 Found 2373 hits with Last Name = 'lai' and Initial = 'y'
Found 2373 hits with Last Name = 'lai' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230846
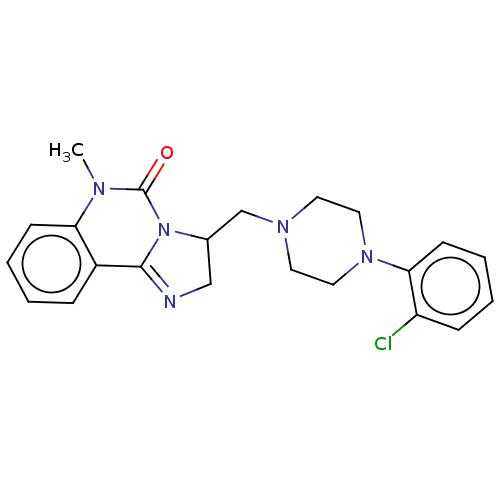
(CHEMBL2114071)Show SMILES CN1C(=O)N2C(CN3CCN(CC3)c3ccccc3Cl)CN=C2c2ccccc12 |c:23| Show InChI InChI=1S/C22H24ClN5O/c1-25-19-8-4-2-6-17(19)21-24-14-16(28(21)22(25)29)15-26-10-12-27(13-11-26)20-9-5-3-7-18(20)23/h2-9,16H,10-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). |
J Med Chem 36: 2196-207 (1993)
Article DOI: 10.1021/jm00067a017
BindingDB Entry DOI: 10.7270/Q298896M |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230859
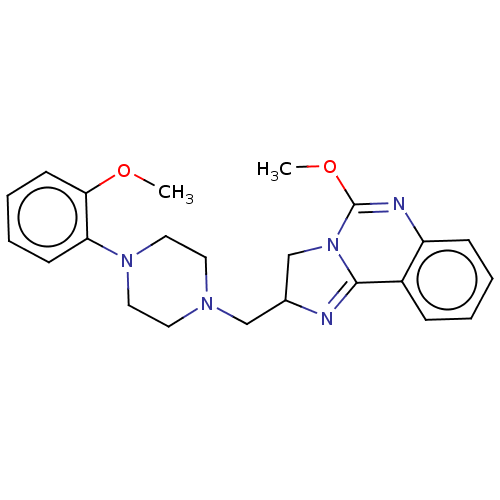
(CHEMBL309150)Show SMILES COC1=Nc2ccccc2C2=NC(CN3CCN(CC3)c3ccccc3OC)CN12 |t:2,11| Show InChI InChI=1S/C23H27N5O2/c1-29-21-10-6-5-9-20(21)27-13-11-26(12-14-27)15-17-16-28-22(24-17)18-7-3-4-8-19(18)25-23(28)30-2/h3-10,17H,11-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). |
J Med Chem 36: 2196-207 (1993)
Article DOI: 10.1021/jm00067a017
BindingDB Entry DOI: 10.7270/Q298896M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 (828-1132) expressed in baculovirus-infected Sf9 cells using EQEDEPEGDYFEWLE as substrate after 1 hr by HTRF assay |
J Med Chem 56: 4521-36 (2013)
Article DOI: 10.1021/jm400266t
BindingDB Entry DOI: 10.7270/Q2VX0HX0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230848
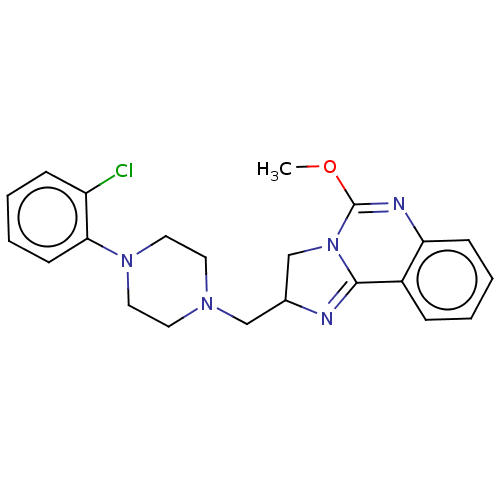
(CHEMBL70872)Show SMILES COC1=Nc2ccccc2C2=NC(CN3CCN(CC3)c3ccccc3Cl)CN12 |t:2,11| Show InChI InChI=1S/C22H24ClN5O/c1-29-22-25-19-8-4-2-6-17(19)21-24-16(15-28(21)22)14-26-10-12-27(13-11-26)20-9-5-3-7-18(20)23/h2-9,16H,10-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). |
J Med Chem 36: 2196-207 (1993)
Article DOI: 10.1021/jm00067a017
BindingDB Entry DOI: 10.7270/Q298896M |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). |
J Med Chem 36: 2196-207 (1993)
Article DOI: 10.1021/jm00067a017
BindingDB Entry DOI: 10.7270/Q298896M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human JAK1 (837-1142) expressed in baculovirus-infected Sf9 cells using EQEDEPEGDYFEWLE as substrate after 1 hr by HTRF assay |
J Med Chem 56: 4521-36 (2013)
Article DOI: 10.1021/jm400266t
BindingDB Entry DOI: 10.7270/Q2VX0HX0 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50066109
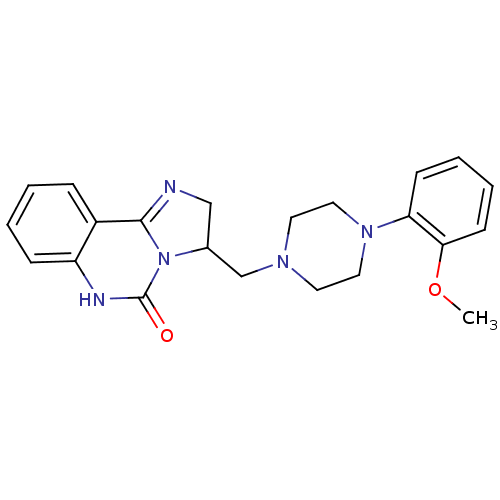
(3-[4-(2-Methoxy-phenyl)-piperazin-1-ylmethyl]-2,6-...)Show SMILES COc1ccccc1N1CCN(CC2CN=C3N2C(=O)Nc2ccccc32)CC1 |c:16| Show InChI InChI=1S/C22H25N5O2/c1-29-20-9-5-4-8-19(20)26-12-10-25(11-13-26)15-16-14-23-21-17-6-2-3-7-18(17)24-22(28)27(16)21/h2-9,16H,10-15H2,1H3,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). |
J Med Chem 36: 2196-207 (1993)
Article DOI: 10.1021/jm00067a017
BindingDB Entry DOI: 10.7270/Q298896M |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50040253
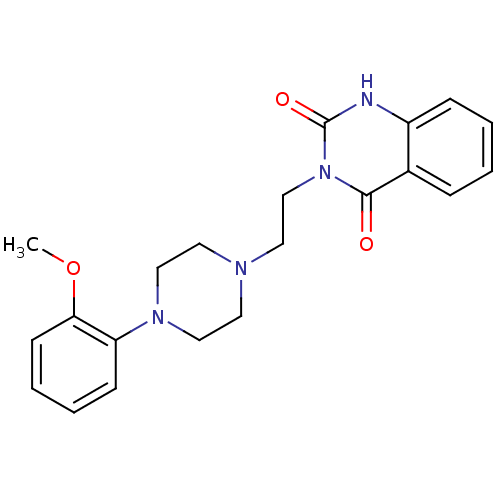
(3-{2-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-ethyl}-...)Show SMILES COc1ccccc1N1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C21H24N4O3/c1-28-19-9-5-4-8-18(19)24-13-10-23(11-14-24)12-15-25-20(26)16-6-2-3-7-17(16)22-21(25)27/h2-9H,10-15H2,1H3,(H,22,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). |
J Med Chem 36: 2196-207 (1993)
Article DOI: 10.1021/jm00067a017
