 Found 1204 hits with Last Name = 'zhai' and Initial = 'y'
Found 1204 hits with Last Name = 'zhai' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50274953
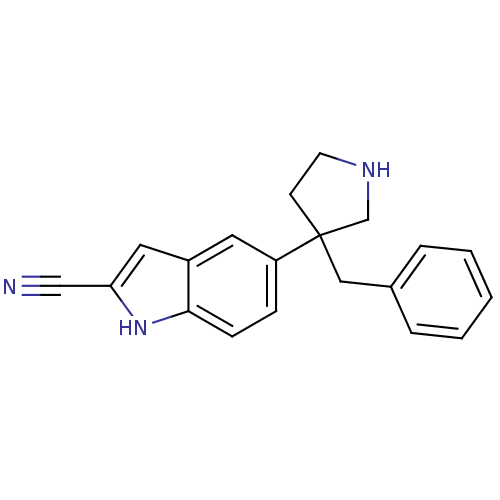
(5-(3-benzylpyrrolidin-3-yl)-1H-indole-2-carbonitri...)Show InChI InChI=1S/C20H19N3/c21-13-18-11-16-10-17(6-7-19(16)23-18)20(8-9-22-14-20)12-15-4-2-1-3-5-15/h1-7,10-11,22-23H,8-9,12,14H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 18: 6062-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.025
BindingDB Entry DOI: 10.7270/Q29886TF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50252010
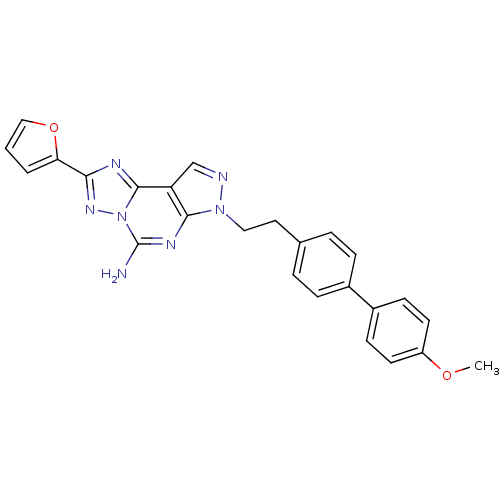
(2-Furan-2-yl-7-[2-(4'-methoxy-biphenyl-4-yl)-ethyl...)Show SMILES COc1ccc(cc1)-c1ccc(CCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)cc1 Show InChI InChI=1S/C25H21N7O2/c1-33-19-10-8-18(9-11-19)17-6-4-16(5-7-17)12-13-31-23-20(15-27-31)24-28-22(21-3-2-14-34-21)30-32(24)25(26)29-23/h2-11,14-15H,12-13H2,1H3,(H2,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 4199-203 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.074
BindingDB Entry DOI: 10.7270/Q2X34X89 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50252063

(2-Furan-2-yl-7-{2-[4-(6-methyl-pyridin-3-yl)-pheny...)Show SMILES Cc1ccc(cn1)-c1ccc(CCn2ncc3c4nc(nn4c(N)nc23)-c2ccco2)cc1 Show InChI InChI=1S/C24H20N8O/c1-15-4-7-18(13-26-15)17-8-5-16(6-9-17)10-11-31-22-19(14-27-31)23-28-21(20-3-2-12-33-20)30-32(23)24(25)29-22/h2-9,12-14H,10-11H2,1H3,(H2,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 4199-203 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.074
BindingDB Entry DOI: 10.7270/Q2X34X89 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50252110

(2-Furan-2-yl-7-[2-(4-pyrazin-2-yl-phenyl)-ethyl]-7...)Show SMILES Nc1nc2n(CCc3ccc(cc3)-c3cnccn3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H17N9O/c23-22-28-20-16(21-27-19(29-31(21)22)18-2-1-11-32-18)12-26-30(20)10-7-14-3-5-15(6-4-14)17-13-24-8-9-25-17/h1-6,8-9,11-13H,7,10H2,(H2,23,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 4199-203 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.074
BindingDB Entry DOI: 10.7270/Q2X34X89 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50275439
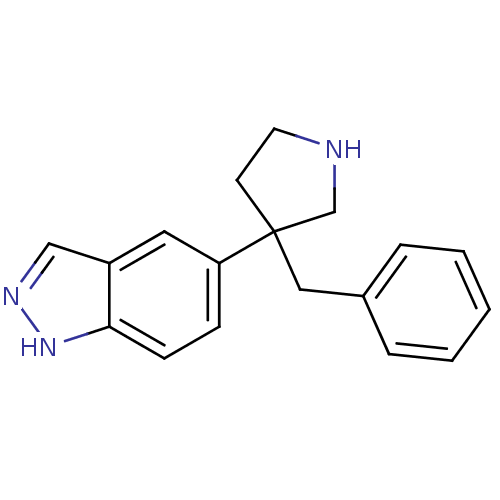
(5-(3-benzylpyrrolidin-3-yl)-1H-indazole | CHEMBL52...)Show InChI InChI=1S/C18H19N3/c1-2-4-14(5-3-1)11-18(8-9-19-13-18)16-6-7-17-15(10-16)12-20-21-17/h1-7,10,12,19H,8-9,11,13H2,(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 18: 6062-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.025
BindingDB Entry DOI: 10.7270/Q29886TF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50237747

(CHEMBL4083298)Show SMILES CC1(C)CCC2(CC1)N[C@H]([C@H](c1cccc(Cl)c1F)[C@]21C(=O)Nc2cc(Cl)ccc12)C(=O)Nc1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C32H30Cl2FN3O4/c1-30(2)12-14-31(15-13-30)32(21-11-8-18(33)16-23(21)37-29(32)42)24(20-4-3-5-22(34)25(20)35)26(38-31)27(39)36-19-9-6-17(7-10-19)28(40)41/h3-11,16,24,26,38H,12-15H2,1-2H3,(H,36,39)(H,37,42)(H,40,41)/t24-,26+,32+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Evaluated for accumulation of cAMP in transfected HEK293 cells expressing human vasopressin V2 receptor |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50237768
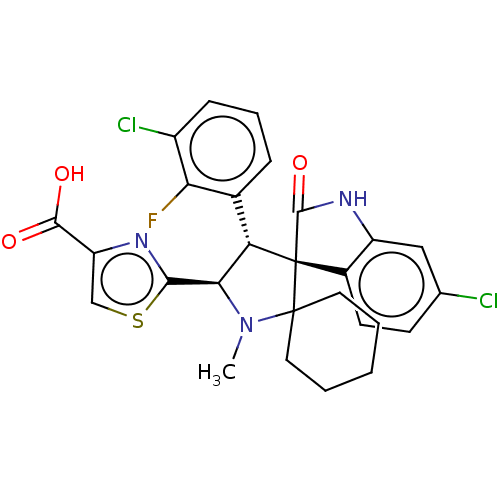
(CHEMBL4063804)Show SMILES CN1[C@H]([C@H](c2cccc(Cl)c2F)[C@]2(C(=O)Nc3cc(Cl)ccc23)C11CCCCC1)c1nc(cs1)C(O)=O |r| Show InChI InChI=1S/C27H24Cl2FN3O3S/c1-33-22(23-31-19(13-37-23)24(34)35)20(15-6-5-7-17(29)21(15)30)27(26(33)10-3-2-4-11-26)16-9-8-14(28)12-18(16)32-25(27)36/h5-9,12-13,20,22H,2-4,10-11H2,1H3,(H,32,36)(H,34,35)/t20-,22+,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50237759

