

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471255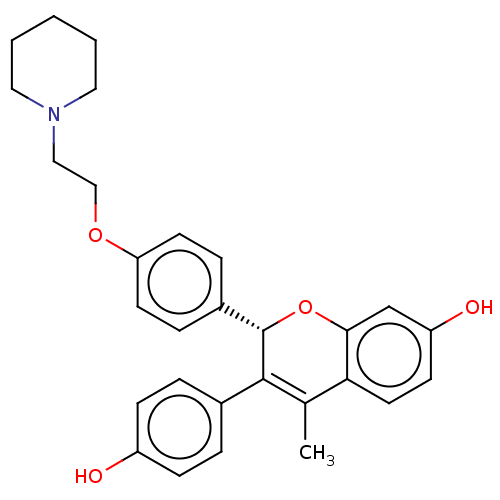 (Acolbifene | EM-652 | SCH-57068) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent binding affinity against estradiol-stimulated T-47D cell proliferation | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471254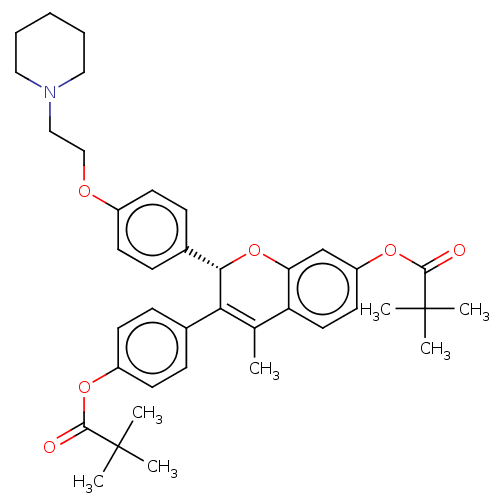 (CHEMBL308234) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent binding affinity against estradiol-stimulated T-47D cell proliferation | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471254 (CHEMBL308234) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Stimulation of alkaline phosphatase activity in human endometrial Ishikawa cells with 1 nM E2 estradiol | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471256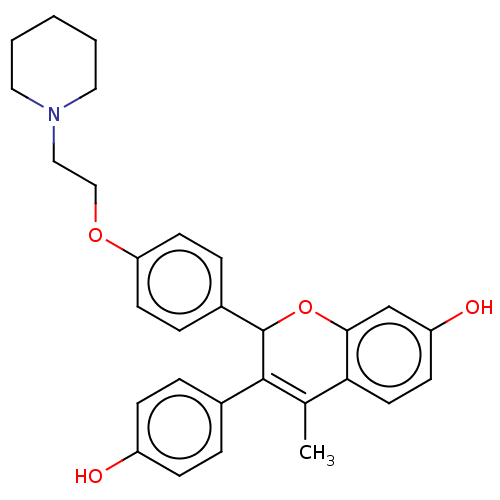 (CHEMBL291808) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent binding affinity against estradiol-stimulated T-47D cell proliferation | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471255 (Acolbifene | EM-652 | SCH-57068) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 3.3%ethanol | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471255 (Acolbifene | EM-652 | SCH-57068) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor in Human Breast cancer cytosol (3.3% ethanol) | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471255 (Acolbifene | EM-652 | SCH-57068) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 2.5%DMF | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471255 (Acolbifene | EM-652 | SCH-57068) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human Breast cancer cytosol in 2.5%DMF | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471255 (Acolbifene | EM-652 | SCH-57068) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent binding affinity against estradiol-stimulated ZR-75-1-cell proliferation | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292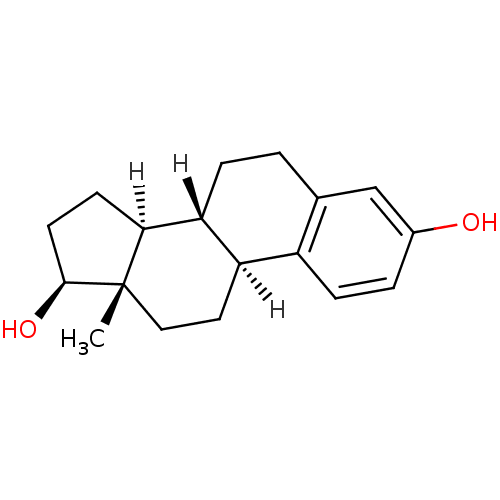 