BindingDB Entry DOI: 10.7270/Q298896M |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM120141
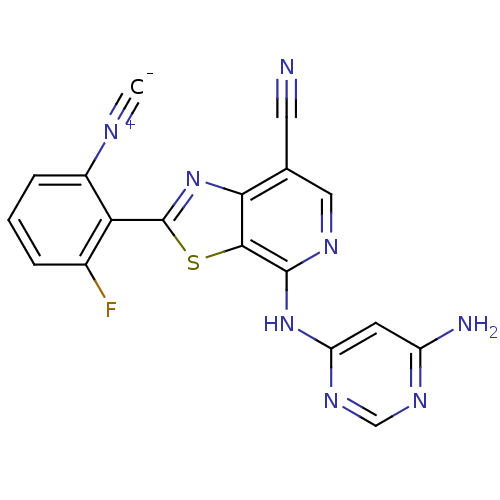
(US8697708, 224)Show SMILES Nc1cc(Nc2ncc(C#N)c3nc(sc23)-c2c(F)cccc2[N+]#[C-])ncn1 Show InChI InChI=1S/C18H9FN8S/c1-22-11-4-2-3-10(19)14(11)18-27-15-9(6-20)7-23-17(16(15)28-18)26-13-5-12(21)24-8-25-13/h2-5,7-8H,(H3,21,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.300 | -53.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
F. Hoffmann-La Roche AG
US Patent
| Assay Description
To determine the inhibition constants (Ki) of Examples 1-240, compounds were diluted serially in DMSO and added to 50 μL kinase reactions contai... |
US Patent US8697708 (2014)
BindingDB Entry DOI: 10.7270/Q2J38R6F |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50261255
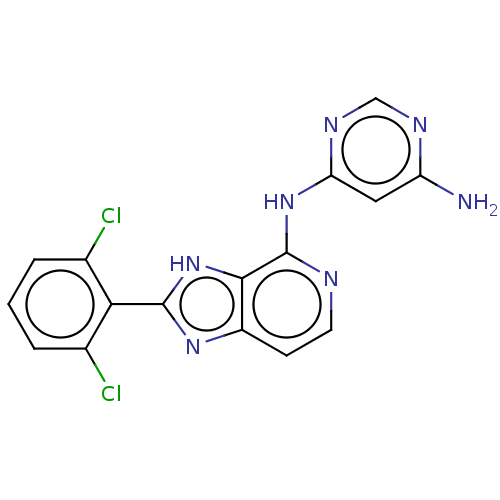
(CHEMBL4084436)Show SMILES Nc1cc(Nc2nccc3nc([nH]c23)-c2c(Cl)cccc2Cl)ncn1 Show InChI InChI=1S/C16H11Cl2N7/c17-8-2-1-3-9(18)13(8)15-23-10-4-5-20-16(14(10)25-15)24-12-6-11(19)21-7-22-12/h1-7H,(H,23,25)(H3,19,20,21,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 (unknown origin) by biochemical assay |
Bioorg Med Chem Lett 27: 4370-4376 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.022
BindingDB Entry DOI: 10.7270/Q2RF5XG8 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM120132
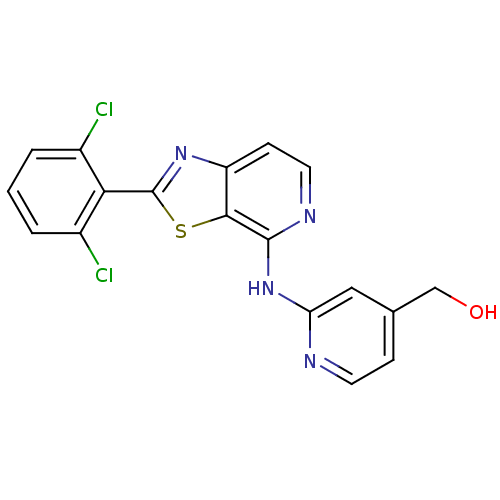
(US8697708, 18)Show SMILES OCc1ccnc(Nc2nccc3nc(sc23)-c2c(Cl)cccc2Cl)c1 Show InChI InChI=1S/C18H12Cl2N4OS/c19-11-2-1-3-12(20)15(11)18-23-13-5-7-22-17(16(13)26-18)24-14-8-10(9-25)4-6-21-14/h1-8,25H,9H2,(H,21,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.300 | -53.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
F. Hoffmann-La Roche AG
US Patent
| Assay Description
To determine the inhibition constants (Ki) of Examples 1-240, compounds were diluted serially in DMSO and added to 50 μL kinase reactions contai... |
US Patent US8697708 (2014)
BindingDB Entry DOI: 10.7270/Q2J38R6F |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230844
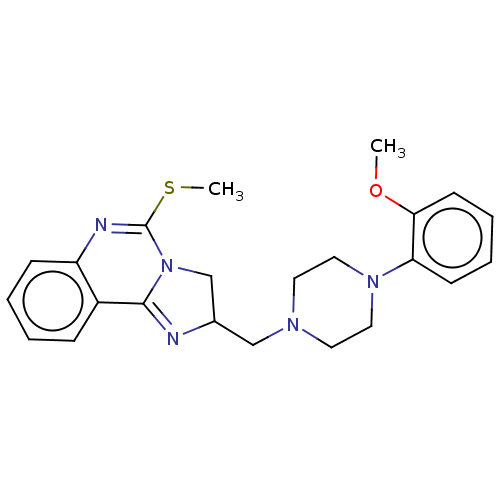
(CHEMBL71470)Show SMILES COc1ccccc1N1CCN(CC2CN3C(=N2)c2ccccc2N=C3SC)CC1 |c:17,27| Show InChI InChI=1S/C23H27N5OS/c1-29-21-10-6-5-9-20(21)27-13-11-26(12-14-27)15-17-16-28-22(24-17)18-7-3-4-8-19(18)25-23(28)30-2/h3-10,17H,11-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). |
J Med Chem 36: 2196-207 (1993)
Article DOI: 10.1021/jm00067a017
BindingDB Entry DOI: 10.7270/Q298896M |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230870
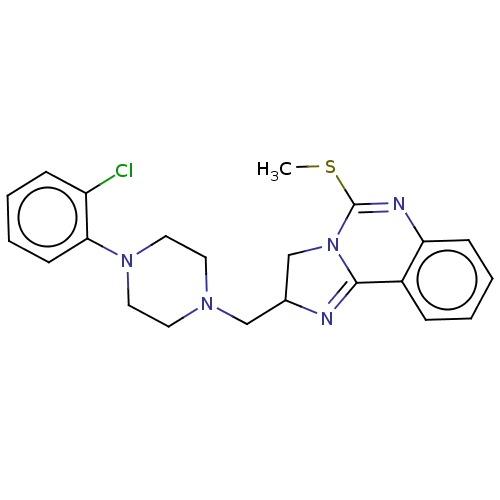
(CHEMBL70498)Show SMILES CSC1=Nc2ccccc2C2=NC(CN3CCN(CC3)c3ccccc3Cl)CN12 |t:2,11| Show InChI InChI=1S/C22H24ClN5S/c1-29-22-25-19-8-4-2-6-17(19)21-24-16(15-28(21)22)14-26-10-12-27(13-11-26)20-9-5-3-7-18(20)23/h2-9,16H,10-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). |
J Med Chem 36: 2196-207 (1993)
Article DOI: 10.1021/jm00067a017
BindingDB Entry DOI: 10.7270/Q298896M |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230861
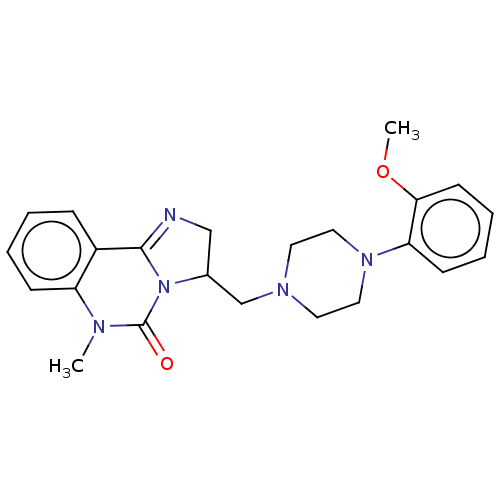
(CHEMBL2114072)Show SMILES COc1ccccc1N1CCN(CC2CN=C3N2C(=O)N(C)c2ccccc32)CC1 |c:16| Show InChI InChI=1S/C23H27N5O2/c1-25-19-8-4-3-7-18(19)22-24-15-17(28(22)23(25)29)16-26-11-13-27(14-12-26)20-9-5-6-10-21(20)30-2/h3-10,17H,11-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). |
J Med Chem 36: 2196-207 (1993)