(CHEMBL4064410)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)NC(=O)[C@@H]1NC2(CCCCC2)[C@]2([C@H]1c1cccc(Cl)c1F)C(=O)Nc1cc(Cl)ccc21 |r,wU:20.33,21.24,6.9,wD:12.12,3.2,(33.7,-7.1,;33.4,-5.59,;34.56,-4.57,;31.94,-5.09,;31.64,-3.58,;30.18,-3.08,;29.04,-4.09,;29.33,-5.61,;30.78,-6.1,;27.58,-3.6,;26.42,-4.62,;24.99,-4.05,;26.8,-6.11,;28.24,-6.65,;28.17,-8.19,;28.56,-9.67,;30.04,-10.07,;31.13,-8.99,;30.73,-7.5,;29.25,-7.1,;26.68,-8.6,;25.75,-7.34,;24.41,-5.82,;24.11,-4.3,;22.65,-3.8,;21.49,-4.81,;21.79,-6.33,;20.62,-7.34,;23.24,-6.83,;24.93,-7.81,;27.6,-9.85,;29,-11.01,;26.69,-11.11,;25.22,-10.64,;23.87,-11.41,;22.54,-10.64,;21.21,-11.41,;22.54,-9.1,;23.87,-8.33,;25.21,-9.09,)| Show InChI InChI=1S/C30H32Cl2FN3O4/c31-17-9-12-20-22(15-17)35-28(40)30(20)23(19-5-4-6-21(32)24(19)33)25(36-29(30)13-2-1-3-14-29)26(37)34-18-10-7-16(8-11-18)27(38)39/h4-6,9,12,15-16,18,23,25,36H,1-3,7-8,10-11,13-14H2,(H,34,37)(H,35,40)(H,38,39)/t16-,18-,23-,25+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V1a receptor |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50237734

(CHEMBL4098834)Show SMILES CS(=O)(=O)NC(=O)C12CCC(CC1)(CC2)NC(=O)[C@@H]1NC2(CCCCC2)[C@]2([C@H]1c1cccc(Cl)c1F)C(=O)Nc1cc(Cl)ccc21 |r| Show InChI InChI=1S/C33H37Cl2FN4O5S/c1-46(44,45)40-28(42)30-12-15-31(16-13-30,17-14-30)39-27(41)26-24(20-6-5-7-22(35)25(20)36)33(32(38-26)10-3-2-4-11-32)21-9-8-19(34)18-23(21)37-29(33)43/h5-9,18,24,26,38H,2-4,10-17H2,1H3,(H,37,43)(H,39,41)(H,40,42)/t24-,26+,30?,31?,33+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Ro- 15-1788 binding to recombinant human gamma-aminobutyric-acid A receptor alpha-3-beta-3-gamma-2 |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50237764
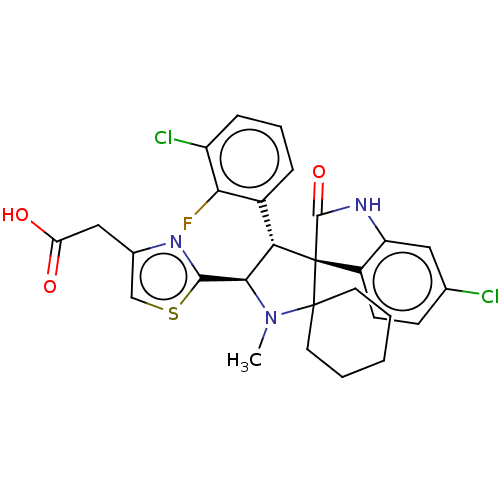
(CHEMBL4060006)Show SMILES CN1[C@H]([C@H](c2cccc(Cl)c2F)[C@]2(C(=O)Nc3cc(Cl)ccc23)C11CCCCC1)c1nc(CC(O)=O)cs1 |r| Show InChI InChI=1S/C28H26Cl2FN3O3S/c1-34-24(25-32-16(14-38-25)13-21(35)36)22(17-6-5-7-19(30)23(17)31)28(27(34)10-3-2-4-11-27)18-9-8-15(29)12-20(18)33-26(28)37/h5-9,12,14,22,24H,2-4,10-11,13H2,1H3,(H,33,37)(H,35,36)/t22-,24+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Ro- 15-1788 binding to human GABA-A receptor alpha-5-beta-3-gamma-2 subunits |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50237744
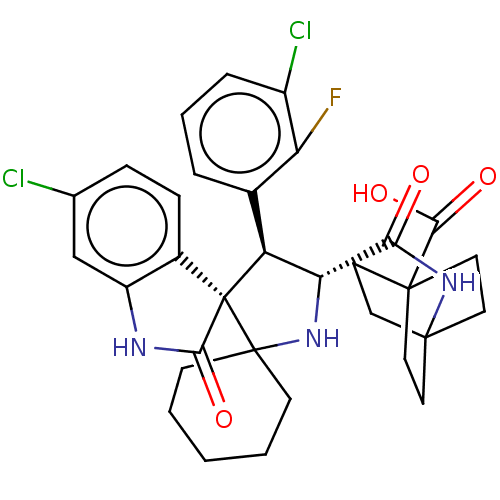
(CHEMBL4072857)Show SMILES OC(=O)C12CCC(CC1)(CC2)NC(=O)[C@@H]1NC2(CCCCC2)[C@]2([C@H]1c1cccc(Cl)c1F)C(=O)Nc1cc(Cl)ccc21 |r| Show InChI InChI=1S/C32H34Cl2FN3O4/c33-18-7-8-20-22(17-18)36-27(40)32(20)23(19-5-4-6-21(34)24(19)35)25(37-31(32)9-2-1-3-10-31)26(39)38-30-14-11-29(12-15-30,13-16-30)28(41)42/h4-8,17,23,25,37H,1-3,9-16H2,(H,36,40)(H,38,39)(H,41,42)/t23-,25+,29?,30?,32+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Ro- 15-1788 binding to human GABA-A receptor alpha-5-beta-3-gamma-2 subunits |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50237765
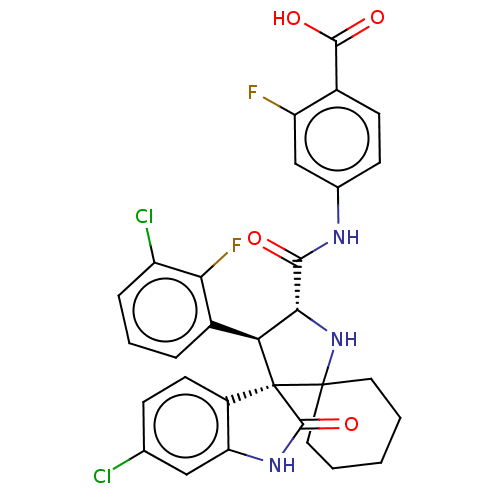
(CHEMBL4077687)Show SMILES OC(=O)c1ccc(NC(=O)[C@@H]2NC3(CCCCC3)[C@]3([C@H]2c2cccc(Cl)c2F)C(=O)Nc2cc(Cl)ccc32)cc1F |r| Show InChI InChI=1S/C30H25Cl2F2N3O4/c31-15-7-10-19-22(13-15)36-28(41)30(19)23(18-5-4-6-20(32)24(18)34)25(37-29(30)11-2-1-3-12-29)26(38)35-16-8-9-17(27(39)40)21(33)14-16/h4-10,13-14,23,25,37H,1-3,11-12H2,(H,35,38)(H,36,41)(H,39,40)/t23-,25+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM112766
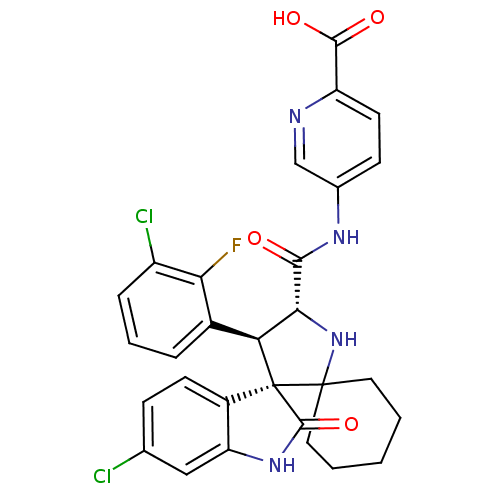
(US8629141, 36)Show SMILES OC(=O)c1ccc(NC(=O)[C@@H]2NC3(CCCCC3)[C@]3([C@H]2c2cccc(Cl)c2F)C(=O)Nc2cc(Cl)ccc32)cn1 |r| Show InChI InChI=1S/C29H25Cl2FN4O4/c30-15-7-9-18-21(13-15)35-27(40)29(18)22(17-5-4-6-19(31)23(17)32)24(36-28(29)11-2-1-3-12-28)25(37)34-16-8-10-20(26(38)39)33-14-16/h4-10,13-14,22,24,36H,1-3,11-12H2,(H,34,37)(H,35,40)(H,38,39)/t22-,24+,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50237738