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human Breast cancer cytosol in 2.5%DMF | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 3.3%ethanol | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM20625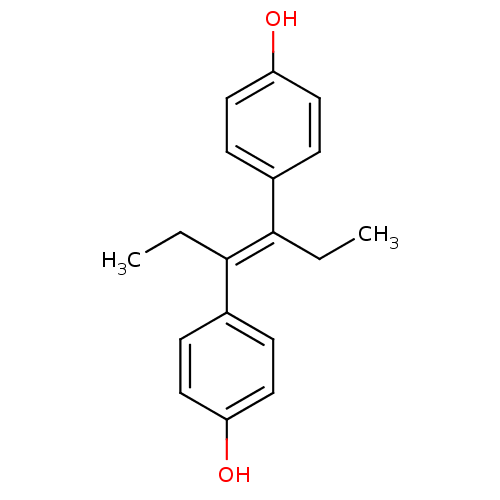 (4-[(3E)-4-(4-hydroxyphenyl)hex-3-en-3-yl]phenol | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor in Human Breast cancer cytosol (3.3% ethanol) | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM20625 (4-[(3E)-4-(4-hydroxyphenyl)hex-3-en-3-yl]phenol | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 3.3%ethanol | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor in Human Breast cancer cytosol (3.3% ethanol) | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 2.5%DMF | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuroendocrine convertase 2 (Homo sapiens (Human)) | BDBM50446944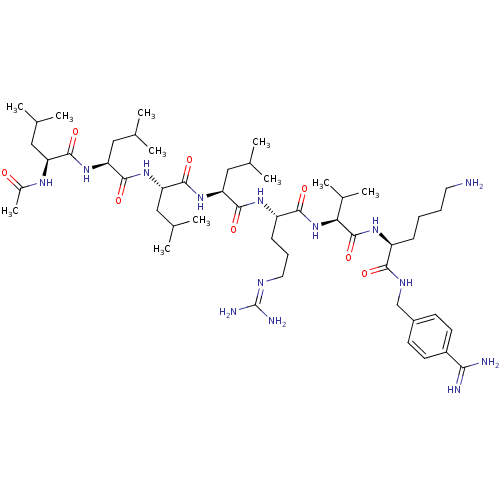 (CHEMBL3115771) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human PC2 expressed in drosophila schneider 2 cells using pyroGlu-Arg-Thr-Lys-Arg-AMC as substrate after 1 hr | J Med Chem 57: 98-109 (2014) Article DOI: 10.1021/jm401457n BindingDB Entry DOI: 10.7270/Q2S1840S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50276802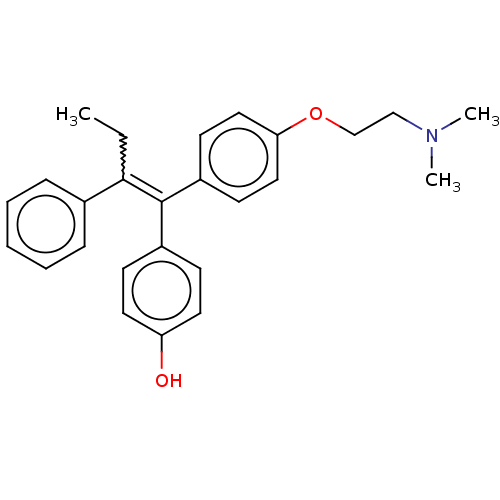 (4-OHT | Afimoxifene | TamoGel) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 0.249 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor in Human Breast cancer cytosol (3.3% ethanol) | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 6 (Homo sapiens (Human)) | BDBM50399749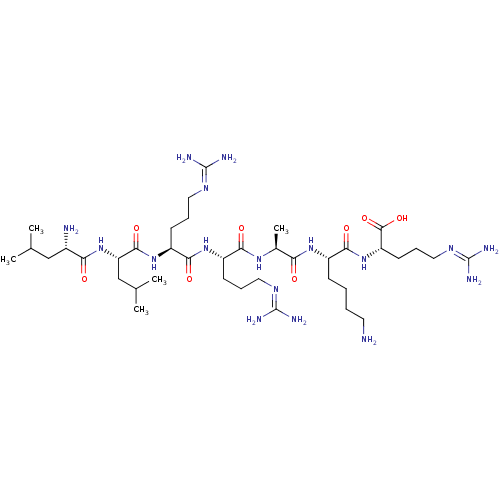 (CHEMBL2179430) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PACE4 expressed in Drosophila S2 cells using pyroGlu-Arg-Val-Lys-Arg-methyl-coumaryl-7-amide as substrate... | J Med Chem 55: 10501-11 (2012) Article DOI: 10.1021/jm3011178 BindingDB Entry DOI: 10.7270/Q2NV9KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50276802 (4-OHT | Afimoxifene | TamoGel) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 