Article DOI: 10.1021/jm00067a017
BindingDB Entry DOI: 10.7270/Q298896M |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230853
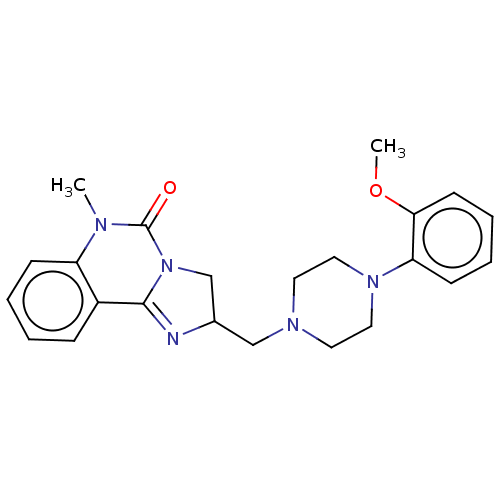
(CHEMBL304165)Show SMILES COc1ccccc1N1CCN(CC2CN3C(=N2)c2ccccc2N(C)C3=O)CC1 |c:17| Show InChI InChI=1S/C23H27N5O2/c1-25-19-8-4-3-7-18(19)22-24-17(16-28(22)23(25)29)15-26-11-13-27(14-12-26)20-9-5-6-10-21(20)30-2/h3-10,17H,11-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). |
J Med Chem 36: 2196-207 (1993)
Article DOI: 10.1021/jm00067a017
BindingDB Entry DOI: 10.7270/Q298896M |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50261268
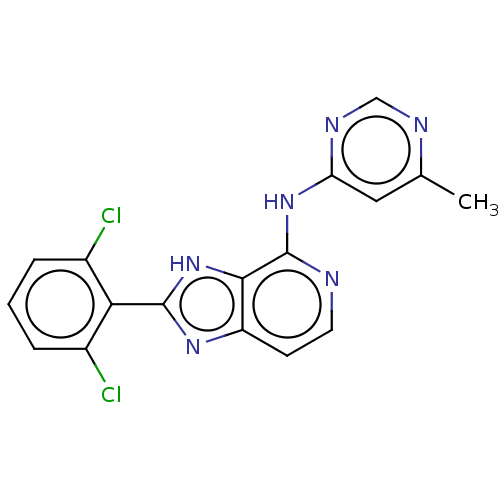
(CHEMBL4062758)Show SMILES Cc1cc(Nc2nccc3nc([nH]c23)-c2c(Cl)cccc2Cl)ncn1 Show InChI InChI=1S/C17H12Cl2N6/c1-9-7-13(22-8-21-9)24-17-15-12(5-6-20-17)23-16(25-15)14-10(18)3-2-4-11(14)19/h2-8H,1H3,(H,23,25)(H,20,21,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 (unknown origin) by biochemical assay |
Bioorg Med Chem Lett 27: 4370-4376 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.022
BindingDB Entry DOI: 10.7270/Q2RF5XG8 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM120140
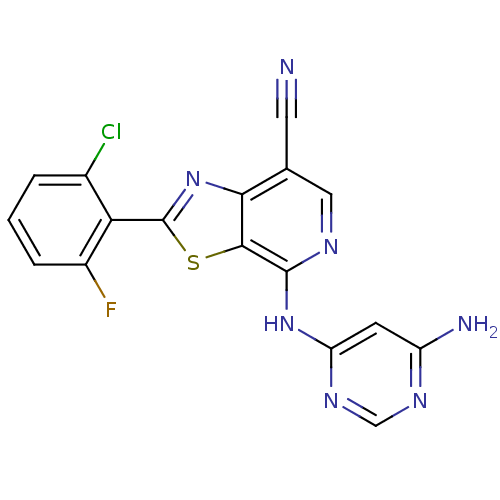
(US8697708, 223)Show SMILES Nc1cc(Nc2ncc(C#N)c3nc(sc23)-c2c(F)cccc2Cl)ncn1 Show InChI InChI=1S/C17H9ClFN7S/c18-9-2-1-3-10(19)13(9)17-26-14-8(5-20)6-22-16(15(14)27-17)25-12-4-11(21)23-7-24-12/h1-4,6-7H,(H3,21,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.400 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
F. Hoffmann-La Roche AG
US Patent
| Assay Description
To determine the inhibition constants (Ki) of Examples 1-240, compounds were diluted serially in DMSO and added to 50 μL kinase reactions contai... |
US Patent US8697708 (2014)
BindingDB Entry DOI: 10.7270/Q2J38R6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human GST-fused JAK3 catalytic domain expressed in baculovirus-infected Sf9 cells using polyglutamic acid-tyrosine as substrate after 3... |
J Med Chem 56: 4521-36 (2013)
Article DOI: 10.1021/jm400266t
BindingDB Entry DOI: 10.7270/Q2VX0HX0 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM120128
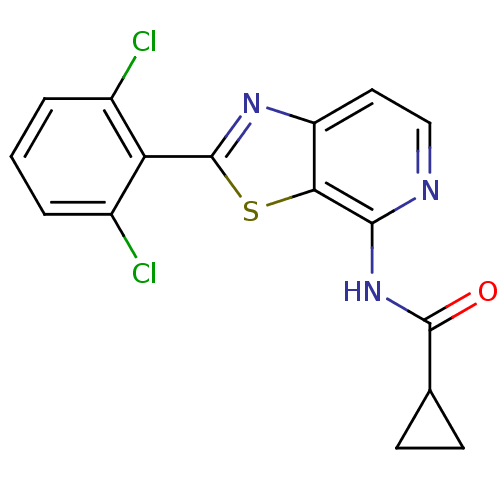
(US8697708, 2)Show InChI InChI=1S/C16H11Cl2N3OS/c17-9-2-1-3-10(18)12(9)16-20-11-6-7-19-14(13(11)23-16)21-15(22)8-4-5-8/h1-3,6-8H,4-5H2,(H,19,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 (unknown origin) assessed as reduction in IL-23 induced STAT3 phosphorylation by cell based ELISA |
Bioorg Med Chem Lett 27: 4370-4376 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.022
BindingDB Entry DOI: 10.7270/Q2RF5XG8 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50261261
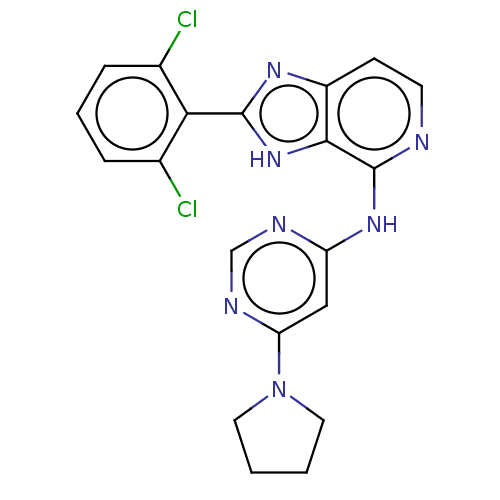
(CHEMBL4075453)Show SMILES Clc1cccc(Cl)c1-c1nc2ccnc(Nc3cc(ncn3)N3CCCC3)c2[nH]1 Show InChI InChI=1S/C20H17Cl2N7/c21-12-4-3-5-13(22)17(12)19-26-14-6-7-23-20(18(14)28-19)27-15-10-16(25-11-24-15)29-8-1-2-9-29/h3-7,10-11H,1-2,8-9H2,(H,26,28)(H,23,24,25,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 (unknown origin) by biochemical assay |
Bioorg Med Chem Lett 27: 4370-4376 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.022
BindingDB Entry DOI: 10.7270/Q2RF5XG8 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM120143