(CHEMBL4064867)Show SMILES CN1[C@H]([C@H](c2cccc(Cl)c2F)[C@]2(C(=O)Nc3cc(Cl)ccc23)C11CCCCC1)C(=O)NC12CCC(CC1)(CC2)C(O)=O |r| Show InChI InChI=1S/C33H36Cl2FN3O4/c1-39-26(27(40)38-31-15-12-30(13-16-31,14-17-31)29(42)43)24(20-6-5-7-22(35)25(20)36)33(32(39)10-3-2-4-11-32)21-9-8-19(34)18-23(21)37-28(33)41/h5-9,18,24,26H,2-4,10-17H2,1H3,(H,37,41)(H,38,40)(H,42,43)/t24-,26+,30?,31?,33+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V1a receptor |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50237750
(CHEMBL4093562)Show SMILES OC(=O)C12CC(C1)(C2)NC(=O)[C@@H]1NC2(CCCCC2)[C@]2([C@H]1c1cccc(Cl)c1F)C(=O)Nc1cc(Cl)ccc21 |r| Show InChI InChI=1S/C29H28Cl2FN3O4/c30-15-7-8-17-19(11-15)33-24(37)29(17)20(16-5-4-6-18(31)21(16)32)22(34-28(29)9-2-1-3-10-28)23(36)35-27-12-26(13-27,14-27)25(38)39/h4-8,11,20,22,34H,1-3,9-10,12-14H2,(H,33,37)(H,35,36)(H,38,39)/t20-,22+,26?,27?,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V1a receptor |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM112759

(US8629141, 29)Show SMILES OC(=O)c1ccc(CNC(=O)[C@@H]2NC3(CCCCC3)[C@]3([C@H]2c2cccc(Cl)c2F)C(=O)Nc2cc(Cl)ccc32)cc1 |r| Show InChI InChI=1S/C31H28Cl2FN3O4/c32-19-11-12-21-23(15-19)36-29(41)31(21)24(20-5-4-6-22(33)25(20)34)26(37-30(31)13-2-1-3-14-30)27(38)35-16-17-7-9-18(10-8-17)28(39)40/h4-12,15,24,26,37H,1-3,13-14,16H2,(H,35,38)(H,36,41)(H,39,40)/t24-,26+,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM112757
(US8629141, 27)Show SMILES OC(=O)CCCNC(=O)[C@@H]1NC2(CCCCC2)[C@]2([C@H]1c1cccc(Cl)c1F)C(=O)Nc1cc(Cl)ccc21 |r| Show InChI InChI=1S/C27H28Cl2FN3O4/c28-15-9-10-17-19(14-15)32-25(37)27(17)21(16-6-4-7-18(29)22(16)30)23(24(36)31-13-5-8-20(34)35)33-26(27)11-2-1-3-12-26/h4,6-7,9-10,14,21,23,33H,1-3,5,8,11-13H2,(H,31,36)(H,32,37)(H,34,35)/t21-,23+,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM112761

(US8629141, 31)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1NC2(CCCCC2)[C@]2([C@H]1c1cccc(Cl)c1F)C(=O)Nc1cc(Cl)ccc21)C(O)=O |r| Show InChI InChI=1S/C31H28Cl2FN3O5/c1-42-23-14-16(28(39)40)8-11-21(23)35-27(38)26-24(18-6-5-7-20(33)25(18)34)31(30(37-26)12-3-2-4-13-30)19-10-9-17(32)15-22(19)36-29(31)41/h5-11,14-15,24,26,37H,2-4,12-13H2,1H3,(H,35,38)(H,36,41)(H,39,40)/t24-,26+,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50237739
(CHEMBL4091801)Show SMILES CCN1[C@H]([C@H](c2cccc(Cl)c2F)[C@]2(C(=O)Nc3cc(Cl)ccc23)C11CCCCC1)C(=O)NC12CCC(CC1)(CC2)C(O)=O |r| Show InChI InChI=1S/C34H38Cl2FN3O4/c1-2-40-27(28(41)39-32-16-13-31(14-17-32,15-18-32)30(43)44)25(21-7-6-8-23(36)26(21)37)34(33(40)11-4-3-5-12-33)22-10-9-20(35)19-24(22)38-29(34)42/h6-10,19,25,27H,2-5,11-18H2,1H3,(H,38,42)(H,39,41)(H,43,44)/t25-,27+,31?,32?,34+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50274951
(5-(3-benzylpyrrolidin-3-yl)-1-methyl-1H-indole | C...)Show InChI InChI=1S/C20H22N2/c1-22-12-9-17-13-18(7-8-19(17)22)20(10-11-21-15-20)14-16-5-3-2-4-6-16/h2-9,12-13,21H,10-11,14-15H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of NET (unknown origin) |
Bioorg Med Chem Lett 18: 6062-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.025
BindingDB Entry DOI: 10.7270/Q29886TF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50236827