0.346 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 3.3%ethanol | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 6 (Homo sapiens (Human)) | BDBM50399753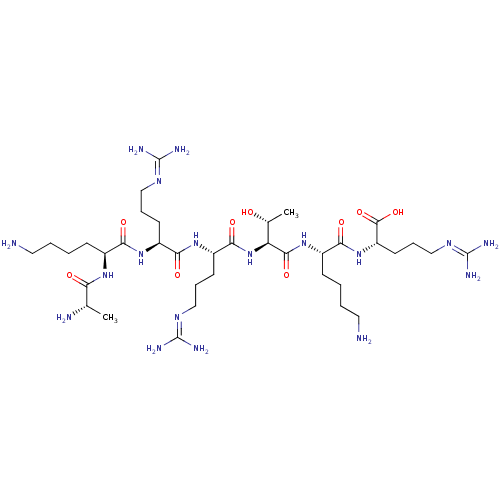 (CHEMBL2179835) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PACE4 expressed in Drosophila S2 cells using pyroGlu-Arg-Val-Lys-Arg-methyl-coumaryl-7-amide as substrate... | J Med Chem 55: 10501-11 (2012) Article DOI: 10.1021/jm3011178 BindingDB Entry DOI: 10.7270/Q2NV9KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471258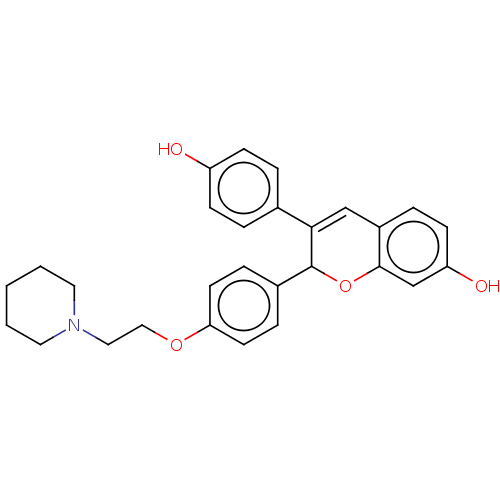 (CHEMBL67783) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.525 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent binding affinity against estradiol-stimulated T-47D cell proliferation | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50238741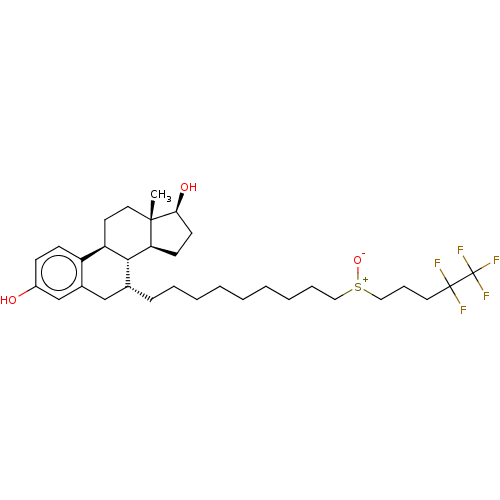 (CHEBI:31638 | Faslodex | Fulvestrant | ICI-182780 ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.668 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 2.5%DMF | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50238741 (CHEBI:31638 | Faslodex | Fulvestrant | ICI-182780 ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.755 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human Breast cancer cytosol in 2.5%DMF | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 6 (Homo sapiens (Human)) | BDBM50399754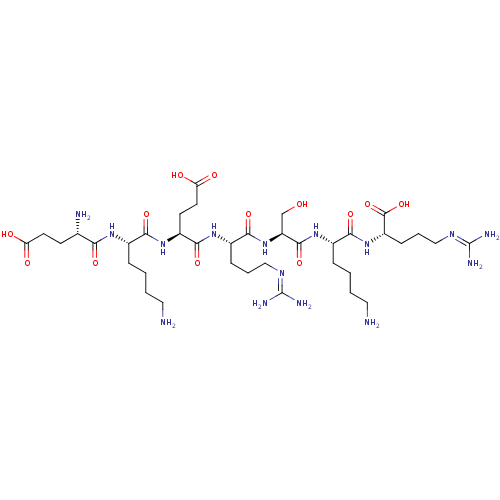 (CHEMBL2179836) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PACE4 expressed in Drosophila S2 cells using pyroGlu-Arg-Val-Lys-Arg-methyl-coumaryl-7-amide as substrate... | J Med Chem 55: 10501-11 (2012) Article DOI: 10.1021/jm3011178 BindingDB Entry DOI: 10.7270/Q2NV9KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 6 (Homo sapiens (Human)) | BDBM50399752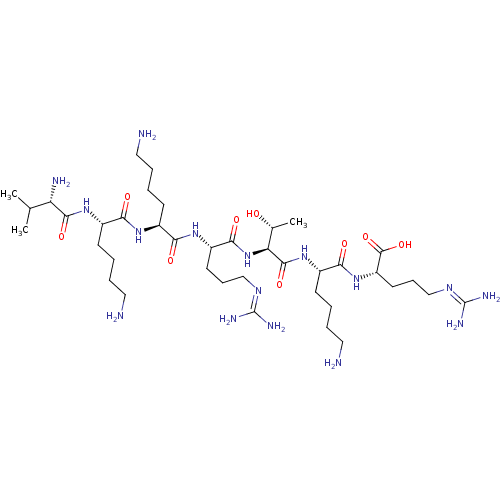 (CHEMBL2179429) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PACE4 expressed in Drosophila S2 cells using