 Found 454 hits with Last Name = 'abbenante' and Initial = 'g'
Found 454 hits with Last Name = 'abbenante' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM578
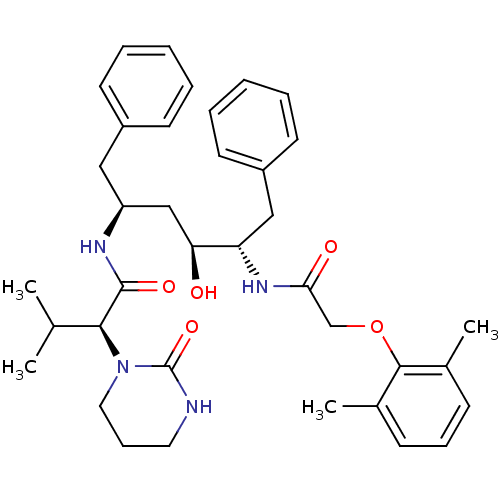
((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...)Show SMILES CC(C)[C@H](N1CCCNC1=O)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N4O5/c1-25(2)34(41-20-12-19-38-37(41)45)36(44)39-30(21-28-15-7-5-8-16-28)23-32(42)31(22-29-17-9-6-10-18-29)40-33(43)24-46-35-26(3)13-11-14-27(35)4/h5-11,13-18,25,30-32,34,42H,12,19-24H2,1-4H3,(H,38,45)(H,39,44)(H,40,43)/t30-,31-,32-,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against ritonavir-resistant strains. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin D
(Homo sapiens (Human)) | BDBM912
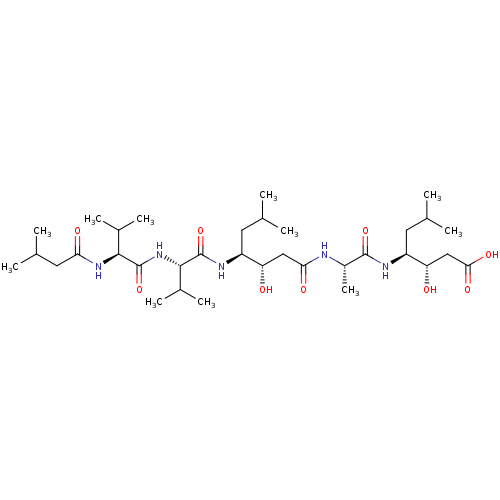
((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against human cathepsin D |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036477

(2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...)Show SMILES COc1cc2c(C(=O)N(COC(=O)c3c(Cl)ccc(OCCN4CCOCC4)c3Cl)S2(=O)=O)c(c1)C(C)C Show InChI InChI=1S/C25H28Cl2N2O8S/c1-15(2)17-12-16(34-3)13-20-21(17)24(30)29(38(20,32)33)14-37-25(31)22-18(26)4-5-19(23(22)27)36-11-8-28-6-9-35-10-7-28/h4-5,12-13,15H,6-11,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Elastase. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM729

((2S,4R)-N-tert-butyl-1-[(2R,3S)-2-hydroxy-3-[(2S)-...)Show SMILES CC(C)[C@H](NC(=O)c1ccc2ccccc2n1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1CC[C@H](C[C@H]1C(=O)NC(C)(C)C)OCc1ccncc1 |r| Show InChI InChI=1S/C41H52N6O5/c1-27(2)37(45-38(49)33-16-15-30-13-9-10-14-32(30)43-33)40(51)44-34(23-28-11-7-6-8-12-28)36(48)25-47-22-19-31(52-26-29-17-20-42-21-18-29)24-35(47)39(50)46-41(3,4)5/h6-18,20-21,27,31,34-37,48H,19,22-26H2,1-5H3,(H,44,51)(H,45,49)(H,46,50)/t31-,34+,35+,36-,37+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against HIV-1 protease . |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50082556
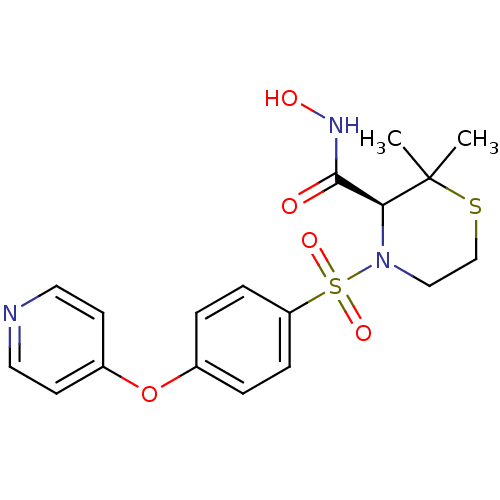
((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-13 (MMP-13). |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50057826

