3ccc(Cl)cc3Cl)c2cc1OCCCCCC(=O)NO Show InChI InChI=1S/C21H22Cl2N4O4/c1-30-18-11-17-14(10-19(18)31-8-4-2-3-5-20(28)27-29)21(25-12-24-17)26-16-7-6-13(22)9-15(16)23/h6-7,9-12,29H,2-5,8H2,1H3,(H,27,28)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Beta Glucosidase from Almonds |
Bioorg Med Chem Lett 25: 5137-41 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.006
BindingDB Entry DOI: 10.7270/Q2CN75QQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50551621
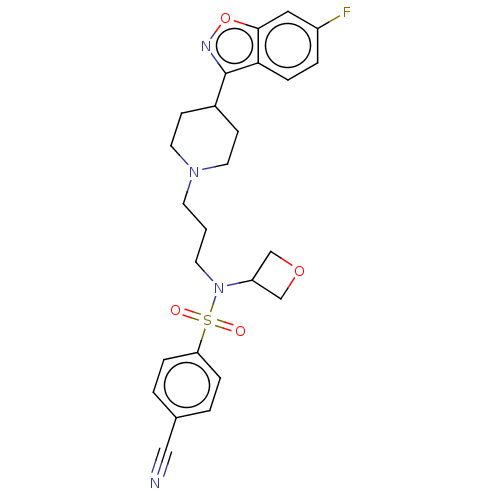
(CHEMBL4747208)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCN(C2COC2)S(=O)(=O)c2ccc(cc2)C#N)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human D2 receptor expressed in HEK293 cells incubated for 60 mins by Eu-cAMP tracer based LANCE ultra cAMP assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112214
BindingDB Entry DOI: 10.7270/Q2959N63 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Mus musculus (mouse)) | BDBM31093

(4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...)Show SMILES OC(=O)c1ccc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2F)cc1 |c:13| Show InChI InChI=1S/C25H15ClF2N4O2/c26-15-6-9-17-18(10-15)23(21-19(27)2-1-3-20(21)28)29-11-14-12-30-25(32-22(14)17)31-16-7-4-13(5-8-16)24(33)34/h1-10,12H,11H2,(H,33,34)(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant mouse Aurora A expressed in Sf9 cells using biotin-GLRRASLG as peptide substrate by [gamma-33S]ATP binding assay |
Eur J Med Chem 78: 65-71 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.027
BindingDB Entry DOI: 10.7270/Q2Z60QKP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50148942
(CHEMBL3769902)Show SMILES Nc1ncc(cn1)-c1nc(N2CCOCC2)c2ccc(Nc3cccnc3C#N)cc2n1 Show InChI InChI=1S/C22H19N9O/c23-11-19-17(2-1-5-25-19)28-15-3-4-16-18(10-15)29-20(14-12-26-22(24)27-13-14)30-21(16)31-6-8-32-9-7-31/h1-5,10,12-13,28H,6-9H2,(H2,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K alpha (unknown origin) using PIP2 as substrate after 1 hr by kinase-Glo luminescent assay |
Eur J Med Chem 108: 644-54 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.038
BindingDB Entry DOI: 10.7270/Q2C53NQ5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50132403
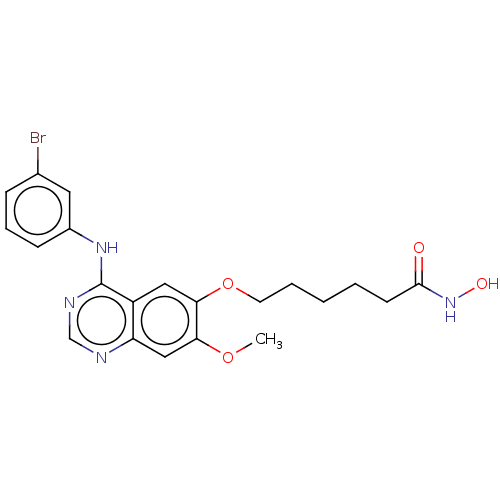
(CHEMBL3633765)Show SMILES COc1cc2ncnc(Nc3cccc(Br)c3)c2cc1OCCCCCC(=O)NO Show InChI InChI=1S/C21H23BrN4O4/c1-29-18-12-17-16(11-19(18)30-9-4-2-3-8-20(27)26-28)21(24-13-23-17)25-15-7-5-6-14(22)10-15/h5-7,10-13,28H,2-4,8-9H2,1H3,(H,26,27)(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Beta Glucosidase from Almonds |
Bioorg Med Chem Lett 25: 5137-41 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.006
BindingDB Entry DOI: 10.7270/Q2CN75QQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50142796