(CHEMBL4066694)Show SMILES COc1cc(NC(=O)[C@@H]2NC3(CCCCC3)[C@]3([C@H]2c2cccc(Cl)c2F)C(=O)Nc2cc(Cl)ccc32)ccc1C(O)=O |r| Show InChI InChI=1S/C31H28Cl2FN3O5/c1-42-23-15-17(9-10-18(23)28(39)40)35-27(38)26-24(19-6-5-7-21(33)25(19)34)31(30(37-26)12-3-2-4-13-30)20-11-8-16(32)14-22(20)36-29(31)41/h5-11,14-15,24,26,37H,2-4,12-13H2,1H3,(H,35,38)(H,36,41)(H,39,40)/t24-,26+,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM112773
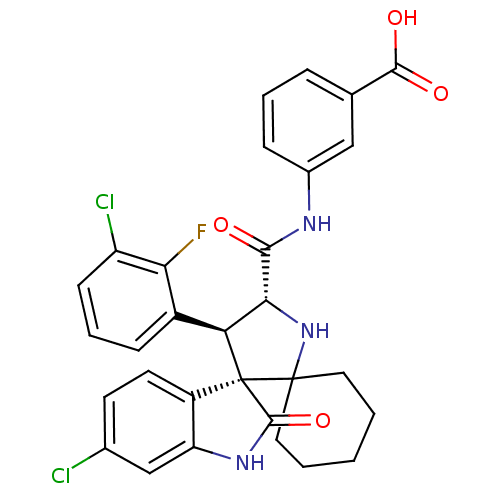
(US8629141, 43)Show SMILES OC(=O)c1cccc(NC(=O)[C@@H]2NC3(CCCCC3)[C@]3([C@H]2c2cccc(Cl)c2F)C(=O)Nc2cc(Cl)ccc32)c1 |r| Show InChI InChI=1S/C30H26Cl2FN3O4/c31-17-10-11-20-22(15-17)35-28(40)30(20)23(19-8-5-9-21(32)24(19)33)25(36-29(30)12-2-1-3-13-29)26(37)34-18-7-4-6-16(14-18)27(38)39/h4-11,14-15,23,25,36H,1-3,12-13H2,(H,34,37)(H,35,40)(H,38,39)/t23-,25+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50237763
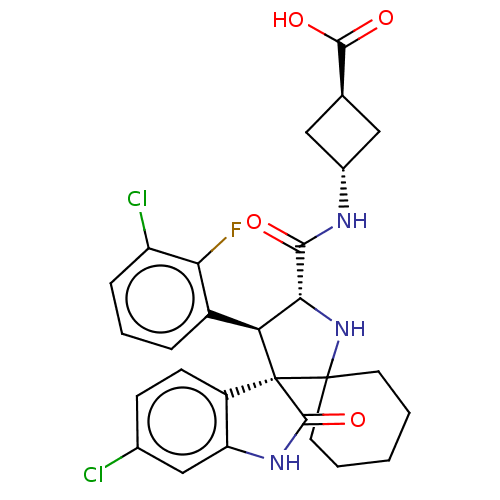
(CHEMBL4084366)Show SMILES OC(=O)[C@H]1C[C@@H](C1)NC(=O)[C@@H]1NC2(CCCCC2)[C@]2([C@H]1c1cccc(Cl)c1F)C(=O)Nc1cc(Cl)ccc21 |r,wU:18.31,19.22,5.7,wD:10.10,3.2,(15.44,-35.61,;15.03,-34.13,;16.12,-33.04,;13.55,-33.74,;12.87,-32.36,;11.49,-33.04,;12.17,-34.42,;10.03,-32.55,;8.88,-33.57,;7.45,-33,;9.26,-35.06,;10.7,-35.61,;10.62,-37.14,;11.02,-38.62,;12.5,-39.02,;13.59,-37.94,;13.19,-36.45,;11.7,-36.05,;9.14,-37.55,;8.2,-36.29,;6.86,-34.77,;6.56,-33.25,;5.11,-32.75,;3.95,-33.76,;4.24,-35.28,;3.08,-36.29,;5.7,-35.78,;7.38,-36.76,;10.05,-38.8,;11.46,-39.96,;9.15,-40.06,;7.67,-39.59,;6.33,-40.36,;5,-39.59,;3.66,-40.36,;5,-38.05,;6.33,-37.28,;7.66,-38.04,)| Show InChI InChI=1S/C28H28Cl2FN3O4/c29-15-7-8-18-20(13-15)33-26(38)28(18)21(17-5-4-6-19(30)22(17)31)23(34-27(28)9-2-1-3-10-27)24(35)32-16-11-14(12-16)25(36)37/h4-8,13-14,16,21,23,34H,1-3,9-12H2,(H,32,35)(H,33,38)(H,36,37)/t14-,16-,21-,23+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50237745
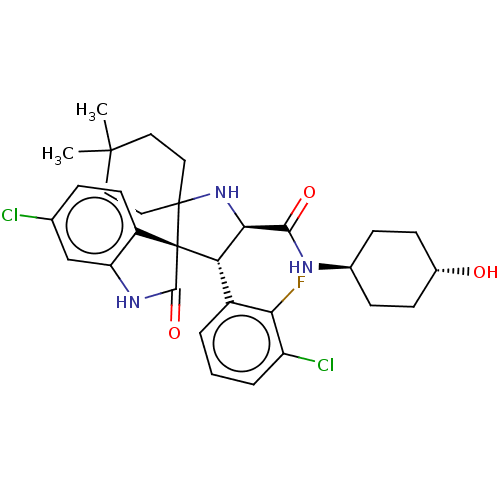
(CHEMBL4064790)Show SMILES CC1(C)CCC2(CC1)N[C@H]([C@H](c1cccc(Cl)c1F)[C@]21C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1CC[C@H](O)CC1 |r,wU:19.22,10.11,33.37,wD:9.34,36.41,(71.57,-7.52,;70.08,-7.92,;71.18,-9.01,;68.99,-9.01,;67.52,-8.61,;67.12,-7.13,;68.2,-6.04,;69.69,-6.44,;67.2,-5.6,;65.76,-5.05,;64.7,-6.28,;63.36,-4.76,;63.06,-3.24,;61.61,-2.75,;60.45,-3.75,;60.74,-5.27,;59.58,-6.28,;62.2,-5.77,;63.88,-6.76,;65.64,-7.54,;66.55,-8.79,;67.96,-9.95,;65.65,-10.05,;64.17,-9.58,;62.83,-10.35,;61.5,-9.58,;60.16,-10.35,;61.5,-8.04,;62.83,-7.27,;64.16,-8.03,;65.35,-3.57,;63.87,-3.17,;66.44,-2.47,;67.93,-2.87,;69,-1.77,;70.49,-2.17,;70.89,-3.66,;72.38,-4.05,;69.8,-4.74,;68.32,-4.35,)| Show InChI InChI=1S/C31H36Cl2FN3O3/c1-29(2)12-14-30(15-13-29)31(21-11-6-17(32)16-23(21)36-28(31)40)24(20-4-3-5-22(33)25(20)34)26(37-30)27(39)35-18-7-9-19(38)10-8-18/h3-6,11,16,18-19,24,26,37-38H,7-10,12-15H2,1-2H3,(H,35,39)(H,36,40)/t18-,19-,24-,26+,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Ro- 15-1788 binding to human GABA-A receptor alpha-5-beta-3-gamma-2 subunits |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50237766

(CHEMBL4085815)Show SMILES CS(=O)(=O)NC(=O)c1ccc(NC(=O)[C@@H]2NC3(CCCCC3)[C@]3([C@H]2c2cccc(Cl)c2F)C(=O)Nc2cc(Cl)ccc32)cc1 |r| Show InChI InChI=1S/C31H29Cl2FN4O5S/c1-44(42,43)38-27(39)17-8-11-19(12-9-17)35-28(40)26-24(20-6-5-7-22(33)25(20)34)31(30(37-26)14-3-2-4-15-30)21-13-10-18(32)16-23(21)36-29(31)41/h5-13,16,24,26,37H,2-4,14-15H2,1H3,(H,35,40)(H,36,41)(H,38,39)/t24-,26+,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Ro- 15-1788 binding to recombinant human gamma-aminobutyric-acid A receptor alpha-1-beta-3-gamma-2 |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50252009
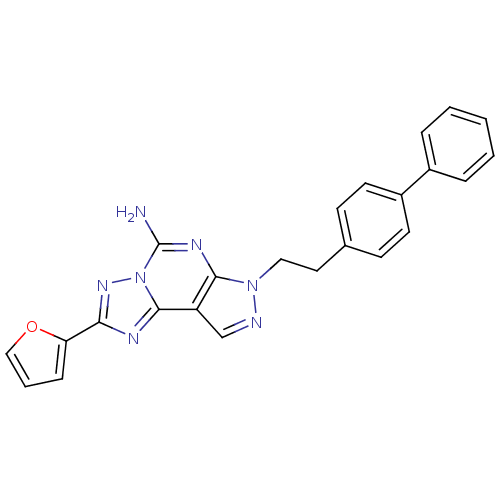
(7-(2-Biphenyl-4-yl-ethyl)-2-furan-2-yl-7H-pyrazolo...)Show SMILES Nc1nc2n(CCc3ccc(cc3)-c3ccccc3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C24H19N7O/c25-24-28-22-19(23-27-21(29-31(23)24)20-7-4-14-32-20)15-26-30(22)13-12-16-8-10-18(11-9-16)17-5-2-1-3-6-17/h1-11,14-15H,12-13H2,(H2,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 4199-203 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.074
BindingDB Entry DOI: 10.7270/Q2X34X89 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50237752

