

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (MOUSE) | BDBM50059997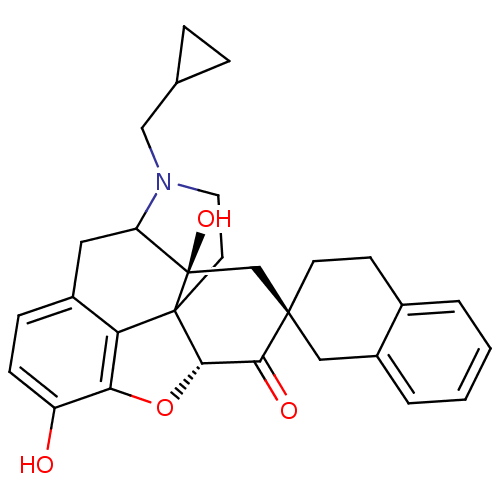 (7 beta-Spirobenzocyclohexylnaltrexone | CHEMBL1016...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-NTI binding to Opioid receptor delta 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50059994 (7 alpha-Spirobenzocyclohexylnaltrexone | CHEMBL105...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-NTI binding to Opioid receptor delta 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50148931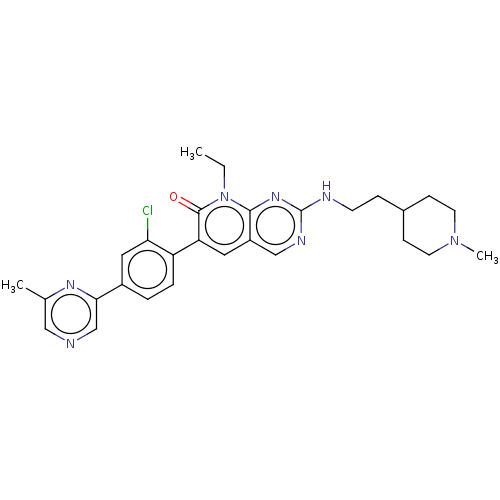 (CHEMBL3770186) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length recombinant human N-terminal GST/His6-tagged PAK1 expressed in sf9 insect cells using tetra LRRWSLG as substrate preincubat... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112517 BindingDB Entry DOI: 10.7270/Q2Q243W7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50007518 ((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Compound was measured for its ability to compete with [125I]NCQ298 binding to the human Dopamine receptor D3 transfected in CHO cells | J Med Chem 46: 4377-92 (2003) Article DOI: 10.1021/jm030085p BindingDB Entry DOI: 10.7270/Q21V5FPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [125I]iodosulpiride from human Dopamine receptor D3 expressed in CHO cells | J Med Chem 46: 4377-92 (2003) Article DOI: 10.1021/jm030085p BindingDB Entry DOI: 10.7270/Q21V5FPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50059997 (7 beta-Spirobenzocyclohexylnaltrexone | CHEMBL1016...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to Opioid receptor mu 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50059994 (7 alpha-Spirobenzocyclohexylnaltrexone | CHEMBL105...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to Opioid receptor mu 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50388994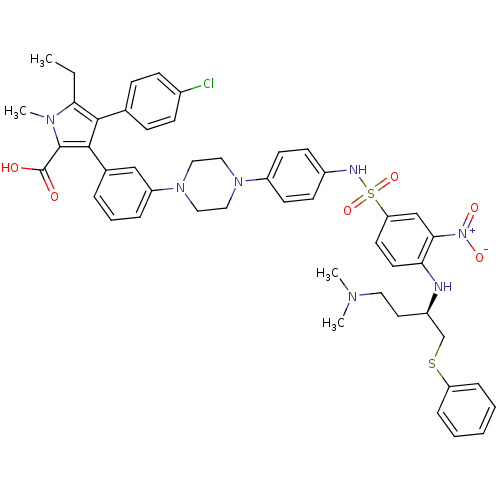 (CHEMBL2063897) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminus 6X His-tagged human Bcl2 expressed in Escherichia coli BL21 (DE3) cells