pyroGlu-Arg-Val-Lys-Arg-methyl-coumaryl-7-amide as substrate... | J Med Chem 55: 10501-11 (2012) Article DOI: 10.1021/jm3011178 BindingDB Entry DOI: 10.7270/Q2NV9KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50399754 (CHEMBL2179836) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant furin expressed in Drosophila S2 cells using pyroGlu-Arg-Val-Lys-Arg-methyl-coumaryl-7-amide as substrate... | J Med Chem 55: 10501-11 (2012) Article DOI: 10.1021/jm3011178 BindingDB Entry DOI: 10.7270/Q2NV9KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471252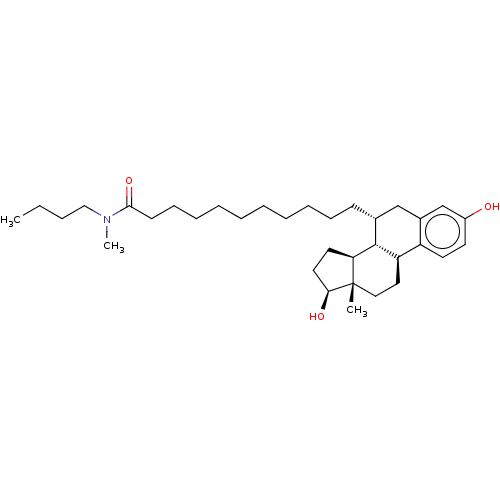 (CHEMBL1222035 | ICI-164384) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human Breast cancer cytosol in 2.5%DMF | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50399753 (CHEMBL2179835) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant furin expressed in Drosophila S2 cells using pyroGlu-Arg-Val-Lys-Arg-methyl-coumaryl-7-amide as substrate... | J Med Chem 55: 10501-11 (2012) Article DOI: 10.1021/jm3011178 BindingDB Entry DOI: 10.7270/Q2NV9KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuroendocrine convertase 2 (Homo sapiens (Human)) | BDBM50446945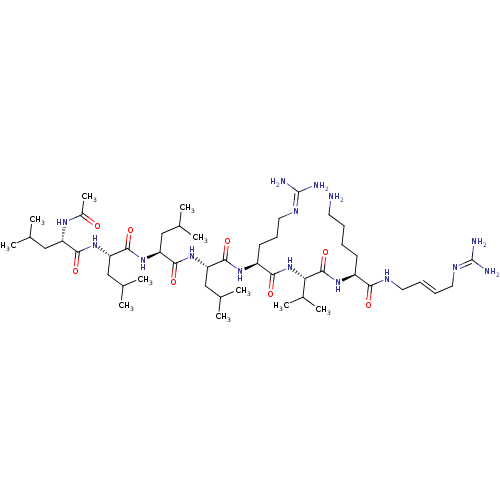 (CHEMBL3115770) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human PC2 expressed in drosophila schneider 2 cells using pyroGlu-Arg-Thr-Lys-Arg-AMC as substrate after 1 hr | J Med Chem 57: 98-109 (2014) Article DOI: 10.1021/jm401457n BindingDB Entry DOI: 10.7270/Q2S1840S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471252 (CHEMBL1222035 | ICI-164384) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 2.5%DMF | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50448473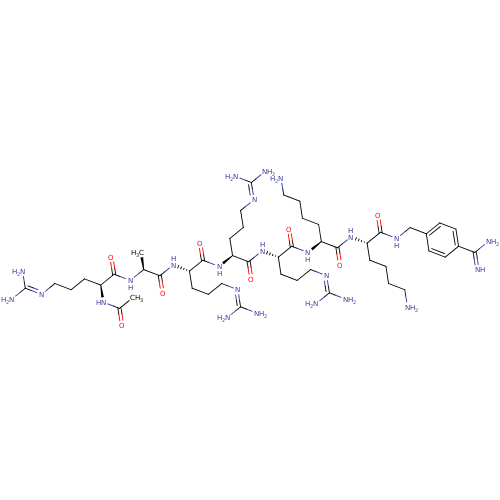 (CHEMBL3126399) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Competitive inhibition of recombinant furin (unknown origin) using as substrate after 60 mins | J Med Chem 57: 29-41 (2014) Article DOI: 10.1021/jm400633d BindingDB Entry DOI: 