(CHEMBL4095706)Show SMILES OC(=O)c1ccc(NC(=O)[C@@H]2NC3(CCCCC3)[C@]3([C@H]2c2cccc(Cl)c2F)C(=O)Nc2cc(Cl)ccc32)c(F)c1 |r| Show InChI InChI=1S/C30H25Cl2F2N3O4/c31-16-8-9-18-22(14-16)36-28(41)30(18)23(17-5-4-6-19(32)24(17)34)25(37-29(30)11-2-1-3-12-29)26(38)35-21-10-7-15(27(39)40)13-20(21)33/h4-10,13-14,23,25,37H,1-3,11-12H2,(H,35,38)(H,36,41)(H,39,40)/t23-,25+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V1a receptor |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50237741

(CHEMBL4071840)Show SMILES OC(=O)C12CCC(CC1)(CC2)NC(=O)[C@@H]1NC2(CCC(F)(F)CC2)[C@]2([C@H]1c1cccc(Cl)c1F)C(=O)Nc1cc(Cl)ccc21 |r| Show InChI InChI=1S/C32H32Cl2F3N3O4/c33-17-4-5-19-21(16-17)38-26(42)32(19)22(18-2-1-3-20(34)23(18)35)24(39-30(32)12-14-31(36,37)15-13-30)25(41)40-29-9-6-28(7-10-29,8-11-29)27(43)44/h1-5,16,22,24,39H,6-15H2,(H,38,42)(H,40,41)(H,43,44)/t22-,24+,28?,29?,32+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Ro- 15-1788 binding to human GABA-A receptor alpha-5-beta-3-gamma-2 subunits |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50252210
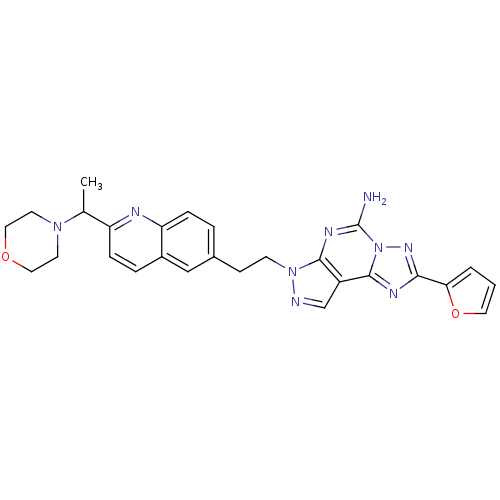
(2-Furan-2-yl-7-{2-[2-(1-morpholin-4-yl-ethyl)-quin...)Show SMILES CC(N1CCOCC1)c1ccc2cc(CCn3ncc4c3nc(N)n3nc(nc43)-c3ccco3)ccc2n1 Show InChI InChI=1S/C27H27N9O2/c1-17(34-10-13-37-14-11-34)21-7-5-19-15-18(4-6-22(19)30-21)8-9-35-25-20(16-29-35)26-31-24(23-3-2-12-38-23)33-36(26)27(28)32-25/h2-7,12,15-17H,8-11,13-14H2,1H3,(H2,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 4199-203 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.074
BindingDB Entry DOI: 10.7270/Q2X34X89 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50252061
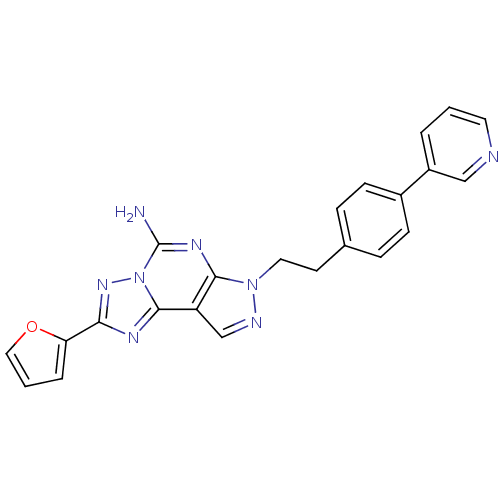
(2-Furan-2-yl-7-[2-(4-pyridin-3-yl-phenyl)-ethyl]-7...)Show SMILES Nc1nc2n(CCc3ccc(cc3)-c3cccnc3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C23H18N8O/c24-23-28-21-18(22-27-20(29-31(22)23)19-4-2-12-32-19)14-26-30(21)11-9-15-5-7-16(8-6-15)17-3-1-10-25-13-17/h1-8,10,12-14H,9,11H2,(H2,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 4199-203 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.074
BindingDB Entry DOI: 10.7270/Q2X34X89 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50252165
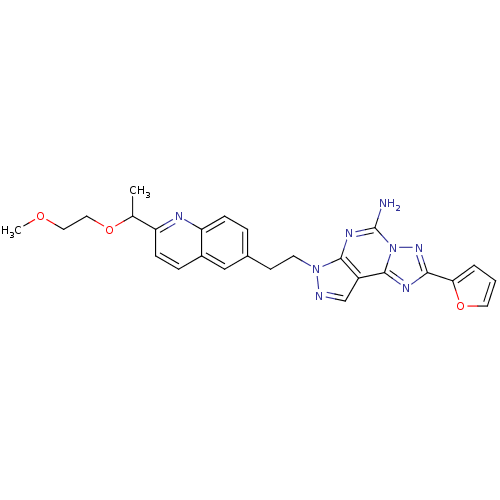
(2-Furan-2-yl-7-(2-{2-[1-(2-methoxy-ethoxy)-ethyl]-...)Show SMILES COCCOC(C)c1ccc2cc(CCn3ncc4c3nc(N)n3nc(nc43)-c3ccco3)ccc2n1 Show InChI InChI=1S/C26H26N8O3/c1-16(36-13-12-35-2)20-8-6-18-14-17(5-7-21(18)29-20)9-10-33-24-19(15-28-33)25-30-23(22-4-3-11-37-22)32-34(25)26(27)31-24/h3-8,11,14-16H,9-10,12-13H2,1-2H3,(H2,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 4199-203 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.074
BindingDB Entry DOI: 10.7270/Q2X34X89 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50252209
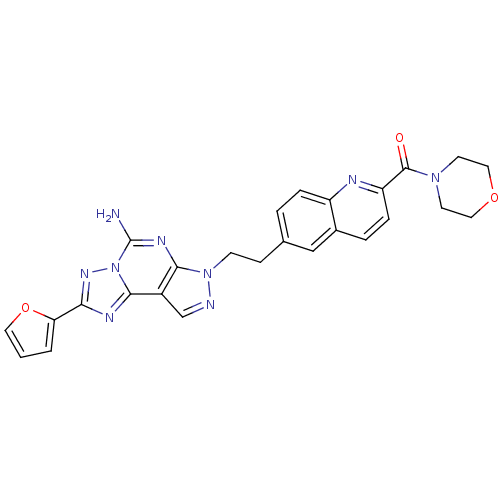
(CHEMBL482138 | {6-[2-(5-Amino-2-furan-2-yl-pyrazol...)Show SMILES Nc1nc2n(CCc3ccc4nc(ccc4c3)C(=O)N3CCOCC3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C26H23N9O3/c27-26-31-23-18(24-30-22(32-35(24)26)21-2-1-11-38-21)15-28-34(23)8-7-16-3-5-19-17(14-16)4-6-20(29-19)25(36)33-9-12-37-13-10-33/h1-6,11,14-15H,7-10,12-13H2,(H2,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 4199-203 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.074
BindingDB Entry DOI: 10.7270/Q2X34X89 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50252211
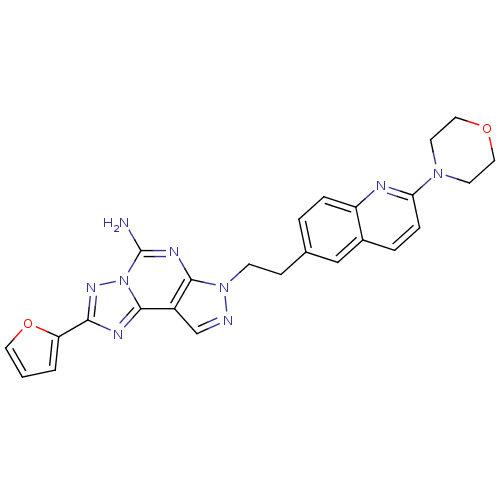
(2-Furan-2-yl-7-[2-(2-morpholin-4-yl-quinolin-6-yl)...)Show SMILES Nc1nc2n(CCc3ccc4nc(ccc4c3)N3CCOCC3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C25H23N9O2/c26-25-30-23-18(24-29-22(31-34(24)25)20-2-1-11-36-20)15-27-33(23)8-7-16-3-5-19-17(14-16)4-6-21(28-19)32-9-12-35-13-10-32/h1-6,11,14-15H,7-10,12-13H2,(H2,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 4199-203 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.074
BindingDB Entry DOI: 10.7270/Q2X34X89 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50237743
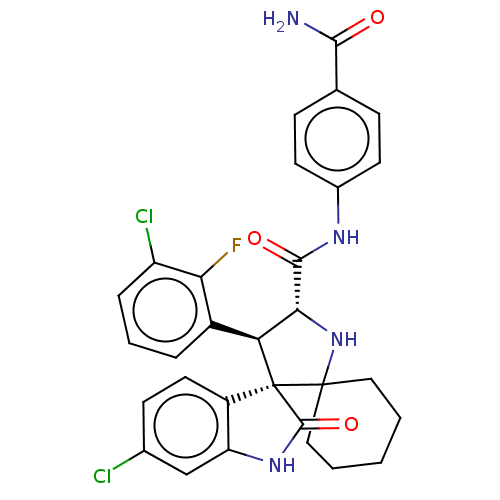
(CHEMBL4102205)Show SMILES NC(=O)c1ccc(NC(=O)[C@@H]2NC3(CCCCC3)[C@]3([C@H]2c2cccc(Cl)c2F)C(=O)Nc2cc(Cl)ccc32)cc1 |r| Show InChI InChI=1S/C30H27Cl2FN4O3/c31-17-9-12-20-22(15-17)36-28(40)30(20)23(19-5-4-6-21(32)24(19)33)25(37-29(30)13-2-1-3-14-29)27(39)35-18-10-7-16(8-11-18)26(34)38/h4-12,15,23,25,37H,1-3,13-14H2,(H2,34,38)(H,35,39)(H,36,40)/t23-,25+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V2 receptor |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50252062