after 2 hrs by fluorescence polarization assay | J Med Chem 55: 4664-82 (2012) Article DOI: 10.1021/jm300178u BindingDB Entry DOI: 10.7270/Q2862HHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50133923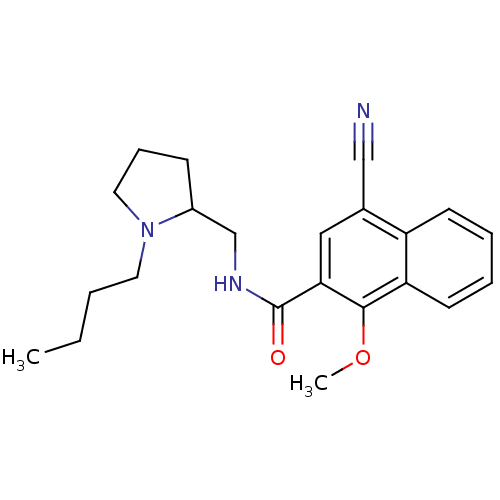 (4-Cyano-1-methoxy-naphthalene-2-carboxylic acid (1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [125I]iodosulpiride from human Dopamine receptor D3 expressed in CHO cells | J Med Chem 46: 4377-92 (2003) Article DOI: 10.1021/jm030085p BindingDB Entry DOI: 10.7270/Q21V5FPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50133933 ((R)-1-Propyl-2,3,10,10a-tetrahydro-1H,4aH-4,9-diox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Compound was measured for its ability to compete with [3H]spiperone binding to the human Dopamine receptor D3 transfected in CHO cells | J Med Chem 46: 4377-92 (2003) Article DOI: 10.1021/jm030085p BindingDB Entry DOI: 10.7270/Q21V5FPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50388990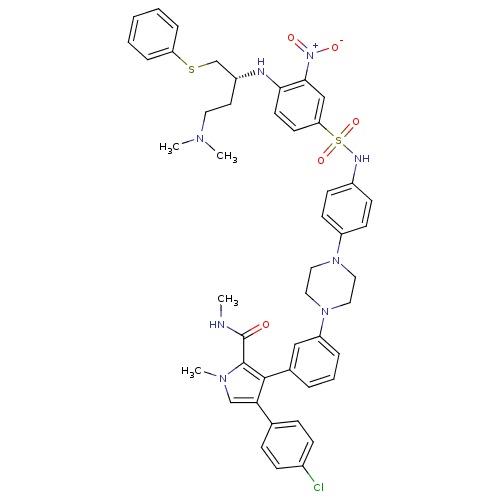 (CHEMBL2063893) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminus 6X His-tagged human Bcl2 expressed in Escherichia coli BL21 (DE3) cells after 2 hrs by fluorescence polarization assay | J Med Chem 55: 4664-82 (2012) Article DOI: 10.1021/jm300178u BindingDB Entry DOI: 10.7270/Q2862HHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50058152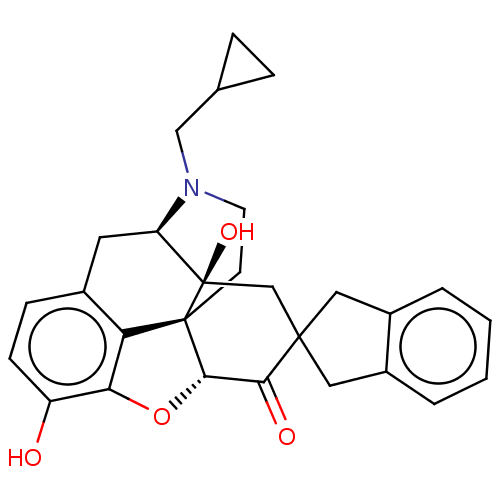 (7-(2''-spiroindanyl)naltrexone | CHEMBL610293) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to Opioid receptor mu 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50058152 (7-(2''-spiroindanyl)naltrexone | CHEMBL610293) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-NTI binding to Opioid receptor delta 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50388986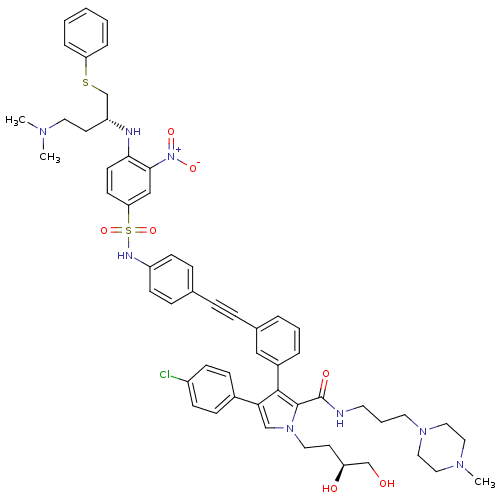 (CHEMBL2063886) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminus 6X His-tagged human Bcl2 expressed in Escherichia coli BL21 (DE3) cells after 2 hrs by fluorescence polarization assay | J Med Chem 55: 4664-82 (2012) Article