(US8697708, 236)Show SMILES Cc1nc(CO)cc(Nc2nccc3nc(sc23)-c2c(Cl)cccc2[N+]#[C-])n1 Show InChI InChI=1S/C19H13ClN6OS/c1-10-23-11(9-27)8-15(24-10)26-18-17-14(6-7-22-18)25-19(28-17)16-12(20)4-3-5-13(16)21-2/h3-8,27H,9H2,1H3,(H,22,23,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.5 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
F. Hoffmann-La Roche AG
US Patent
| Assay Description
To determine the inhibition constants (Ki) of Examples 1-240, compounds were diluted serially in DMSO and added to 50 μL kinase reactions contai... |
US Patent US8697708 (2014)
BindingDB Entry DOI: 10.7270/Q2J38R6F |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50261256
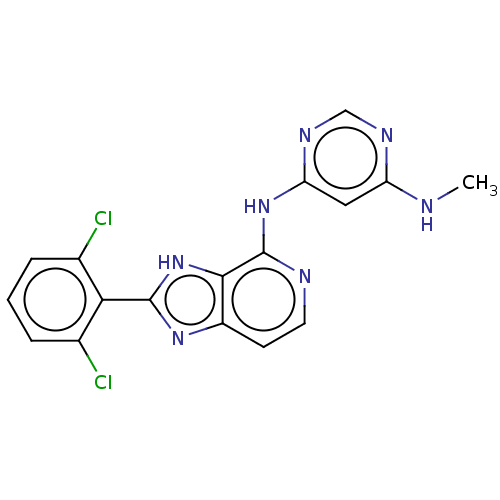
(CHEMBL4071399)Show SMILES CNc1cc(Nc2nccc3nc([nH]c23)-c2c(Cl)cccc2Cl)ncn1 Show InChI InChI=1S/C17H13Cl2N7/c1-20-12-7-13(23-8-22-12)25-17-15-11(5-6-21-17)24-16(26-15)14-9(18)3-2-4-10(14)19/h2-8H,1H3,(H,24,26)(H2,20,21,22,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 (unknown origin) by biochemical assay |
Bioorg Med Chem Lett 27: 4370-4376 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.022
BindingDB Entry DOI: 10.7270/Q2RF5XG8 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM120128

(US8697708, 2)Show InChI InChI=1S/C16H11Cl2N3OS/c17-9-2-1-3-10(18)12(9)16-20-11-6-7-19-14(13(11)23-16)21-15(22)8-4-5-8/h1-3,6-8H,4-5H2,(H,19,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.5 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
F. Hoffmann-La Roche AG
US Patent
| Assay Description
To determine the inhibition constants (Ki) of Examples 1-240, compounds were diluted serially in DMSO and added to 50 μL kinase reactions contai... |
US Patent US8697708 (2014)
BindingDB Entry DOI: 10.7270/Q2J38R6F |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230847
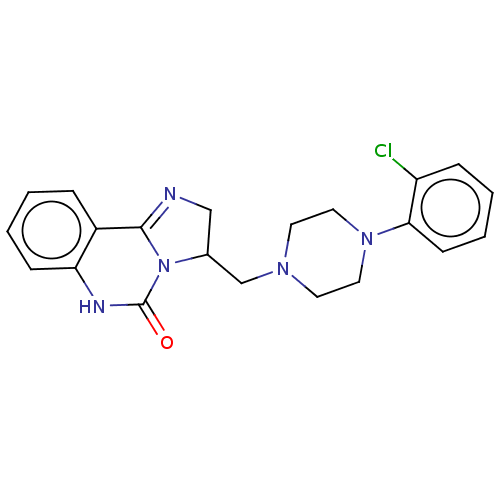
(CHEMBL2114069)Show SMILES Clc1ccccc1N1CCN(CC2CN=C3N2C(=O)Nc2ccccc32)CC1 |c:15| Show InChI InChI=1S/C21H22ClN5O/c22-17-6-2-4-8-19(17)26-11-9-25(10-12-26)14-15-13-23-20-16-5-1-3-7-18(16)24-21(28)27(15)20/h1-8,15H,9-14H2,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). |
J Med Chem 36: 2196-207 (1993)
Article DOI: 10.1021/jm00067a017
BindingDB Entry DOI: 10.7270/Q298896M |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50261254
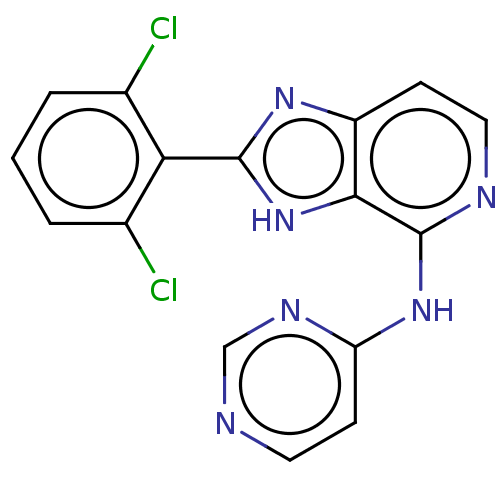
(CHEMBL4074130)Show InChI InChI=1S/C16H10Cl2N6/c17-9-2-1-3-10(18)13(9)15-22-11-4-7-20-16(14(11)24-15)23-12-5-6-19-8-21-12/h1-8H,(H,22,24)(H,19,20,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 (unknown origin) assessed as reduction in IL-23 induced STAT3 phosphorylation by cell based ELISA |
Bioorg Med Chem Lett 27: 4370-4376 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.022
BindingDB Entry DOI: 10.7270/Q2RF5XG8 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50261260
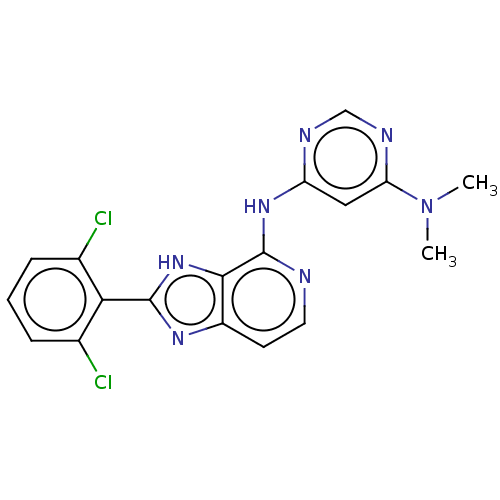
(CHEMBL4069942)Show SMILES CN(C)c1cc(Nc2nccc3nc([nH]c23)-c2c(Cl)cccc2Cl)ncn1 Show InChI InChI=1S/C18H15Cl2N7/c1-27(2)14-8-13(22-9-23-14)25-18-16-12(6-7-21-18)24-17(26-16)15-10(19)4-3-5-11(15)20/h3-9H,1-2H3,(H,24,26)(H,21,22,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 (unknown origin) assessed as reduction in IL-23 induced STAT3 phosphorylation by cell based ELISA |
Bioorg Med Chem Lett 27: 4370-4376 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.022
BindingDB Entry DOI: 10.7270/Q2RF5XG8 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50261274
