

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50594821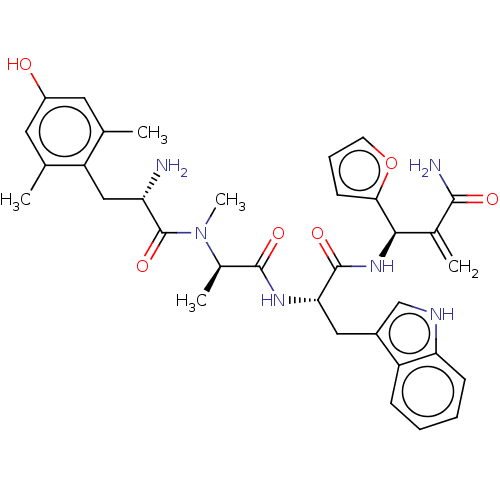 (CHEMBL5175179) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01915 BindingDB Entry DOI: 10.7270/Q22B931S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50594822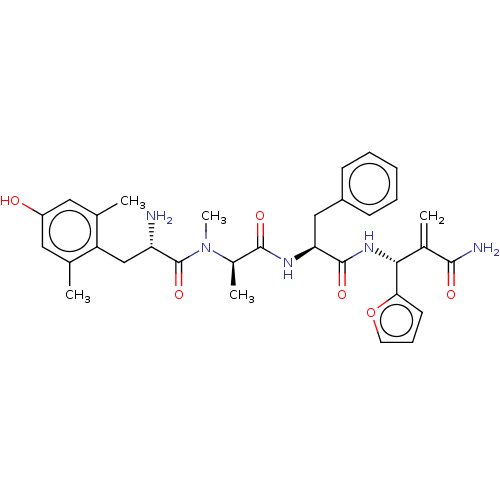 (CHEMBL5182849) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01915 BindingDB Entry DOI: 10.7270/Q22B931S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50207116 (CHEMBL3905247 | US9550741, I-4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50207116 (CHEMBL3905247 | US9550741, I-4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50594820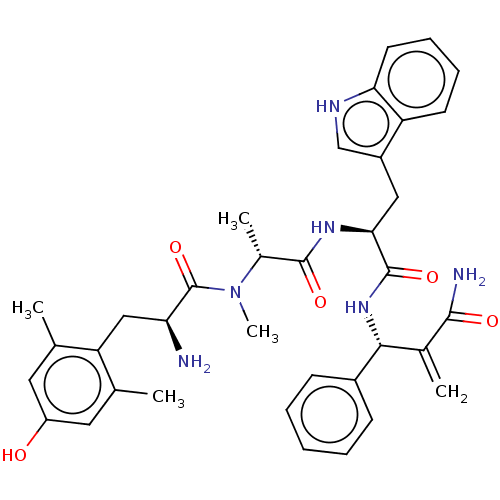 (CHEMBL5176887) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01915 BindingDB Entry DOI: 10.7270/Q22B931S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263372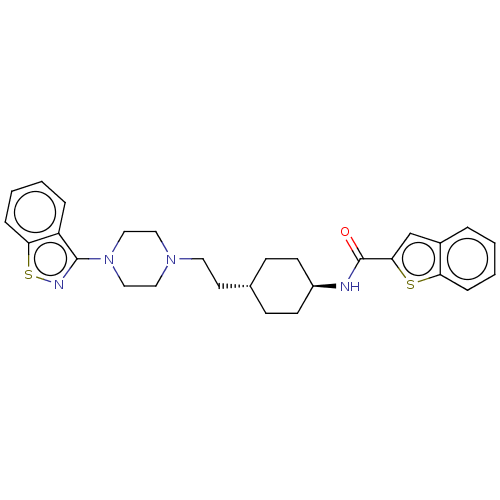 (US9550741, I-6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263372 (US9550741, I-6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50207143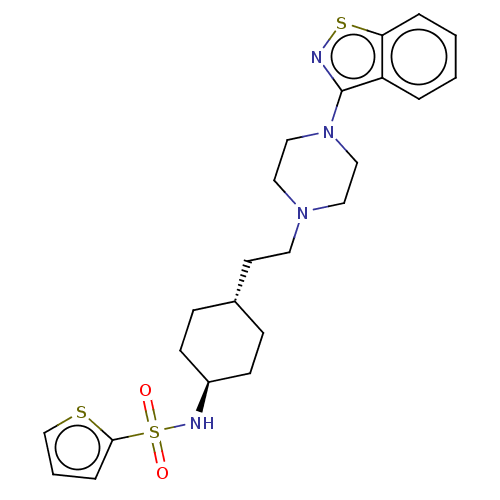 (CHEMBL3966842 | US9550741, II-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50207143 (CHEMBL3966842 | US9550741, II-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50408664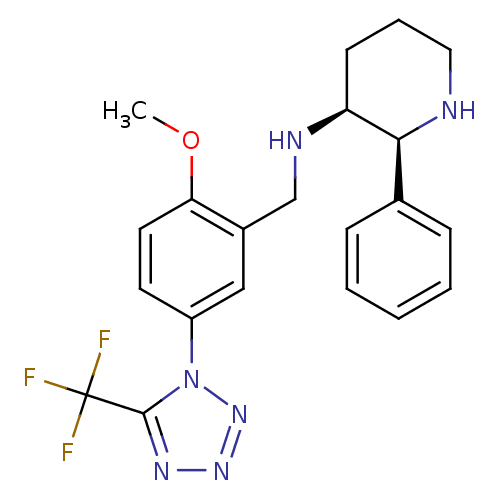 (GR-205171 | VOFOPITANT) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human recombinant NK1 receptor expressed in CHO cells | J Med Chem 52: 3238-47 (2009) Article DOI: 10.1021/jm900023b BindingDB Entry DOI: 10.7270/Q2BP0425 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50594825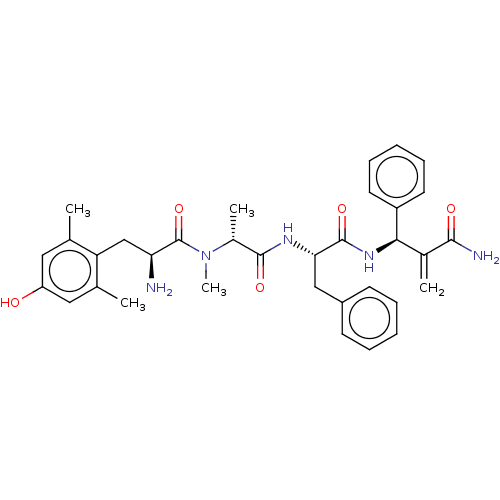 (CHEMBL5198856) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0511 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01915 BindingDB Entry DOI: 10.7270/Q22B931S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50207094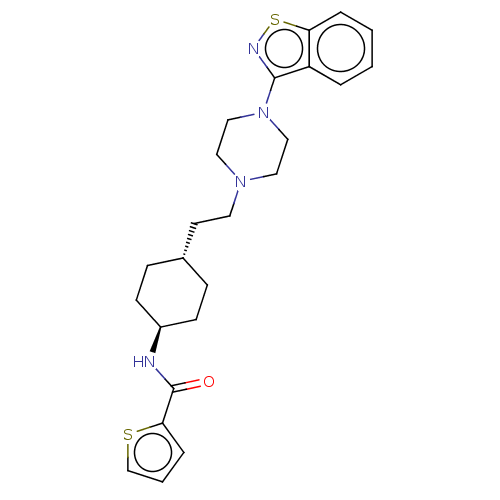 (CHEMBL3976282 | US9550741, I-2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50207094 (CHEMBL3976282 | US9550741, I-2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50207158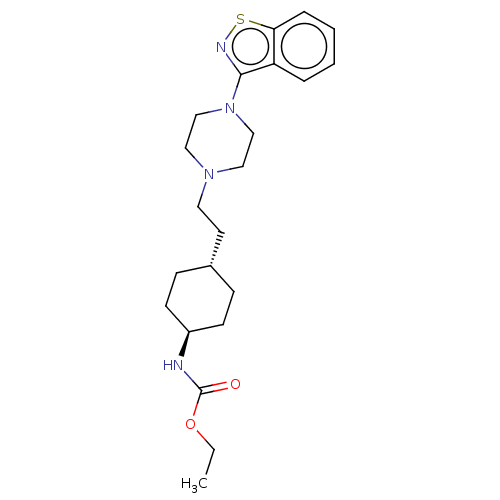 (CHEMBL3982486 | US9550741, III-2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50207158 (CHEMBL3982486 | US9550741, III-2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263429 (US9550741, III-11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263429 (US9550741, III-11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263393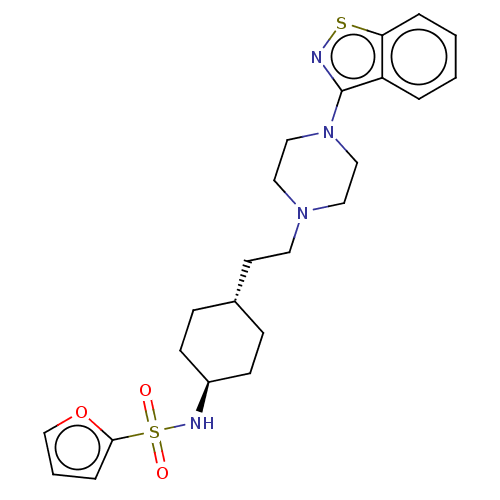 (US9550741, II-3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263393 (US9550741, II-3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50207113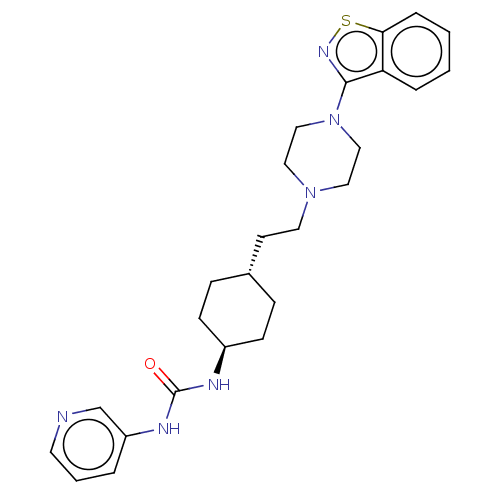 (CHEMBL3896937 | US9550741, IV-3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50207113 (CHEMBL3896937 | US9550741, IV-3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263388 (US9550741, I-22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263388 (US9550741, I-22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263371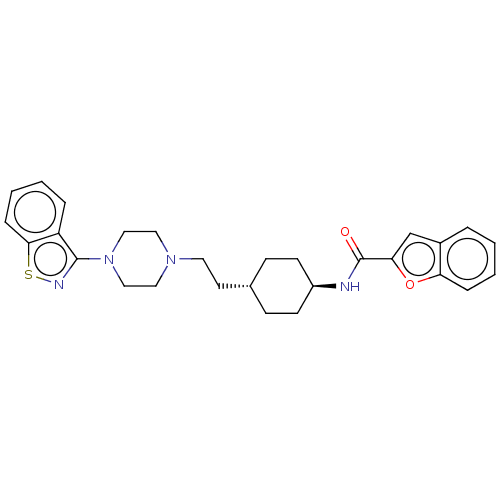 (US9550741, I-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263371 (US9550741, I-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50417977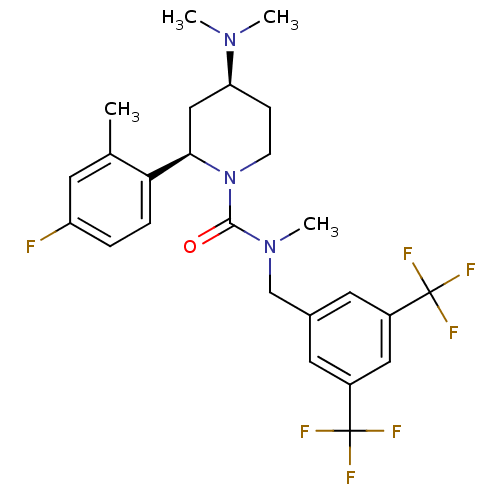 (CHEMBL1672044) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 54: 1071-9 (2011) Article DOI: 10.1021/jm1013264 BindingDB Entry DOI: 10.7270/Q2W66M1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50417978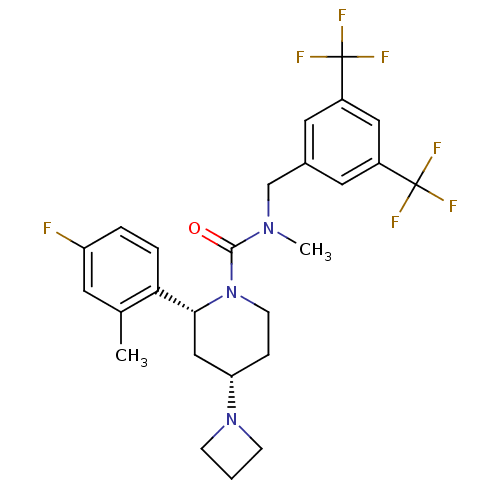 (CHEMBL1672047) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0813 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 54: 1071-9 (2011) Article DOI: 10.1021/jm1013264 BindingDB Entry DOI: 10.7270/Q2W66M1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263395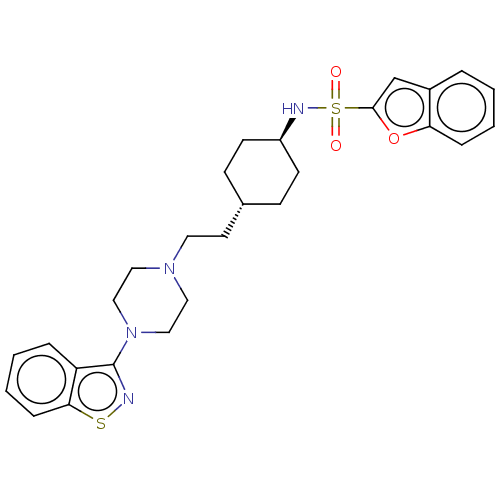 (US9550741, II-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263395 (US9550741, II-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263432 (US9550741, III-14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263432 (US9550741, III-14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50594846 (CHEMBL5209004) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01915 BindingDB Entry DOI: 10.7270/Q22B931S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263449 (US9550741, RGH-188) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263412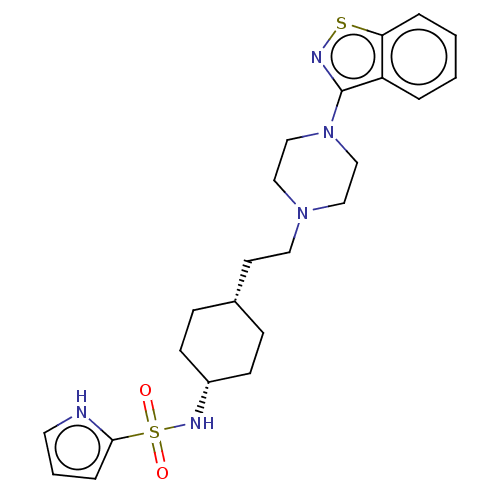 (US9550741, II-17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263421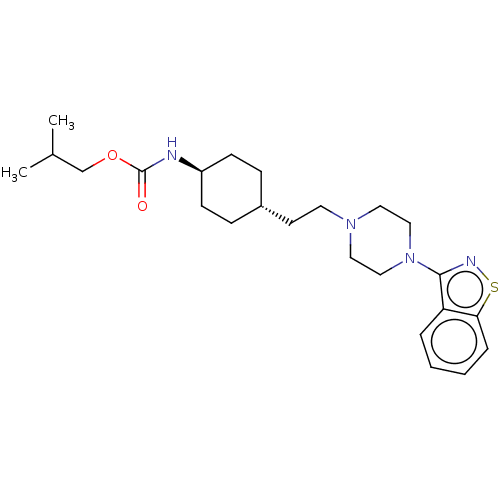 (US9550741, III-3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263412 (US9550741, II-17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263421 (US9550741, III-3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50091350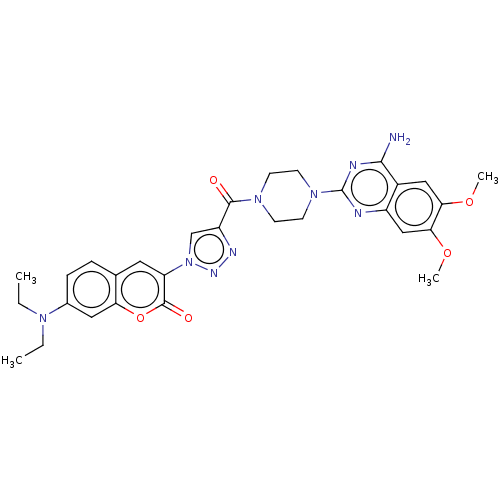 (CHEMBL3582270) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Displacement of [3H]-Prazosin from human alpha-1B adrenergic receptor transfected in CHO cell membranes after 2 hrs by microplate scintillation count... | ACS Med Chem Lett 6: 502-6 (2015) Article DOI: 10.1021/ml5004298 BindingDB Entry DOI: 10.7270/Q2VM4DZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263383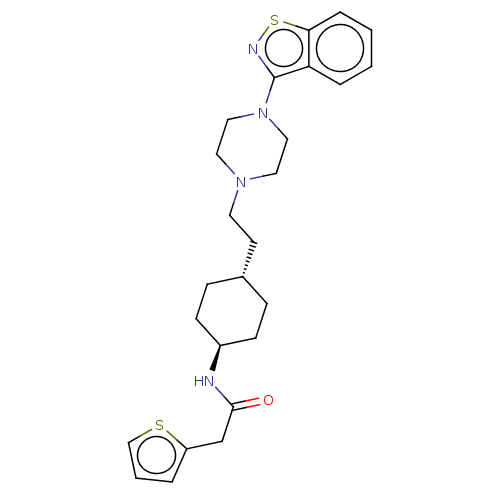 (US9550741, I-17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263383 (US9550741, I-17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263419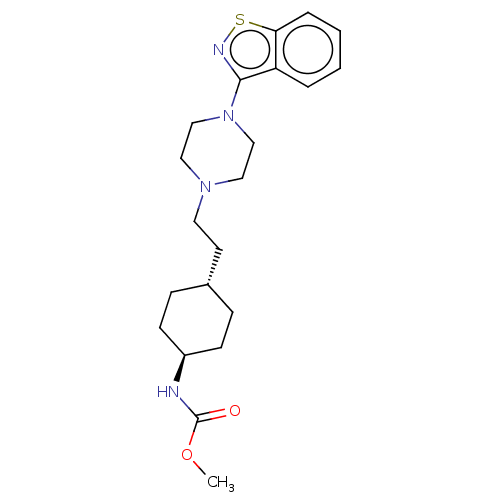 (US9550741, III-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263419 (US9550741, III-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50417973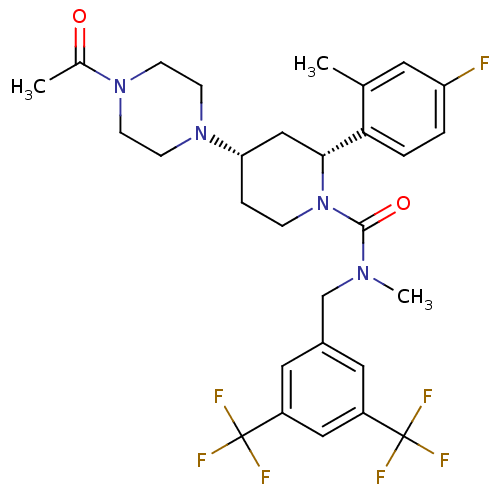 (CHEMBL1672053) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 54: 1071-9 (2011) Article DOI: 10.1021/jm1013264 BindingDB Entry DOI: 10.7270/Q2W66M1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50336575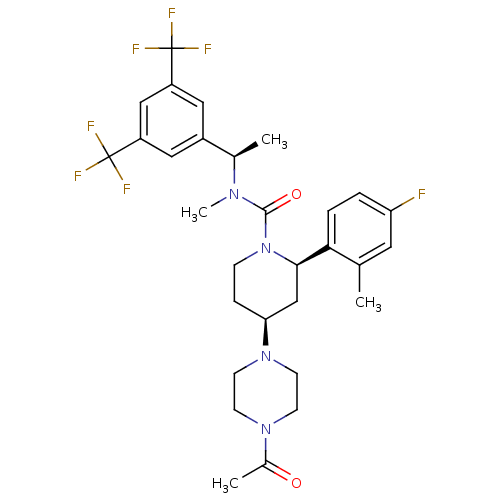 (CHEMBL1672054 | cis-(1'-Acetyl-N-{(1R)-1-[3,5-bis(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]GR205171 