DOI: 10.1021/jm300178u BindingDB Entry DOI: 10.7270/Q2862HHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50388986 (CHEMBL2063886) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminus 8X His-tagged human Bcl-xL expressed in Escherichia coli BL21 (DE3) cells after 2 hrs by fluorescence polarization ass... | J Med Chem 55: 4664-82 (2012) Article DOI: 10.1021/jm300178u BindingDB Entry DOI: 10.7270/Q2862HHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50388992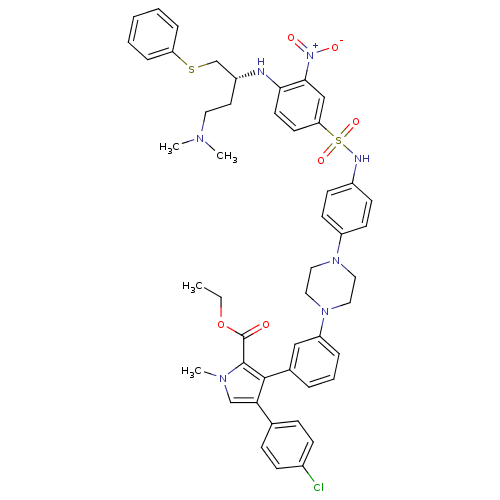 (CHEMBL2063895) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminus 8X His-tagged human Bcl-xL expressed in Escherichia coli BL21 (DE3) cells after 2 hrs by fluorescence polarization ass... | J Med Chem 55: 4664-82 (2012) Article DOI: 10.1021/jm300178u BindingDB Entry DOI: 10.7270/Q2862HHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50388982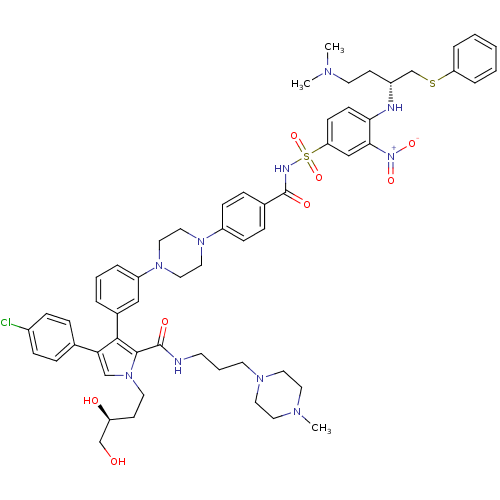 (CHEMBL2063882) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminus 6X His-tagged human Bcl2 expressed in Escherichia coli BL21 (DE3) cells after 2 hrs by fluorescence polarization assay | J Med Chem 55: 4664-82 (2012) Article DOI: 10.1021/jm300178u BindingDB Entry DOI: 10.7270/Q2862HHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [125I]iodosulpiride from human Dopamine receptor D3 expressed in CHO cells | J Med Chem 46: 4377-92 (2003) Article DOI: 10.1021/jm030085p BindingDB Entry DOI: 10.7270/Q21V5FPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50054067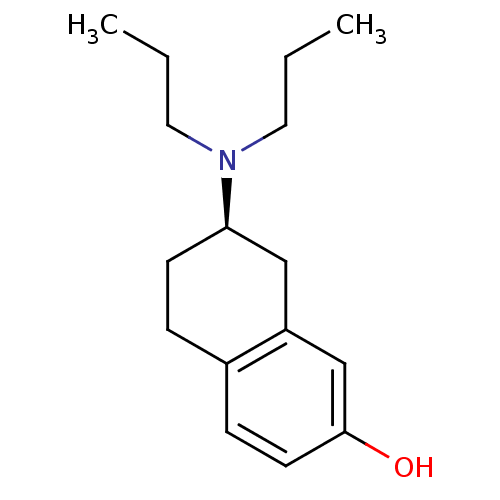 ((2R)-7-Dipropylamino-5,6,7,8-tetrahydro-naphthalen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [125I]iodosulpiride from human Dopamine receptor D3 expressed in CHO cells | J Med Chem 46: 4377-92 (2003) Article DOI: 10.1021/jm030085p BindingDB Entry DOI: 10.7270/Q21V5FPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50061669 ((R)-2-(Benzylamino-methyl)-chroman-7-ol; oxalic ac...