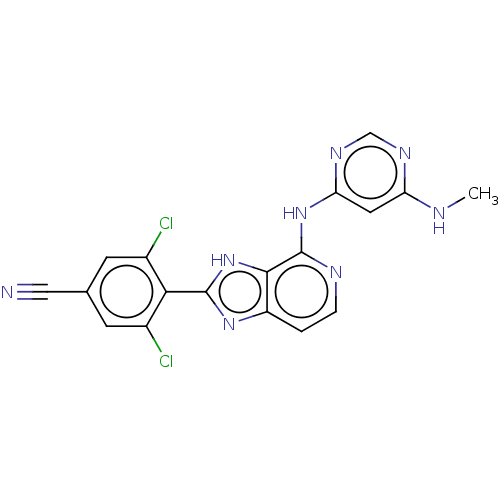
(CHEMBL4062680)Show SMILES CNc1cc(Nc2nccc3nc([nH]c23)-c2c(Cl)cc(cc2Cl)C#N)ncn1 Show InChI InChI=1S/C18H12Cl2N8/c1-22-13-6-14(25-8-24-13)27-18-16-12(2-3-23-18)26-17(28-16)15-10(19)4-9(7-21)5-11(15)20/h2-6,8H,1H3,(H,26,28)(H2,22,23,24,25,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 (unknown origin) by biochemical assay |
Bioorg Med Chem Lett 27: 4370-4376 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.022
BindingDB Entry DOI: 10.7270/Q2RF5XG8 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50261276
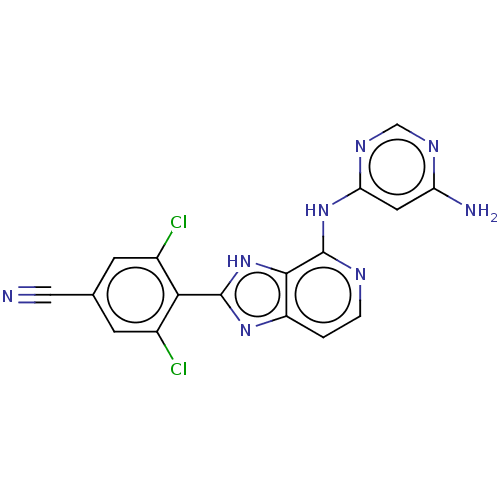
(CHEMBL4092116)Show SMILES Nc1cc(Nc2nccc3nc([nH]c23)-c2c(Cl)cc(cc2Cl)C#N)ncn1 Show InChI InChI=1S/C17H10Cl2N8/c18-9-3-8(6-20)4-10(19)14(9)16-25-11-1-2-22-17(15(11)27-16)26-13-5-12(21)23-7-24-13/h1-5,7H,(H,25,27)(H3,21,22,23,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 (unknown origin) by biochemical assay |
Bioorg Med Chem Lett 27: 4370-4376 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.022
BindingDB Entry DOI: 10.7270/Q2RF5XG8 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50261270
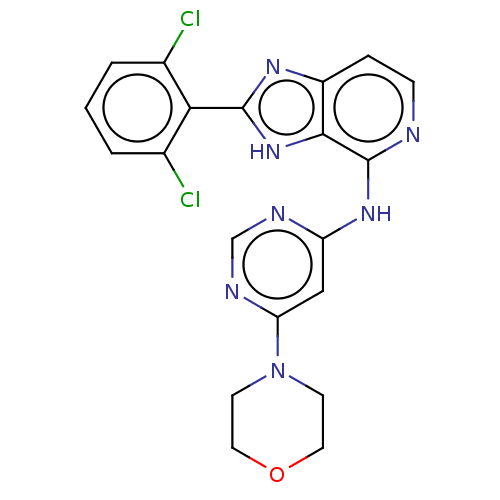
(CHEMBL4093872)Show SMILES Clc1cccc(Cl)c1-c1nc2ccnc(Nc3cc(ncn3)N3CCOCC3)c2[nH]1 Show InChI InChI=1S/C20H17Cl2N7O/c21-12-2-1-3-13(22)17(12)19-26-14-4-5-23-20(18(14)28-19)27-15-10-16(25-11-24-15)29-6-8-30-9-7-29/h1-5,10-11H,6-9H2,(H,26,28)(H,23,24,25,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 (unknown origin) by biochemical assay |
Bioorg Med Chem Lett 27: 4370-4376 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.022
BindingDB Entry DOI: 10.7270/Q2RF5XG8 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230851
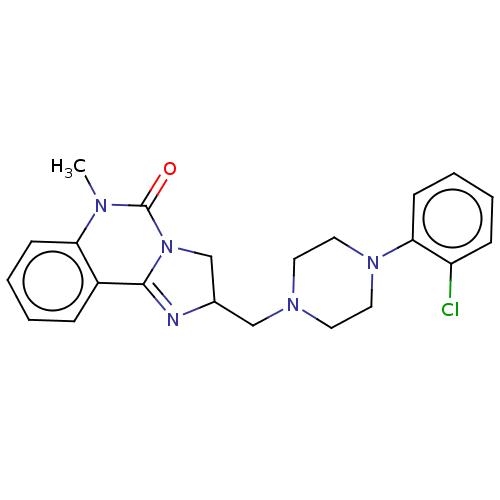
(CHEMBL70347)Show SMILES CN1C(=O)N2CC(CN3CCN(CC3)c3ccccc3Cl)N=C2c2ccccc12 |c:23| Show InChI InChI=1S/C22H24ClN5O/c1-25-19-8-4-2-6-17(19)21-24-16(15-28(21)22(25)29)14-26-10-12-27(13-11-26)20-9-5-3-7-18(20)23/h2-9,16H,10-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). |
J Med Chem 36: 2196-207 (1993)
Article DOI: 10.1021/jm00067a017
BindingDB Entry DOI: 10.7270/Q298896M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human GST-fused JAK2 catalytic domain expressed in baculovirus-infected Sf9 cells using polyglutamic acid-tyrosine as substrate after 3... |
J Med Chem 56: 4521-36 (2013)
Article DOI: 10.1021/jm400266t
BindingDB Entry DOI: 10.7270/Q2VX0HX0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50261269
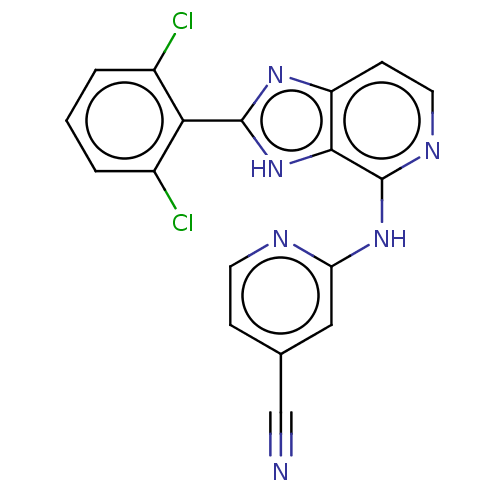
(CHEMBL4092191)Show SMILES Clc1cccc(Cl)c1-c1nc2ccnc(Nc3cc(ccn3)C#N)c2[nH]1 Show InChI InChI=1S/C18H10Cl2N6/c19-11-2-1-3-12(20)15(11)17-24-13-5-7-23-18(16(13)26-17)25-14-8-10(9-21)4-6-22-14/h1-8H,(H,24,26)(H,22,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 (unknown origin) by biochemical assay |
Bioorg Med Chem Lett 27: 4370-4376 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.022
BindingDB Entry DOI: 10.7270/Q2RF5XG8 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50261273
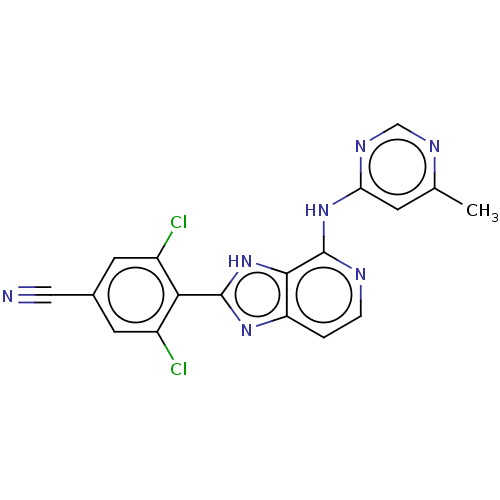
(CHEMBL4076947)Show SMILES Cc1cc(Nc2nccc3nc([nH]c23)-c2c(Cl)cc(cc2Cl)C#N)ncn1 Show InChI InChI=1S/C18H11Cl2N7/c1-9-4-14(24-8-23-9)26-18-16-13(2-3-22-18)25-17(27-16)15-11(19)5-10(7-21)6-12(15)20/h2-6,8H,1H3,(H,25,27)(H,22,23,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 (unknown origin) assessed as reduction in IL-23 induced STAT3 phosphorylation by cell based ELISA |