10.7270/Q27M09FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471257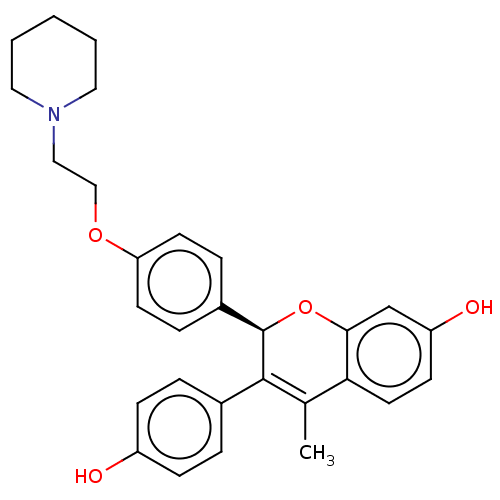 (CHEMBL67837) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 3.3%ethanol | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 6 (Homo sapiens (Human)) | BDBM50399751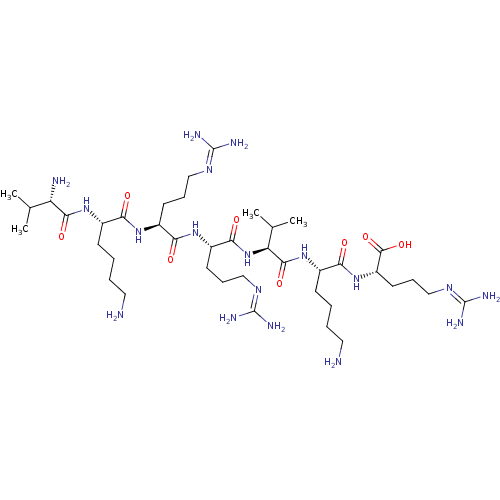 (CHEMBL2179431) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PACE4 expressed in Drosophila S2 cells using pyroGlu-Arg-Val-Lys-Arg-methyl-coumaryl-7-amide as substrate... | J Med Chem 55: 10501-11 (2012) Article DOI: 10.1021/jm3011178 BindingDB Entry DOI: 10.7270/Q2NV9KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471257 (CHEMBL67837) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor in Human Breast cancer cytosol (3.3% ethanol) | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471252 (CHEMBL1222035 | ICI-164384) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 3.3%ethanol | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proprotein convertase subtilisin/kexin type 6 (Homo sapiens (Human)) | BDBM50466079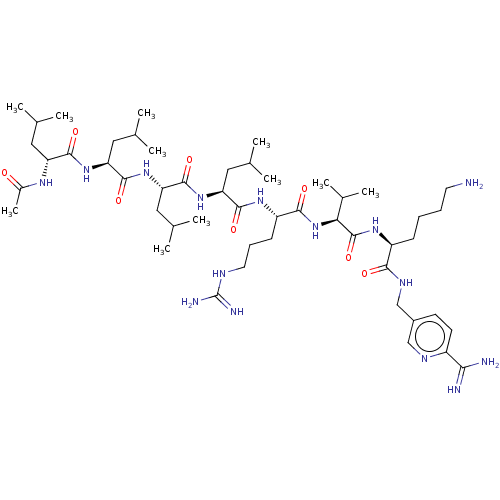 (CHEMBL4286124) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human PACE4 using pGlu-Arg-Thr-Lys-Arg-AMC peptide as substrate by spectrofluorometry | J Med Chem 61: 11250-11260 (2018) Article DOI: 10.1021/acs.jmedchem.8b01381 BindingDB Entry DOI: 10.7270/Q2TQ646X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50399752 (CHEMBL2179429) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant furin expressed in Drosophila S2 cells using pyroGlu-Arg-Val-Lys-Arg-methyl-coumaryl-7-amide as substrate... | J Med Chem 55: 10501-11 (2012) Article DOI: 10.1021/jm3011178 BindingDB Entry DOI: 10.7270/Q2NV9KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 6 (Homo sapiens (Human)) | BDBM50446944 (CHEMBL3115771) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant PACE4 expressed in drosophila schneider 2 cells using pyroGlu-Arg-Thr-Lys-Arg-AMC as substrate after 1 hr | J Med Chem 57: 98-109 (2014) Article DOI: 