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [125I]iodosulpiride from human Dopamine receptor D3 expressed in CHO cells | J Med Chem 46: 4377-92 (2003) Article DOI: 10.1021/jm030085p BindingDB Entry DOI: 10.7270/Q21V5FPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50297170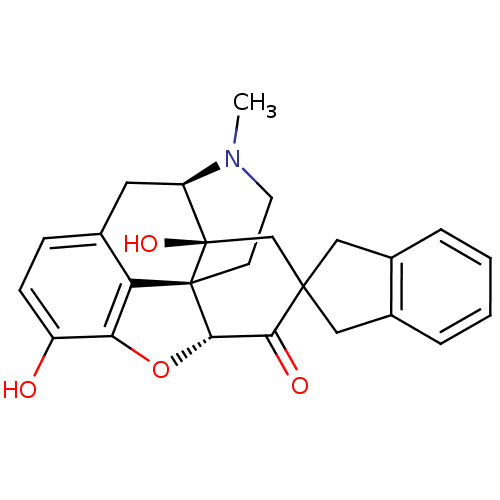 (7-(2'-spiroindanyl)oxymorphone | 7-(Spiroindano)Ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-NTI binding to Opioid receptor delta 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50059996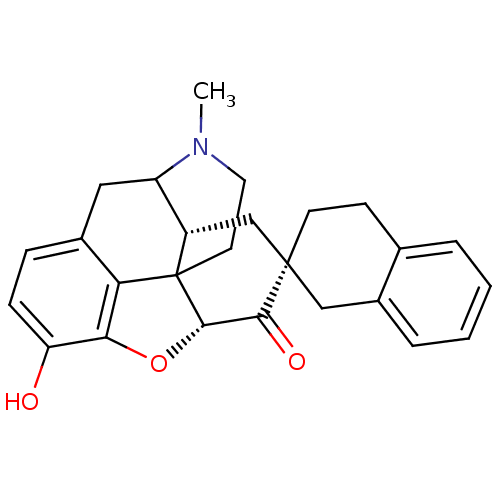 (7 beta-Spirobenzocyclohexylhydromorphone | CHEMBL1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to Opioid receptor mu 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [125I]iodosulpiride from human Dopamine receptor D3 expressed in CHO cells | J Med Chem 46: 4377-92 (2003) Article DOI: 10.1021/jm030085p BindingDB Entry DOI: 10.7270/Q21V5FPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50241107 (1-(3-(4-(5-chloro-2-oxo-2,3-dihydrobenzo[d]imidazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [125I]iodosulpiride from human Dopamine receptor D3 expressed in CHO cells | J Med Chem 46: 4377-92 (2003) Article DOI: 10.1021/jm030085p BindingDB Entry DOI: 10.7270/Q21V5FPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50005118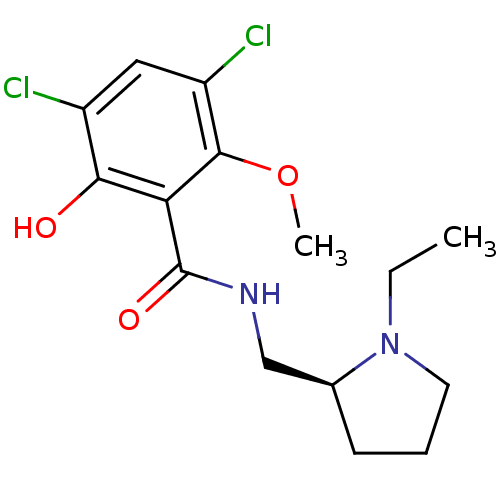 ((S)-3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Compound was measured for its ability to compete with [3H]spiperone binding to the human Dopamine receptor D3 transfected in CHO cells | J Med Chem 46: 4377-92 (2003) Article DOI: 10.1021/jm030085p BindingDB Entry DOI: 10.7270/Q21V5FPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50059995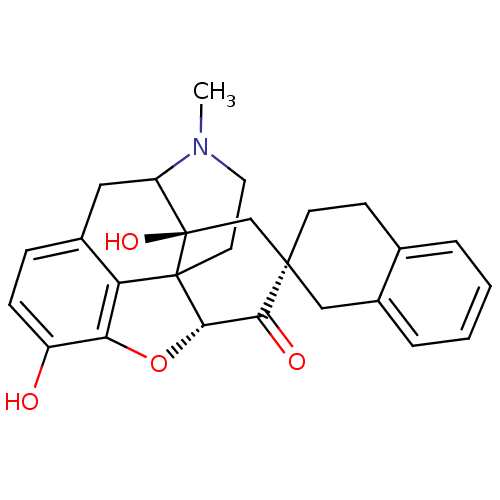 (7 beta-Spirobenzocyclohexyloxymorphone | CHEMBL104...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-NTI binding to Opioid receptor delta 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM11638 (CHEMBL26 | Compound 7 | N-[(1-ethylpyrrolidin-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [125I]iodosulpiride from human Dopamine receptor D3 expressed in CHO cells | J Med Chem 46: 4377-92 (2003) Article DOI: 10.1021/jm030085p BindingDB Entry DOI: 