(2-Furan-2-yl-7-{2-[4-(6-methoxy-pyridin-3-yl)-phen...)Show SMILES COc1ccc(cn1)-c1ccc(CCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)cc1 Show InChI InChI=1S/C24H20N8O2/c1-33-20-9-8-17(13-26-20)16-6-4-15(5-7-16)10-11-31-22-18(14-27-31)23-28-21(19-3-2-12-34-19)30-32(23)24(25)29-22/h2-9,12-14H,10-11H2,1H3,(H2,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 4199-203 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.074
BindingDB Entry DOI: 10.7270/Q2X34X89 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50048466
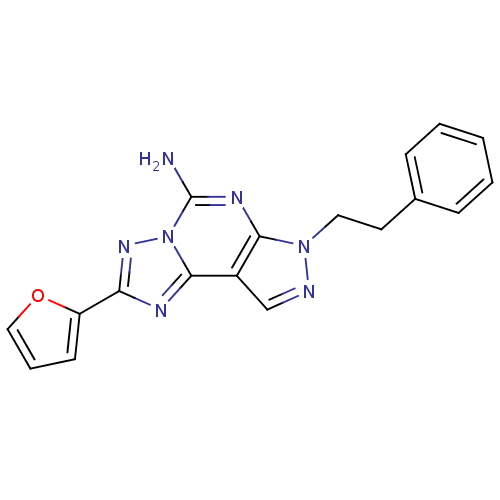
(2-(furan-2-yl)-7-phenethyl-7H-pyrazolo[4,3-e][1,2,...)Show InChI InChI=1S/C18H15N7O/c19-18-22-16-13(11-20-24(16)9-8-12-5-2-1-3-6-12)17-21-15(23-25(17)18)14-7-4-10-26-14/h1-7,10-11H,8-9H2,(H2,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 4199-203 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.074
BindingDB Entry DOI: 10.7270/Q2X34X89 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50274954
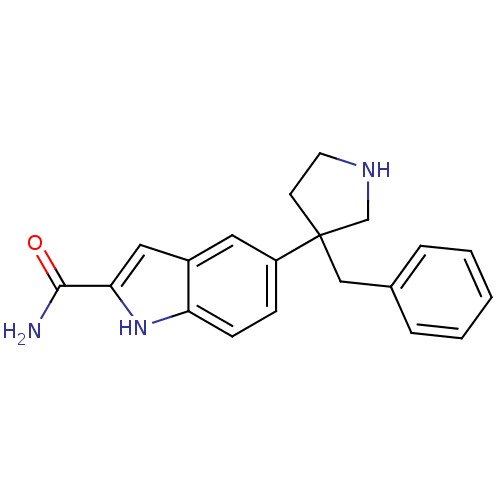
(5-(3-benzylpyrrolidin-3-yl)-1H-indole-2-carboxamid...)Show InChI InChI=1S/C20H21N3O/c21-19(24)18-11-15-10-16(6-7-17(15)23-18)20(8-9-22-13-20)12-14-4-2-1-3-5-14/h1-7,10-11,22-23H,8-9,12-13H2,(H2,21,24) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 18: 6062-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.025
BindingDB Entry DOI: 10.7270/Q29886TF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50237762
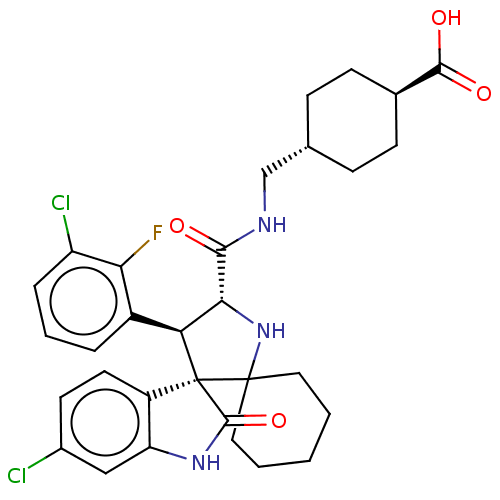
(CHEMBL4072993)Show SMILES OC(=O)[C@H]1CC[C@H](CNC(=O)[C@@H]2NC3(CCCCC3)[C@]3([C@H]2c2cccc(Cl)c2F)C(=O)Nc2cc(Cl)ccc32)CC1 |r,wU:19.31,20.22,6.6,wD:11.10,3.2,(34.1,-31.43,;32.64,-30.93,;32.34,-29.42,;31.48,-31.94,;30.02,-31.45,;28.86,-32.46,;29.17,-33.97,;28.02,-34.98,;26.56,-34.49,;25.4,-35.51,;23.97,-34.95,;25.78,-37,;27.22,-37.55,;27.15,-39.08,;27.54,-40.57,;29.02,-40.96,;30.11,-39.88,;29.71,-38.4,;28.23,-37.99,;25.66,-39.49,;24.73,-38.23,;23.39,-36.71,;23.09,-35.19,;21.63,-34.7,;20.47,-35.71,;20.77,-37.22,;19.6,-38.23,;22.22,-37.72,;23.91,-38.71,;26.58,-40.74,;27.98,-41.9,;25.67,-42,;24.2,-41.53,;22.86,-42.3,;21.52,-41.53,;20.19,-42.3,;21.52,-39.99,;22.85,-39.22,;24.19,-39.98,;30.63,-34.46,;31.78,-33.45,)| Show InChI InChI=1S/C31H34Cl2FN3O4/c32-19-11-12-21-23(15-19)36-29(41)31(21)24(20-5-4-6-22(33)25(20)34)26(37-30(31)13-2-1-3-14-30)27(38)35-16-17-7-9-18(10-8-17)28(39)40/h4-6,11-12,15,17-18,24,26,37H,1-3,7-10,13-14,16H2,(H,35,38)(H,36,41)(H,39,40)/t17-,18-,24-,26+,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Evaluated for accumulation of cAMP in transfected HEK293 cells expressing human vasopressin V2 receptor |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50237757
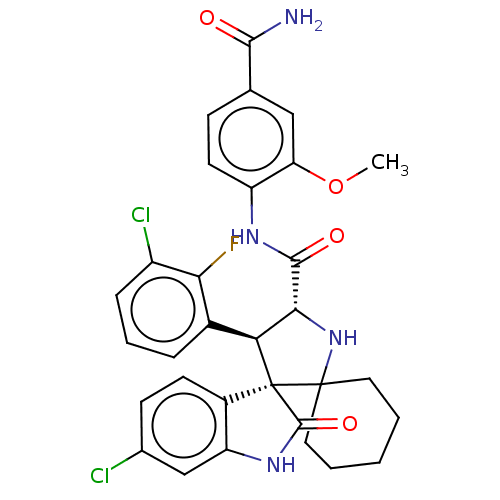
(CHEMBL4101295)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1NC2(CCCCC2)[C@]2([C@H]1c1cccc(Cl)c1F)C(=O)Nc1cc(Cl)ccc21)C(N)=O |r| Show InChI InChI=1S/C31H29Cl2FN4O4/c1-42-23-14-16(27(35)39)8-11-21(23)36-28(40)26-24(18-6-5-7-20(33)25(18)34)31(30(38-26)12-3-2-4-13-30)19-10-9-17(32)15-22(19)37-29(31)41/h5-11,14-15,24,26,38H,2-4,12-13H2,1H3,(H2,35,39)(H,36,40)(H,37,41)/t24-,26+,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Ro- 15-1788 binding to human GABA-A receptor alpha-5-beta-3-gamma-2 subunits |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50252166
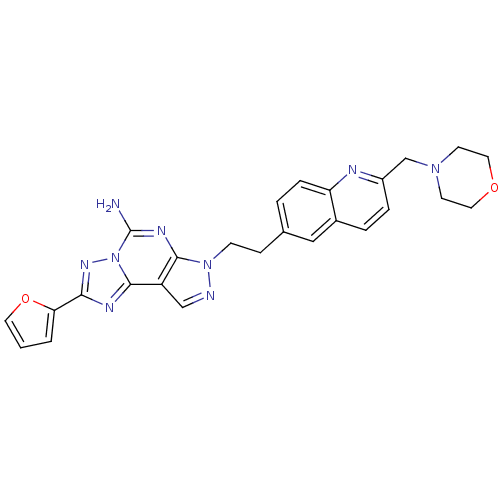
(2-Furan-2-yl-7-[2-(2-morpholin-4-ylmethyl-quinolin...)Show SMILES Nc1nc2n(CCc3ccc4nc(CN5CCOCC5)ccc4c3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C26H25N9O2/c27-26-31-24-20(25-30-23(32-35(25)26)22-2-1-11-37-22)15-28-34(24)8-7-17-3-6-21-18(14-17)4-5-19(29-21)16-33-9-12-36-13-10-33/h1-6,11,14-15H,7-10,12-13,16H2,(H2,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 4199-203 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.074
BindingDB Entry DOI: 10.7270/Q2X34X89 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50237735
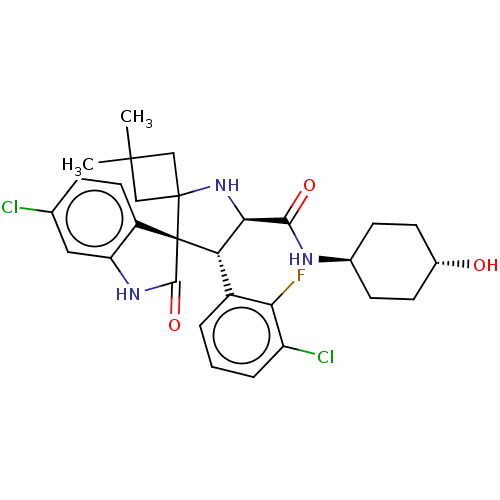
(CHEMBL4100233)Show SMILES CC1(C)CC2(C1)N[C@H]([C@H](c1cccc(Cl)c1F)[C@]21C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1CC[C@H](O)CC1 |r,wU:17.20,8.9,31.35,wD:7.32,34.39,(50.67,-37.89,;49.19,-38.3,;50.28,-39.38,;47.86,-39.06,;47.1,-37.73,;48.43,-36.97,;47.17,-36.2,;45.73,-35.65,;44.68,-36.88,;43.34,-35.36,;43.04,-33.84,;41.58,-33.34,;40.42,-34.35,;40.72,-35.87,;39.55,-36.88,;42.17,-36.37,;43.86,-37.35,;45.61,-38.14,;46.53,-39.39,;47.93,-40.55,;45.62,-40.65,;44.14,-40.18,;42.8,-40.95,;41.47,-40.18,;40.14,-40.95,;41.47,-38.64,;42.8,-37.87,;44.14,-38.63,;45.33,-34.17,;43.84,-33.77,;46.41,-33.07,;47.9,-33.47,;48.97,-32.37,;50.47,-32.77,;50.87,-34.26,;52.35,-34.65,;49.78,-35.34,;48.3,-34.95,)| Show InChI InChI=1S/C29H32Cl2FN3O3/c1-27(2)13-28(14-27)29(19-11-6-15(30)12-21(19)34-26(29)38)22(18-4-3-5-20(31)23(18)32)24(35-28)25(37)33-16-7-9-17(36)10-8-16/h3-6,11-12,16-17,22,24,35-36H,7-10,13-14H2,1-2H3,(H,33,37)(H,34,38)/t16-,17-,22-,24+,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50252113
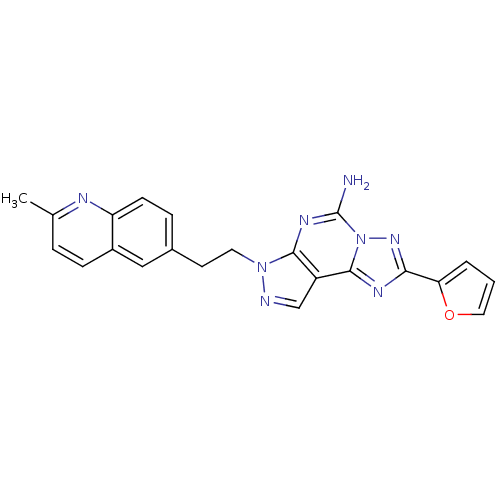
(2-Furan-2-yl-7-[2-(2-methyl-quinolin-6-yl)-ethyl]-...)Show SMILES Cc1ccc2cc(CCn3ncc4c5nc(nn5c(N)nc34)-c3ccco3)ccc2n1 Show InChI InChI=1S/C22H18N8O/c1-13-4-6-15-11-14(5-7-17(15)25-13)8-9-29-20-16(12-24-29)21-26-19(18-3-2-10-31-18)28-30(21)22(23)27-20/h2-7,10-12H,8-9H2,1H3,(H2,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 4199-203 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.074
BindingDB Entry DOI: 10.7270/Q2X34X89 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50236970
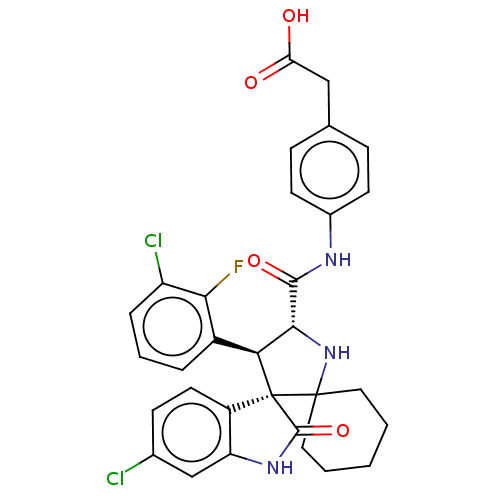
(CHEMBL4080118)Show SMILES OC(=O)Cc1ccc(NC(=O)[C@@H]2NC3(CCCCC3)[C@]3([C@H]2c2cccc(Cl)c2F)C(=O)Nc2cc(Cl)ccc32)cc1 |r| Show InChI InChI=1S/C31H28Cl2FN3O4/c32-18-9-12-21-23(16-18)36-29(41)31(21)25(20-5-4-6-22(33)26(20)34)27(37-30(31)13-2-1-3-14-30)28(40)35-19-10-7-17(8-11-19)15-24(38)39/h4-12,16,25,27,37H,1-3,13-15H2,(H,35,40)(H,36,41)(H,38,39)/t25-,27+,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50237753