Bioorg Med Chem Lett 27: 4370-4376 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.022
BindingDB Entry DOI: 10.7270/Q2RF5XG8 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50261257
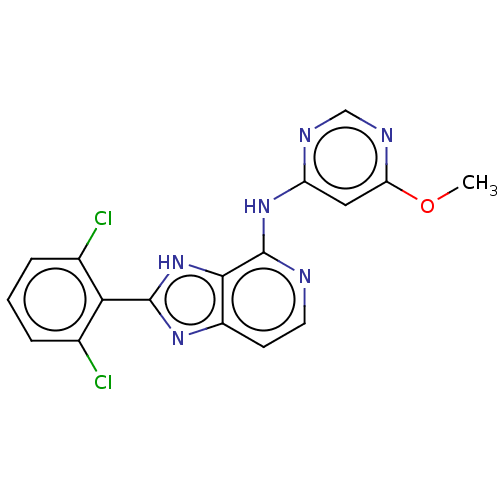
(CHEMBL4099854)Show SMILES COc1cc(Nc2nccc3nc([nH]c23)-c2c(Cl)cccc2Cl)ncn1 Show InChI InChI=1S/C17H12Cl2N6O/c1-26-13-7-12(21-8-22-13)24-17-15-11(5-6-20-17)23-16(25-15)14-9(18)3-2-4-10(14)19/h2-8H,1H3,(H,23,25)(H,20,21,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 (unknown origin) by biochemical assay |
Bioorg Med Chem Lett 27: 4370-4376 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.022
BindingDB Entry DOI: 10.7270/Q2RF5XG8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human GST-fused JAK1 catalytic domain expressed in baculovirus-infected Sf9 cells using polyglutamic acid-tyrosine as substrate after 3... |
J Med Chem 56: 4521-36 (2013)
Article DOI: 10.1021/jm400266t
BindingDB Entry DOI: 10.7270/Q2VX0HX0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM120142

(US8697708, 227)Show SMILES Cc1cc(Nc2nccc3nc(sc23)-c2c(cc(cc2[N+]#[C-])C#N)[N+]#[C-])nc(C)n1 Show InChI InChI=1S/C21H12N8S/c1-11-7-17(27-12(2)26-11)29-20-19-14(5-6-25-20)28-21(30-19)18-15(23-3)8-13(10-22)9-16(18)24-4/h5-9H,1-2H3,(H,25,26,27,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.800 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
F. Hoffmann-La Roche AG
US Patent
| Assay Description
To determine the inhibition constants (Ki) of Examples 1-240, compounds were diluted serially in DMSO and added to 50 μL kinase reactions contai... |
US Patent US8697708 (2014)
BindingDB Entry DOI: 10.7270/Q2J38R6F |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50261283
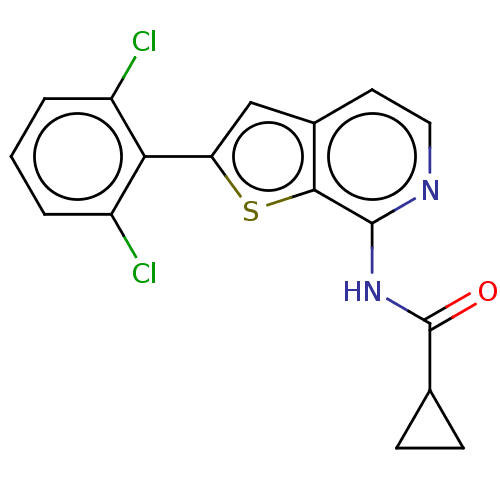
(CHEMBL4093172)Show InChI InChI=1S/C17H12Cl2N2OS/c18-11-2-1-3-12(19)14(11)13-8-10-6-7-20-16(15(10)23-13)21-17(22)9-4-5-9/h1-3,6-9H,4-5H2,(H,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 (unknown origin) assessed as reduction in IL-23 induced STAT3 phosphorylation by cell based ELISA |
Bioorg Med Chem Lett 27: 4370-4376 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.022
BindingDB Entry DOI: 10.7270/Q2RF5XG8 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50261262
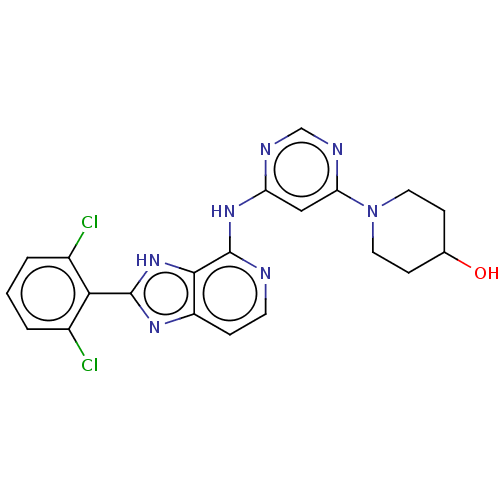
(CHEMBL4070262)Show SMILES OC1CCN(CC1)c1cc(Nc2nccc3nc([nH]c23)-c2c(Cl)cccc2Cl)ncn1 Show InChI InChI=1S/C21H19Cl2N7O/c22-13-2-1-3-14(23)18(13)20-27-15-4-7-24-21(19(15)29-20)28-16-10-17(26-11-25-16)30-8-5-12(31)6-9-30/h1-4,7,10-12,31H,5-6,8-9H2,(H,27,29)(H,24,25,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 (unknown origin) by biochemical assay |
Bioorg Med Chem Lett 27: 4370-4376 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.022
BindingDB Entry DOI: 10.7270/Q2RF5XG8 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM120133

(US8697708, 22)Show SMILES Cc1cc(Nc2nccc3nc(sc23)-c2c(Cl)cc(CO)cc2Cl)ncn1 Show InChI InChI=1S/C18H13Cl2N5OS/c1-9-4-14(23-8-22-9)25-17-16-13(2-3-21-17)24-18(27-16)15-11(19)5-10(7-26)6-12(15)20/h2-6,8,26H,7H2,1H3,(H,21,22,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
F. Hoffmann-La Roche AG
US Patent
| Assay Description