10.7270/Q21V5FPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50388991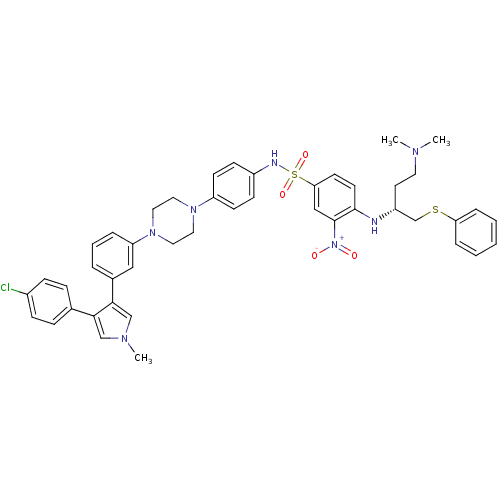 (CHEMBL2063894) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminus 8X His-tagged human Bcl-xL expressed in Escherichia coli BL21 (DE3) cells after 2 hrs by fluorescence polarization ass... | J Med Chem 55: 4664-82 (2012) Article DOI: 10.1021/jm300178u BindingDB Entry DOI: 10.7270/Q2862HHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50059996 (7 beta-Spirobenzocyclohexylhydromorphone | CHEMBL1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-NTI binding to Opioid receptor delta 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50388987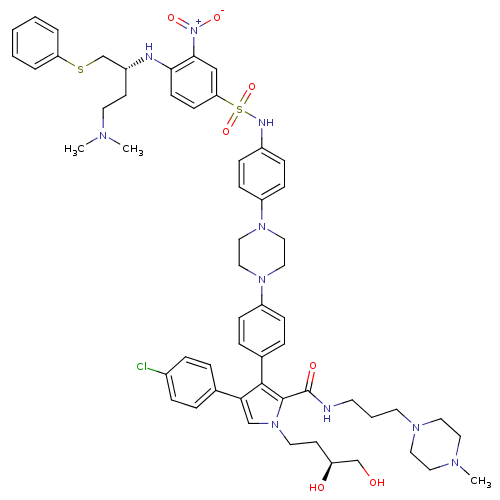 (CHEMBL2063888) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminus 6X His-tagged human Bcl2 expressed in Escherichia coli BL21 (DE3) cells after 2 hrs by fluorescence polarization assay | J Med Chem 55: 4664-82 (2012) Article DOI: 10.1021/jm300178u BindingDB Entry DOI: 10.7270/Q2862HHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50059990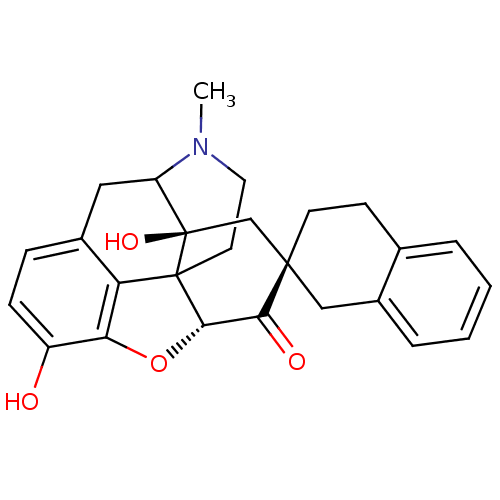 (7 alpha-Spirobenzocyclohexyloxymorphone | CHEMBL31...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-NTI binding to Opioid receptor delta 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50133931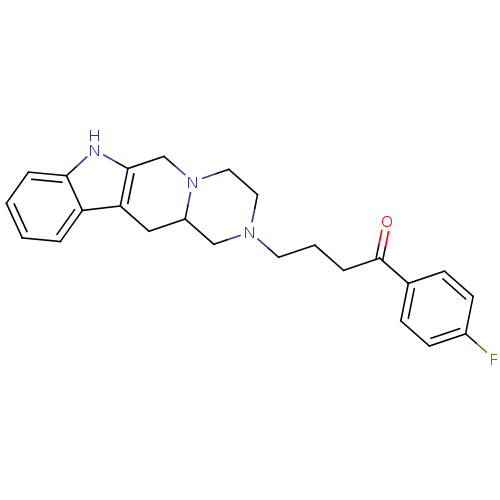 (1-(4-fluoro-phenyl)-4-(3,4,6,7,12,12a-hexahydro-1H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Ability to compete with [3H]YM-09151-2 binding to the human dopamine receptor D3 transfected in CHO cells | J Med Chem 46: 4377-92 (2003) Article DOI: 10.1021/jm030085p BindingDB Entry DOI: 10.7270/Q21V5FPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50388977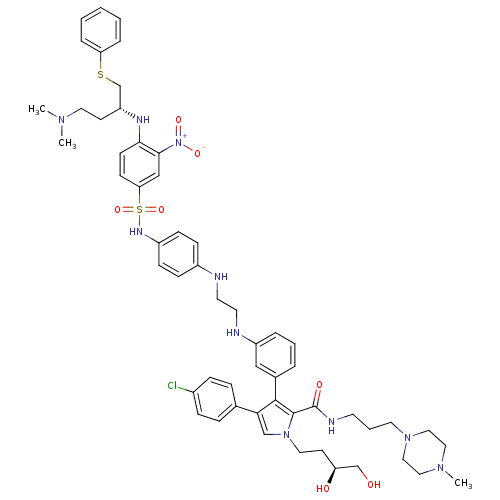 (CHEMBL2063887) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminus 8X His-tagged human Bcl-xL expressed in Escherichia coli BL21 (DE3) cells after 2 hrs by fluorescence polarization ass... | J Med Chem 55: 4664-82 (2012) Article DOI: 10.1021/jm300178u BindingDB Entry DOI: 