from human NK1 receptor in cortex homogenate by liquid scintillation counting | J Med Chem 54: 1071-9 (2011) Article DOI: 10.1021/jm1013264 BindingDB Entry DOI: 10.7270/Q2W66M1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50207091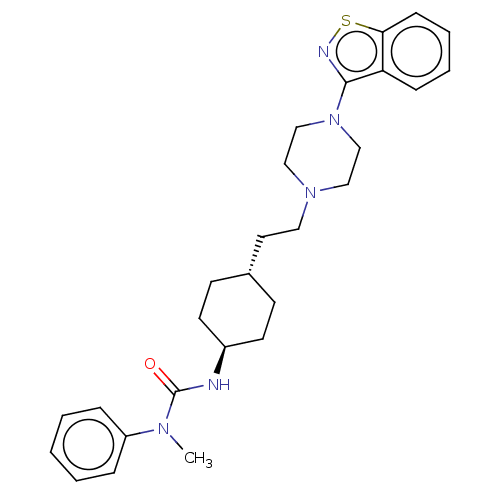 (CHEMBL3953773 | US9550741, IV-6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50207091 (CHEMBL3953773 | US9550741, IV-6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50207145 (CHEMBL3946995 | US9550741, I-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50207145 (CHEMBL3946995 | US9550741, I-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50367123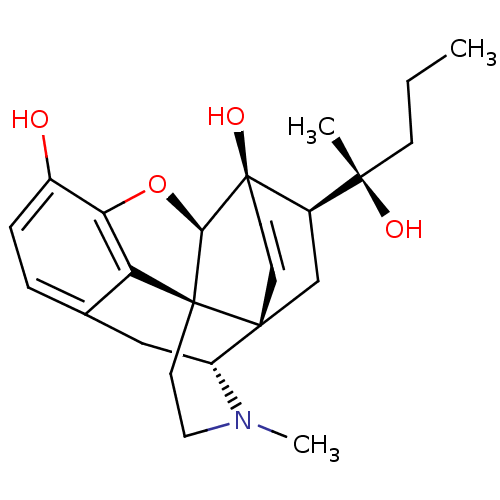 (ETORPHINE | M99) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 1301-4 (2009) Article DOI: 10.1016/j.bmcl.2009.01.078 BindingDB Entry DOI: 10.7270/Q2MP546B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123599 (ETORPHINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 25: 4689-92 (2015) Article DOI: 10.1016/j.bmcl.2015.06.092 BindingDB Entry DOI: 10.7270/Q2NV9M2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 13631 total ) | Next | Last >> |