To determine the inhibition constants (Ki) of Examples 1-240, compounds were diluted serially in DMSO and added to 50 μL kinase reactions contai... |
US Patent US8697708 (2014)
BindingDB Entry DOI: 10.7270/Q2J38R6F |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230850
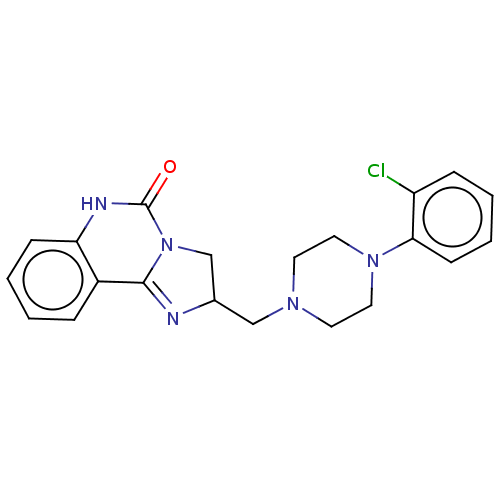
(CHEMBL309149)Show SMILES Clc1ccccc1N1CCN(CC2CN3C(=N2)c2ccccc2NC3=O)CC1 |c:16| Show InChI InChI=1S/C21H22ClN5O/c22-17-6-2-4-8-19(17)26-11-9-25(10-12-26)13-15-14-27-20(23-15)16-5-1-3-7-18(16)24-21(27)28/h1-8,15H,9-14H2,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). |
J Med Chem 36: 2196-207 (1993)
Article DOI: 10.1021/jm00067a017
BindingDB Entry DOI: 10.7270/Q298896M |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50261271
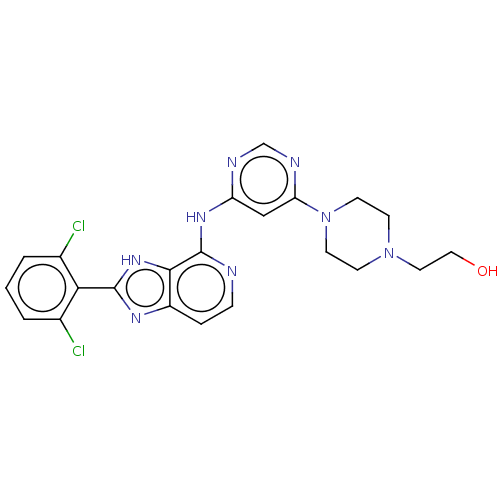
(CHEMBL4080904)Show SMILES OCCN1CCN(CC1)c1cc(Nc2nccc3nc([nH]c23)-c2c(Cl)cccc2Cl)ncn1 Show InChI InChI=1S/C22H22Cl2N8O/c23-14-2-1-3-15(24)19(14)21-28-16-4-5-25-22(20(16)30-21)29-17-12-18(27-13-26-17)32-8-6-31(7-9-32)10-11-33/h1-5,12-13,33H,6-11H2,(H,28,30)(H,25,26,27,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 (unknown origin) assessed as reduction in IL-23 induced STAT3 phosphorylation by cell based ELISA |
Bioorg Med Chem Lett 27: 4370-4376 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.022
BindingDB Entry DOI: 10.7270/Q2RF5XG8 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50261267
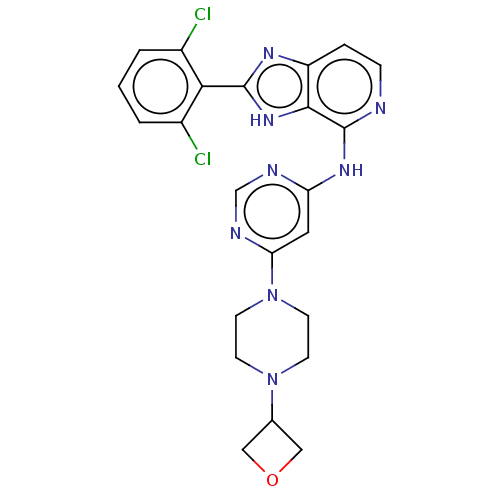
(CHEMBL4097446)Show SMILES Clc1cccc(Cl)c1-c1nc2ccnc(Nc3cc(ncn3)N3CCN(CC3)C3COC3)c2[nH]1 Show InChI InChI=1S/C23H22Cl2N8O/c24-15-2-1-3-16(25)20(15)22-29-17-4-5-26-23(21(17)31-22)30-18-10-19(28-13-27-18)33-8-6-32(7-9-33)14-11-34-12-14/h1-5,10,13-14H,6-9,11-12H2,(H,29,31)(H,26,27,28,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 (unknown origin) by biochemical assay |
Bioorg Med Chem Lett 27: 4370-4376 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.022
BindingDB Entry DOI: 10.7270/Q2RF5XG8 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50040260
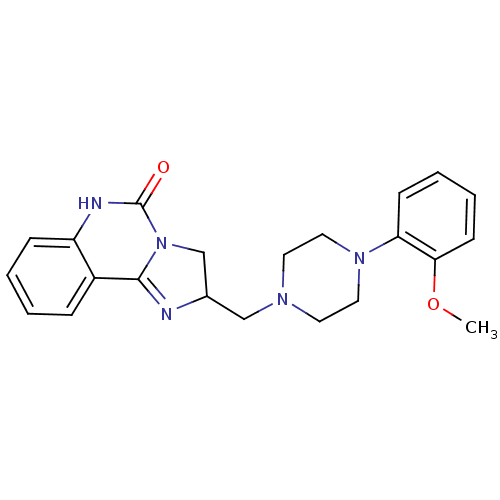
(2-[4-(2-Methoxy-phenyl)-piperazin-1-ylmethyl]-2,6-...)Show SMILES COc1ccccc1N1CCN(CC2CN3C(=N2)c2ccccc2NC3=O)CC1 |c:17| Show InChI InChI=1S/C22H25N5O2/c1-29-20-9-5-4-8-19(20)26-12-10-25(11-13-26)14-16-15-27-21(23-16)17-6-2-3-7-18(17)24-22(27)28/h2-9,16H,10-15H2,1H3,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). |
J Med Chem 36: 2196-207 (1993)
Article DOI: 10.1021/jm00067a017
BindingDB Entry DOI: 10.7270/Q298896M |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50261253
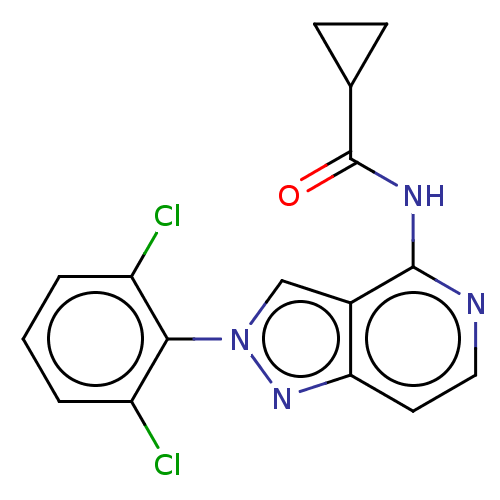
(CHEMBL4100889)Show InChI InChI=1S/C16H12Cl2N4O/c17-11-2-1-3-12(18)14(11)22-8-10-13(21-22)6-7-19-15(10)20-16(23)9-4-5-9/h1-3,6-9H,4-5H2,(H,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 (unknown origin) assessed as reduction in IL-23 induced STAT3 phosphorylation by cell based ELISA |
Bioorg Med Chem Lett 27: 4370-4376 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.022
BindingDB Entry DOI: 10.7270/Q2RF5XG8 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM120131

(US8697708, 16)Show InChI InChI=1S/C16H12ClFN4OS/c17-9-2-1-3-10(18)12(9)15-21-11-6-7-19-14(13(11)24-15)22-16(23)20-8-4-5-8/h1-3,6-8H,4-5H2,(H2,19,20,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.40 | -50.0 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