10.7270/Q2862HHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50388992 (CHEMBL2063895) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminus 6X His-tagged human Bcl2 expressed in Escherichia coli BL21 (DE3) cells after 2 hrs by fluorescence polarization assay | J Med Chem 55: 4664-82 (2012) Article DOI: 10.1021/jm300178u BindingDB Entry DOI: 10.7270/Q2862HHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50133937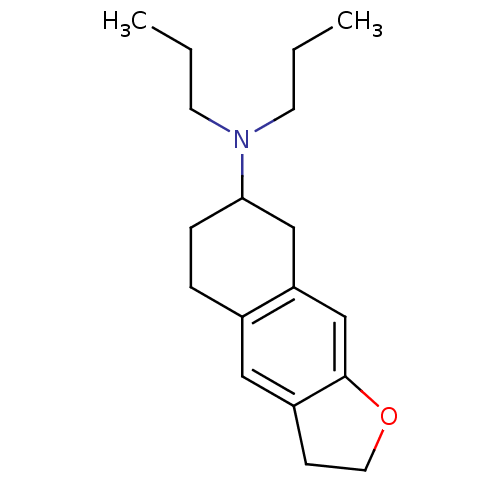 ((2,3,5,6,7,8-Hexahydro-naphtho[2,3-b]furan-7-yl)-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [125I]iodosulpiride from human Dopamine receptor D3 expressed in CHO cells | J Med Chem 46: 4377-92 (2003) Article DOI: 10.1021/jm030085p BindingDB Entry DOI: 10.7270/Q21V5FPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50297170 (7-(2'-spiroindanyl)oxymorphone | 7-(Spiroindano)Ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to Opioid receptor mu 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50388977 (CHEMBL2063887) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminus 6X His-tagged human Bcl2 expressed in Escherichia coli BL21 (DE3) cells after 2 hrs by fluorescence polarization assay | J Med Chem 55: 4664-82 (2012) Article DOI: 10.1021/jm300178u BindingDB Entry DOI: 10.7270/Q2862HHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50048299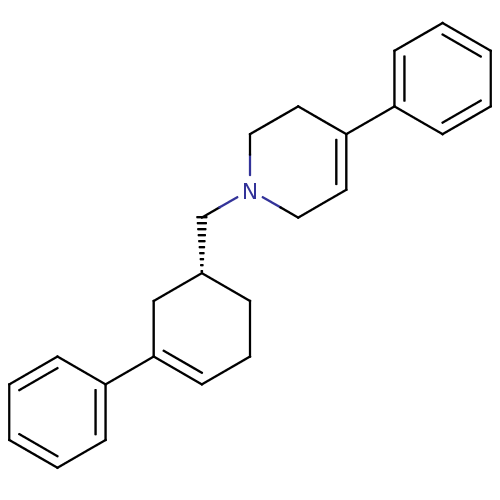 (4-Phenyl-1-((R)-3-phenyl-cyclohex-3-enylmethyl)-1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Compound was measured for its ability to compete with [3H]spiperone binding to the human Dopamine receptor D3 transfected in CHO cells | J Med Chem 46: 4377-92 (2003) Article DOI: 10.1021/jm030085p BindingDB Entry DOI: 10.7270/Q21V5FPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50059995 (7 beta-Spirobenzocyclohexyloxymorphone | CHEMBL104...