(CHEMBL4085388)Show SMILES CN1[C@H]([C@H](c2cccc(Cl)c2F)[C@]2(C(=O)Nc3cc(Cl)ccc23)C11CCCCC1)c1nc(co1)C(O)=O |r| Show InChI InChI=1S/C27H24Cl2FN3O4/c1-33-22(23-31-19(13-37-23)24(34)35)20(15-6-5-7-17(29)21(15)30)27(26(33)10-3-2-4-11-26)16-9-8-14(28)12-18(16)32-25(27)36/h5-9,12-13,20,22H,2-4,10-11H2,1H3,(H,32,36)(H,34,35)/t20-,22+,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V2 receptor |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50252163
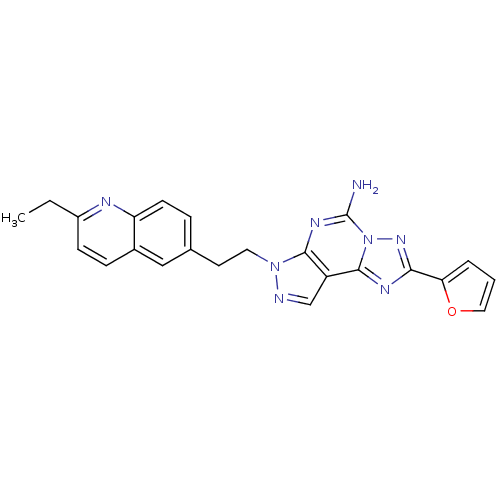
(7-[2-(2-Ethyl-quinolin-6-yl)-ethyl]-2-furan-2-yl-7...)Show SMILES CCc1ccc2cc(CCn3ncc4c5nc(nn5c(N)nc34)-c3ccco3)ccc2n1 Show InChI InChI=1S/C23H20N8O/c1-2-16-7-6-15-12-14(5-8-18(15)26-16)9-10-30-21-17(13-25-30)22-27-20(19-4-3-11-32-19)29-31(22)23(24)28-21/h3-8,11-13H,2,9-10H2,1H3,(H2,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 4199-203 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.074
BindingDB Entry DOI: 10.7270/Q2X34X89 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50252164