((S)-1-((R)-2-Amino-3,3-dicyclohexyl-propionyl)-pyr...)Show SMILES N[C@H](C(C1CCCCC1)C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1 |wU:21.24,wD:1.0,(8.57,-5.29,;9.89,-4.53,;9.89,-2.99,;11.24,-2.22,;12.55,-2.99,;13.9,-2.22,;13.9,-.68,;12.58,.09,;11.23,-.68,;8.57,-2.21,;7.23,-2.98,;5.9,-2.21,;5.9,-.67,;7.23,.1,;8.57,-.67,;11.24,-5.3,;11.24,-6.84,;12.72,-4.89,;13.26,-3.44,;14.8,-3.51,;15.22,-5,;13.92,-5.85,;13.86,-7.39,;12.49,-8.1,;15.15,-8.21,;16.52,-7.51,;17.82,-8.32,;17.78,-9.66,;18.86,-11.05,;17.78,-12.17,;18.86,-13.25,;17.85,-10.92,;16.76,-9.44,)| Show InChI InChI=1S/C27H48N4O2/c28-22-15-13-19(14-16-22)18-30-26(32)23-12-7-17-31(23)27(33)25(29)24(20-8-3-1-4-9-20)21-10-5-2-6-11-21/h19-25H,1-18,28-29H2,(H,30,32)/t19?,22?,23-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Thrombin. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-2 (MMP-2). |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50084664

(({3-(5-Carbamimidoyl-2-hydroxy-phenoxy)-2,6-difluo...)Show SMILES CN(CC(O)=O)c1c(F)c(Oc2cccc(c2)C2=NCCN2C)cc(Oc2cc(ccc2O)C(N)=N)c1F |t:18| Show InChI InChI=1S/C26H25F2N5O5/c1-32-9-8-31-26(32)15-4-3-5-16(10-15)37-19-12-20(23(28)24(22(19)27)33(2)13-21(35)36)38-18-11-14(25(29)30)6-7-17(18)34/h3-7,10-12,34H,8-9,13H2,1-2H3,(H3,29,30)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Coagulation factor X |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM729

((2S,4R)-N-tert-butyl-1-[(2R,3S)-2-hydroxy-3-[(2S)-...)Show SMILES CC(C)[C@H](NC(=O)c1ccc2ccccc2n1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1CC[C@H](C[C@H]1C(=O)NC(C)(C)C)OCc1ccncc1 |r| Show InChI InChI=1S/C41H52N6O5/c1-27(2)37(45-38(49)33-16-15-30-13-9-10-14-32(30)43-33)40(51)44-34(23-28-11-7-6-8-12-28)36(48)25-47-22-19-31(52-26-29-17-20-42-21-18-29)24-35(47)39(50)46-41(3,4)5/h6-18,20-21,27,31,34-37,48H,19,22-26H2,1-5H3,(H,44,51)(H,45,49)(H,46,50)/t31-,34+,35+,36-,37+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against HIV-1 protease . |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50084682