(CHEMBL3759186)Show SMILES COc1cc2ncnc(Nc3ccc(Br)cc3)c2cc1OCCCCCCC(=O)NO Show InChI InChI=1S/C22H25BrN4O4/c1-30-19-13-18-17(12-20(19)31-11-5-3-2-4-6-21(28)27-29)22(25-14-24-18)26-16-9-7-15(23)8-10-16/h7-10,12-14,29H,2-6,11H2,1H3,(H,27,28)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) preincubated for 15 mins followed by addition of Fluor de Lys as substrate for 1 hr by fluorometric assay |
Eur J Med Chem 109: 1-12 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.033
BindingDB Entry DOI: 10.7270/Q2377BJX |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration of compound for 50% displacement of [3H]QNB from Muscarinic acetylcholine receptor in rat brain |
J Med Chem 23: 865-73 (1980)
BindingDB Entry DOI: 10.7270/Q2542QTR |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50004231
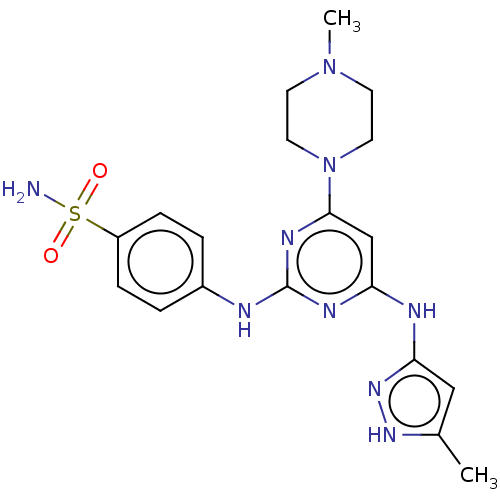
(CHEMBL3237167)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Nc2ccc(cc2)S(N)(=O)=O)n1 Show InChI InChI=1S/C19H25N9O2S/c1-13-11-17(26-25-13)22-16-12-18(28-9-7-27(2)8-10-28)24-19(23-16)21-14-3-5-15(6-4-14)31(20,29)30/h3-6,11-12H,7-10H2,1-2H3,(H2,20,29,30)(H3,21,22,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition measured after... |
Eur J Med Chem 78: 65-71 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.027
BindingDB Entry DOI: 10.7270/Q2Z60QKP |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM31046
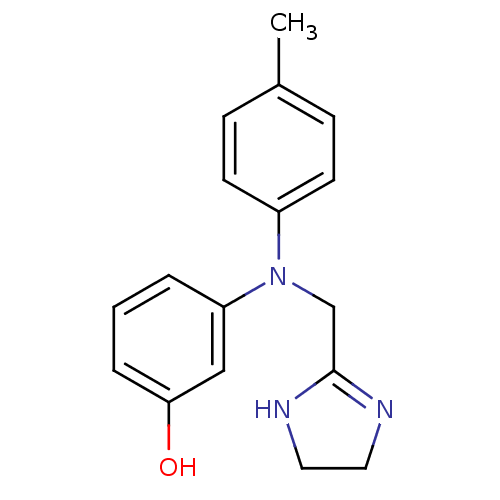
(3-[4,5-dihydro-1H-imidazol-2-ylmethyl-(4-methylphe...)Show InChI InChI=1S/C17H19N3O/c1-13-5-7-14(8-6-13)20(12-17-18-9-10-19-17)15-3-2-4-16(21)11-15/h2-8,11,21H,9-10,12H2,1H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration of compound for 50% displacement of [3H]WB-4101 from Alpha-1 adrenergic receptor of rat brain |
J Med Chem 23: 865-73 (1980)
BindingDB Entry DOI: 10.7270/Q2542QTR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50132484

(CHEMBL3633769)Show SMILES COc1cc2ncnc(Nc3ccc(Cl)cc3Cl)c2cc1OCCCCCC(=O)NO Show InChI InChI=1S/C21H22Cl2N4O4/c1-30-18-11-17-14(10-19(18)31-8-4-2-3-5-20(28)27-29)21(25-12-24-17)26-16-7-6-13(22)9-15(16)23/h6-7,9-12,29H,2-5,8H2,1H3,(H,27,28)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibitory activity against metabotropic glutamate receptor 5 (mGluR5) |
Bioorg Med Chem Lett 25: 5137-41 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.006
BindingDB Entry DOI: 10.7270/Q2CN75QQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50132345
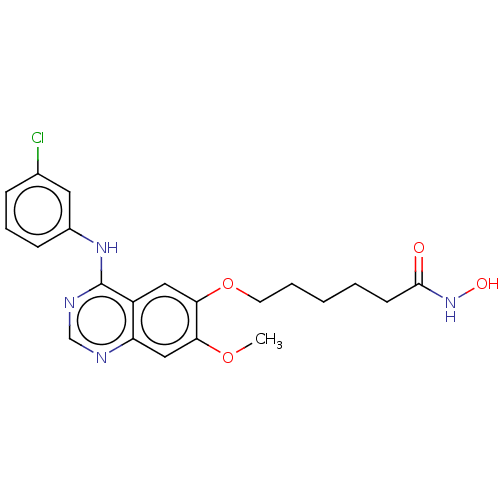
(CHEMBL3633761)Show SMILES COc1cc2ncnc(Nc3cccc(Cl)c3)c2cc1OCCCCCC(=O)NO Show InChI InChI=1S/C21H23ClN4O4/c1-29-18-12-17-16(11-19(18)30-9-4-2-3-8-20(27)26-28)21(24-13-23-17)25-15-7-5-6-14(22)10-15/h5-7,10-13,28H,2-4,8-9H2,1H3,(H,26,27)(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity for prostanoid EP4 receptor |
Bioorg Med Chem Lett 25: 5137-41 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.006
BindingDB Entry DOI: 10.7270/Q2CN75QQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K alpha by scintillation proximity radiometric assay |
Eur J Med Chem 108: 644-54 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.038
BindingDB Entry DOI: 10.7270/Q2C53NQ5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data