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to Opioid receptor mu 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50388991 (CHEMBL2063894) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminus 6X His-tagged human Bcl2 expressed in Escherichia coli BL21 (DE3) cells after 2 hrs by fluorescence polarization assay | J Med Chem 55: 4664-82 (2012) Article DOI: 10.1021/jm300178u BindingDB Entry DOI: 10.7270/Q2862HHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50388988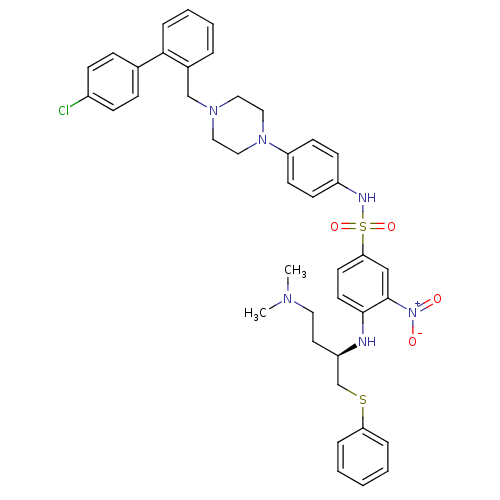 (CHEMBL2063889) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminus 6X His-tagged human Bcl2 expressed in Escherichia coli BL21 (DE3) cells after 2 hrs by fluorescence polarization assay | J Med Chem 55: 4664-82 (2012) Article DOI: 10.1021/jm300178u BindingDB Entry DOI: 10.7270/Q2862HHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50388988 (CHEMBL2063889) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminus 8X His-tagged human Bcl-xL expressed in Escherichia coli BL21 (DE3) cells after 2 hrs by fluorescence polarization ass... | J Med Chem 55: 4664-82 (2012) Article DOI: 10.1021/jm300178u BindingDB Entry DOI: 10.7270/Q2862HHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50133917 (((6S,7R)-6-Methyl-6,7,8,9-tetrahydro-naphtho[1,2-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Compound was measured for its ability to compete with [3H]spiperone binding to the human Dopamine receptor D3 transfected in CHO cells | J Med Chem 46: 4377-92 (2003) Article DOI: 10.1021/jm030085p BindingDB Entry DOI: 10.7270/Q21V5FPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM48320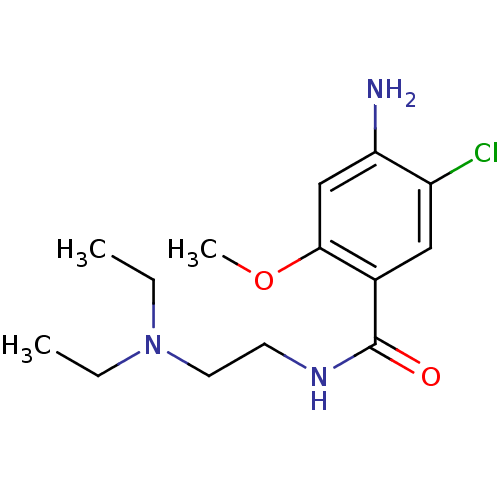 (4-amino-5-chloro-N-[2-(diethylamino)ethyl]-2-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [125I]iodosulpiride from human Dopamine receptor D3 expressed in CHO cells | J Med Chem 46: 4377-92 (2003) Article DOI: 10.1021/jm030085p BindingDB Entry DOI: 10.7270/Q21V5FPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50059990 (7 alpha-Spirobenzocyclohexyloxymorphone | CHEMBL31...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to Opioid receptor mu 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31197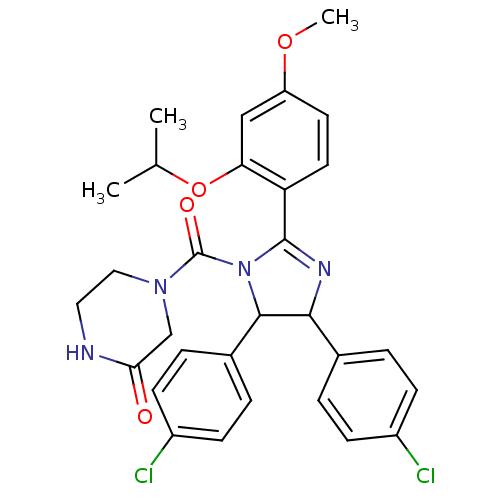 (CHEMBL211045 | Nutlin-3 | med.21724, Compound 186) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 36 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 23 |