10.1021/jm401457n BindingDB Entry DOI: 10.7270/Q2S1840S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471253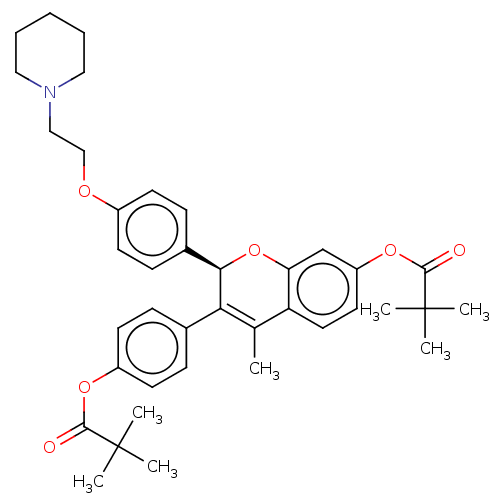 (CHEMBL441622) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent binding affinity against estradiol-stimulated T-47D cell proliferation | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50446944 (CHEMBL3115771) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant furin expressed in drosophila schneider 2 cells using pyroGlu-Arg-Thr-Lys-Arg-AMC as substrate after 1 hr | J Med Chem 57: 98-109 (2014) Article DOI: 10.1021/jm401457n BindingDB Entry DOI: 10.7270/Q2S1840S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471252 (CHEMBL1222035 | ICI-164384) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor in Human Breast cancer cytosol (3.3% ethanol) | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471254 (CHEMBL308234) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor in Human Breast cancer cytosol (3.3% ethanol) | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 6 (Homo sapiens (Human)) | BDBM50264998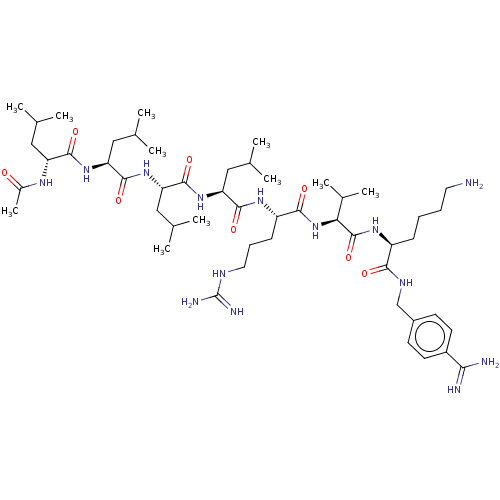 (CHEMBL4103458) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human PACE4 using pGlu-Arg-Thr-Lys-Arg-AMC peptide as substrate by spectrofluorometry | J Med Chem 61: 11250-11260 (2018) Article DOI: 10.1021/acs.jmedchem.8b01381 BindingDB Entry DOI: 10.7270/Q2TQ646X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 6 (Homo sapiens (Human)) | BDBM50264998 (CHEMBL4103458) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of recombinant human PACE4 expressed in S2 insect cells using pyroGlu-Arg-Thr-Lys-Arg-AMC peptide as substrate after 1 hr by spectrofluoro... | J Med Chem 61: 8457-8467 (2018) Article DOI: 10.1021/acs.jmedchem.8b01144 BindingDB Entry DOI: 10.7270/Q2SJ1P8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 6 (Homo sapiens (Human)) | BDBM50446945 (CHEMBL3115770) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant PACE4 expressed in drosophila