F. Hoffmann-La Roche AG
US Patent
| Assay Description
To determine the inhibition constants (Ki) of Examples 1-240, compounds were diluted serially in DMSO and added to 50 μL kinase reactions contai... |
US Patent US8697708 (2014)
BindingDB Entry DOI: 10.7270/Q2J38R6F |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM120129

(US8697708, 9)Show InChI InChI=1S/C14H9Cl2N3O2S/c1-21-14(20)19-12-11-9(5-6-17-12)18-13(22-11)10-7(15)3-2-4-8(10)16/h2-6H,1H3,(H,17,19,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.40 | -50.0 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
F. Hoffmann-La Roche AG
US Patent
| Assay Description
To determine the inhibition constants (Ki) of Examples 1-240, compounds were diluted serially in DMSO and added to 50 μL kinase reactions contai... |
US Patent US8697708 (2014)
BindingDB Entry DOI: 10.7270/Q2J38R6F |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230871
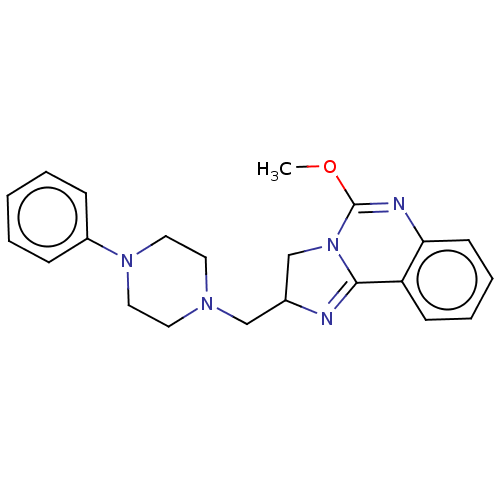
(CHEMBL307961)Show SMILES COC1=Nc2ccccc2C2=NC(CN3CCN(CC3)c3ccccc3)CN12 |t:2,11| Show InChI InChI=1S/C22H25N5O/c1-28-22-24-20-10-6-5-9-19(20)21-23-17(16-27(21)22)15-25-11-13-26(14-12-25)18-7-3-2-4-8-18/h2-10,17H,11-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). |
J Med Chem 36: 2196-207 (1993)
Article DOI: 10.1021/jm00067a017
BindingDB Entry DOI: 10.7270/Q298896M |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM120139

(US8697708, 213)Show SMILES Fc1cnc(NC(=O)C2CC2)c2sc(nc12)-c1c(Cl)cc(cc1Cl)C#N Show InChI InChI=1S/C17H9Cl2FN4OS/c18-9-3-7(5-21)4-10(19)12(9)17-23-13-11(20)6-22-15(14(13)26-17)24-16(25)8-1-2-8/h3-4,6,8H,1-2H2,(H,22,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.5 | -49.9 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
F. Hoffmann-La Roche AG
US Patent
| Assay Description
To determine the inhibition constants (Ki) of Examples 1-240, compounds were diluted serially in DMSO and added to 50 μL kinase reactions contai... |
US Patent US8697708 (2014)
BindingDB Entry DOI: 10.7270/Q2J38R6F |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM120137
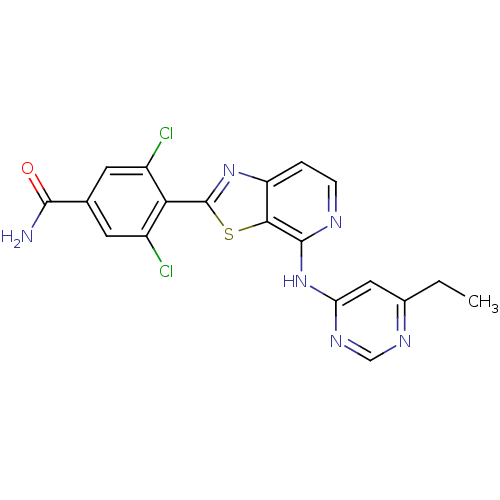
(US8697708, 129)Show SMILES CCc1cc(Nc2nccc3nc(sc23)-c2c(Cl)cc(cc2Cl)C(N)=O)ncn1 Show InChI InChI=1S/C19H14Cl2N6OS/c1-2-10-7-14(25-8-24-10)27-18-16-13(3-4-23-18)26-19(29-16)15-11(20)5-9(17(22)28)6-12(15)21/h3-8H,2H2,1H3,(H2,22,28)(H,23,24,25,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.60 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
F. Hoffmann-La Roche AG
US Patent
| Assay Description
To determine the inhibition constants (Ki) of Examples 1-240, compounds were diluted serially in DMSO and added to 50 μL kinase reactions contai... |
US Patent US8697708 (2014)
BindingDB Entry DOI: 10.7270/Q2J38R6F |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50261215

(CHEMBL2387223)Show SMILES F[C@@H]1C[C@@H]1C(=O)Nc1cc(NC(=O)c2c(F)cc(cc2Cl)C#N)ccn1 |r| Show InChI InChI=1S/C17H11ClF2N4O2/c18-11-3-8(7-21)4-13(20)15(11)17(26)23-9-1-2-22-14(5-9)24-16(25)10-6-12(10)19/h1-5,10,12H,6H2,(H2,22,23,24,25,26)/t10-,12+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 (unknown origin) by biochemical assay |
Bioorg Med Chem Lett 27: 4370-4376 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.022
BindingDB Entry DOI: 10.7270/Q2RF5XG8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data