(2-Furan-2-yl-7-{2-[2-(2-methoxy-ethoxymethyl)-quin...)Show SMILES COCCOCc1ccc2cc(CCn3ncc4c3nc(N)n3nc(nc43)-c3ccco3)ccc2n1 Show InChI InChI=1S/C25H24N8O3/c1-34-11-12-35-15-18-6-5-17-13-16(4-7-20(17)28-18)8-9-32-23-19(14-27-32)24-29-22(21-3-2-10-36-21)31-33(24)25(26)30-23/h2-7,10,13-14H,8-9,11-12,15H2,1H3,(H2,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 4199-203 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.074
BindingDB Entry DOI: 10.7270/Q2X34X89 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50237736

(CHEMBL4103467)Show SMILES OC(=O)c1ccc(NC(=O)[C@@H]2NC3(CCCCC3)[C@]3([C@H]2c2cccc(Cl)c2F)C(=O)Nc2cc(Cl)ccc32)o1 |r| Show InChI InChI=1S/C28H24Cl2FN3O5/c29-14-7-8-16-18(13-14)32-26(38)28(16)21(15-5-4-6-17(30)22(15)31)23(34-27(28)11-2-1-3-12-27)24(35)33-20-10-9-19(39-20)25(36)37/h4-10,13,21,23,34H,1-3,11-12H2,(H,32,38)(H,33,35)(H,36,37)/t21-,23+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50275440
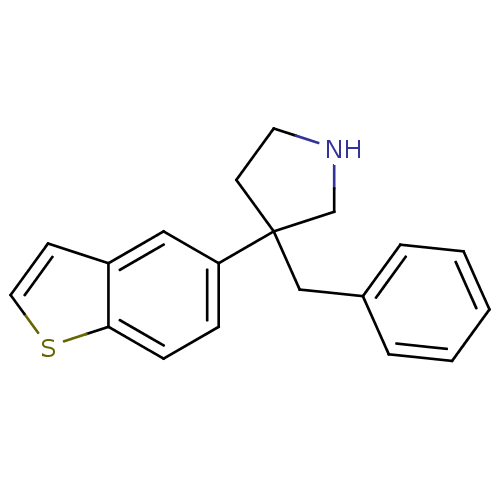
(3-(benzo[b]thiophen-5-yl)-3-benzylpyrrolidine | CH...)Show InChI InChI=1S/C19H19NS/c1-2-4-15(5-3-1)13-19(9-10-20-14-19)17-6-7-18-16(12-17)8-11-21-18/h1-8,11-12,20H,9-10,13-14H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of NET (unknown origin) |
Bioorg Med Chem Lett 18: 6062-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.025
BindingDB Entry DOI: 10.7270/Q29886TF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50237751
(CHEMBL4066543)Show SMILES CN1[C@H]([C@H](c2cccc(Cl)c2F)[C@]2(C(=O)Nc3cc(Cl)ccc23)C11CCCCC1)c1nc(C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C28H26Cl2FN3O4/c1-14-22(25(35)36)33-24(38-14)23-20(16-7-6-8-18(30)21(16)31)28(27(34(23)2)11-4-3-5-12-27)17-10-9-15(29)13-19(17)32-26(28)37/h6-10,13,20,23H,3-5,11-12H2,1-2H3,(H,32,37)(H,35,36)/t20-,23+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Ro- 15-1788 binding to recombinant human gamma-aminobutyric-acid A receptor alpha-1-beta-3-gamma-2 |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM112768

(US8629141, 38)Show SMILES CC(=O)N1CC(C1)NC(=O)[C@@H]1NC2(CCCCC2)[C@]2([C@H]1c1cccc(Cl)c1F)C(=O)Nc1cc(Cl)ccc21 |r| Show InChI InChI=1S/C28H29Cl2FN4O3/c1-15(36)35-13-17(14-35)32-25(37)24-22(18-6-5-7-20(30)23(18)31)28(27(34-24)10-3-2-4-11-27)19-9-8-16(29)12-21(19)33-26(28)38/h5-9,12,17,22,24,34H,2-4,10-11,13-14H2,1H3,(H,32,37)(H,33,38)/t22-,24+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... |
J Med Chem 60: 2819-2839 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01665
BindingDB Entry DOI: 10.7270/Q25Q4ZCF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data