schneider 2 cells using pyroGlu-Arg-Thr-Lys-Arg-AMC as substrate after 1 hr | J Med Chem 57: 98-109 (2014) Article DOI: 10.1021/jm401457n BindingDB Entry DOI: 10.7270/Q2S1840S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50399751 (CHEMBL2179431) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant furin expressed in Drosophila S2 cells using pyroGlu-Arg-Val-Lys-Arg-methyl-coumaryl-7-amide as substrate... | J Med Chem 55: 10501-11 (2012) Article DOI: 10.1021/jm3011178 BindingDB Entry DOI: 10.7270/Q2NV9KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50552678 (CHEMBL4759036) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant soluble human furin using Phac-Arg-Val-Arg-Arg-AMC as substrate | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00386 BindingDB Entry DOI: 10.7270/Q2CN77KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50552879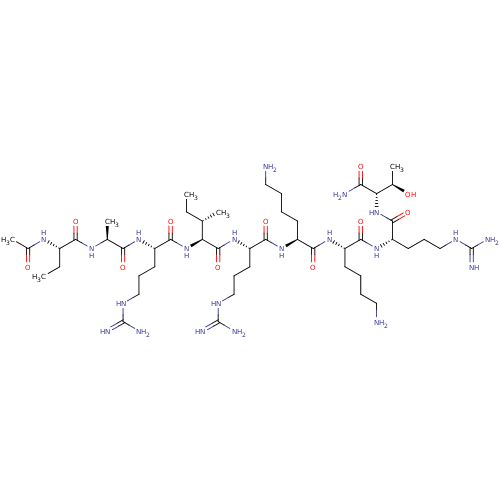 (CHEMBL4747516) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant soluble human furin using pyroGlu-Arg-Thr-Lys-Arg-methyl-coumaryl-7-amide as substrate measured after 1 hr | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00386 BindingDB Entry DOI: 10.7270/Q2CN77KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50552793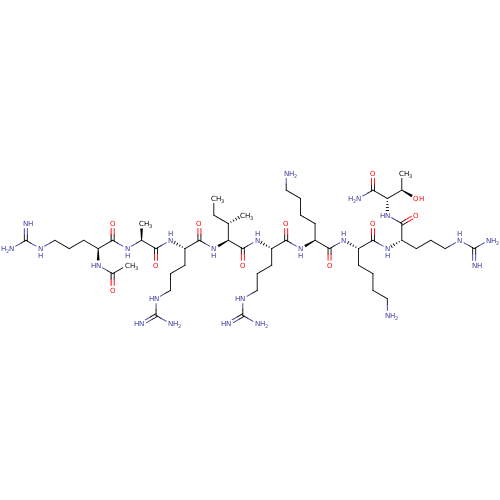 (CHEMBL4793978) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant soluble human furin using pyroGlu-Arg-Thr-Lys-Arg-methyl-coumaryl-7-amide as substrate measured after 1 hr | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00386 BindingDB Entry DOI: 10.7270/Q2CN77KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50448472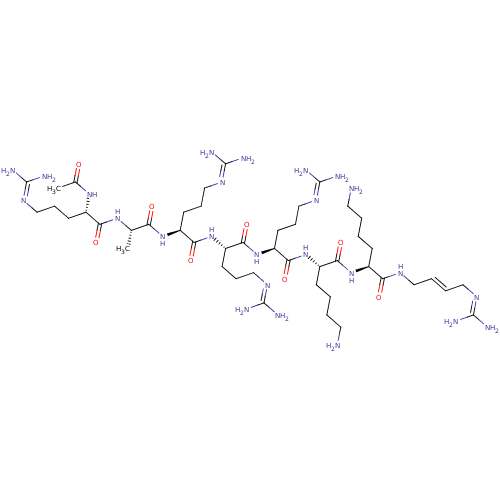 (CHEMBL3126398) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Competitive inhibition of recombinant furin (unknown origin) using as substrate after 60 mins | J Med Chem 57: 29-41 (2014) Article DOI: 10.1021/jm400633d BindingDB Entry DOI: 10.7270/Q27M09FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 318 total ) | Next | Last >> |