University of Michigan | Assay Description The dose-dependent binding experiments were carried out with serial dilutions of the tested compounds in DMSO. A 5 ul sample of the tested samples an... | J Med Chem 49: 3759-62 (2006) Article DOI: 10.1021/jm060023+ BindingDB Entry DOI: 10.7270/Q20P0XCM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50133925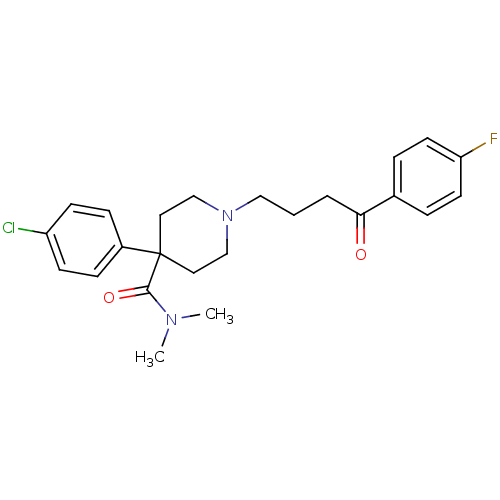 (4-(4-Chloro-phenyl)-1-[4-(4-fluoro-phenyl)-4-oxo-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 43.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Ability to compete with [3H]YM-09151-2 binding to the human dopamine receptor D3 transfected in CHO cells | J Med Chem 46: 4377-92 (2003) Article DOI: 10.1021/jm030085p BindingDB Entry DOI: 10.7270/Q21V5FPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50405961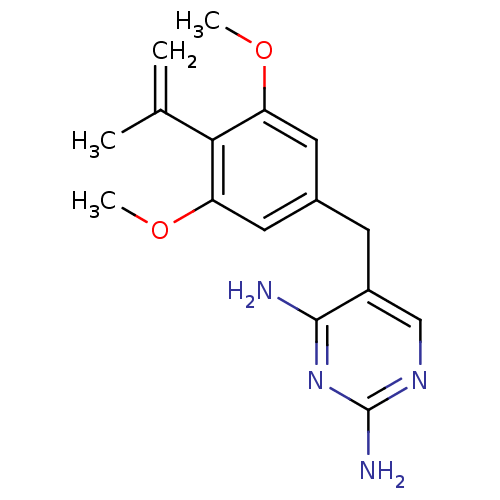 (CHEMBL57787) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pomona College Curated by ChEMBL | Assay Description Inhibitory activity against Lactobacillus casei dihydrofolate reductase | J Med Chem 32: 1895-905 (1989) BindingDB Entry DOI: 10.7270/Q2QR4ZB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50029763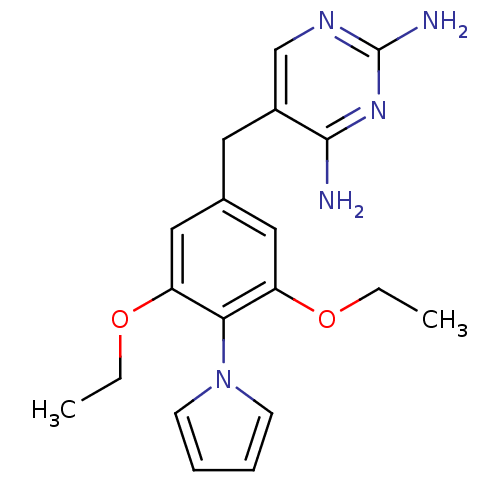 (5-(3,5-Diethoxy-4-pyrrol-1-yl-benzyl)-pyrimidine-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pomona College Curated by ChEMBL | Assay Description Inhibitory activity against Lactobacillus casei dihydrofolate reductase | J Med Chem 32: 1895-905 (1989) BindingDB Entry DOI: 10.7270/Q2QR4ZB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50027970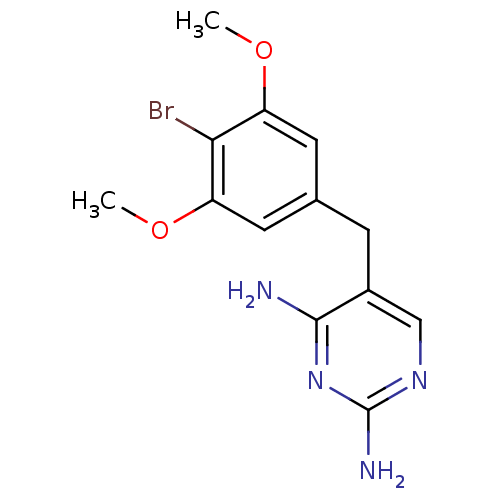 (5-(4-Bromo-3,5-dimethoxy-benzyl)-pyrimidine-2,4-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pomona College Curated by ChEMBL | Assay Description Inhibitory activity against Lactobacillus casei dihydrofolate reductase | J Med Chem 32: 1895-905 (1989) BindingDB Entry DOI: 10.7270/Q2QR4ZB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1140 total ) | Next | Last >> |