(1-[3-(4-Carbamimidoyl-phenyl)-2-(2-methyl-1,2,3,4-...)Show SMILES Cc1cc(ccc1C(=O)N[C@@H](CCCNC(N)=N)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1)-c1ccccc1S(=O)(=O)NC(C)(C)C |TLB:30:28:25:23| Show InChI InChI=1S/C33H48BN5O5S/c1-20-17-21(24-11-8-9-12-25(24)45(41,42)39-31(2,3)4)14-15-23(20)29(40)38-28(13-10-16-37-30(35)36)34-43-27-19-22-18-26(32(22,5)6)33(27,7)44-34/h8-9,11-12,14-15,17,22,26-28,39H,10,13,16,18-19H2,1-7H3,(H,38,40)(H4,35,36,37)/t22?,26?,27-,28+,33+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Thrombin. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11863

(4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CCOCC1 Show InChI InChI=1S/C19H20ClNO6S/c20-14-1-3-15(4-2-14)27-16-5-7-17(8-6-16)28(24,25)13-19(18(22)21-23)9-11-26-12-10-19/h1-8,23H,9-13H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-2 (MMP-2). |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-3 (MMP-3). |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50037991

(CHEMBL325166 | NAPSAGATRAN | Ro-46-6240 | {[(S)-3-...)Show SMILES NC(=N)N1CCC[C@@H](CNC(=O)C[C@H](NS(=O)(=O)c2ccc3ccccc3c2)C(=O)N(CC(O)=O)C2CC2)C1 Show InChI InChI=1S/C26H34N6O6S/c27-26(28)31-11-3-4-17(15-31)14-29-23(33)13-22(25(36)32(16-24(34)35)20-8-9-20)30-39(37,38)21-10-7-18-5-1-2-6-19(18)12-21/h1-2,5-7,10,12,17,20,22,30H,3-4,8-9,11,13-16H2,(H3,27,28)(H,29,33)(H,34,35)/t17-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Thrombin. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50068972
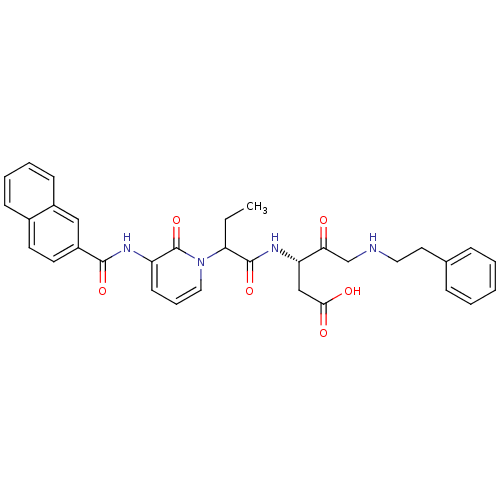
((S)-3-(2-{3-[(Naphthalene-2-carbonyl)-amino]-2-oxo...)Show SMILES CCC(C(=O)N[C@@H](CC(O)=O)C(=O)CNCCc1ccccc1)n1cccc(NC(=O)c2ccc3ccccc3c2)c1=O Show InChI InChI=1S/C33H34N4O6/c1-2-28(32(42)36-27(20-30(39)40)29(38)21-34-17-16-22-9-4-3-5-10-22)37-18-8-13-26(33(37)43)35-31(41)25-15-14-23-11-6-7-12-24(23)19-25/h3-15,18-19,27-28,34H,2,16-17,20-21H2,1H3,(H,35,41)(H,36,42)(H,39,40)/t27-,28?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
The binding affinity against IL-1 beta converting enzyme |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058491

(3-{4-[2-(4-{1-[4-(2-Carboxy-2-methyl-propane-1-sul...)Show SMILES CC(C)(CS(=O)(=O)c1ccc(OC(=O)C(C)(C)c2ccc(cc2)C(C)(C)C(=O)Oc2ccc(cc2)S(=O)(=O)CC(C)(C)C(O)=O)cc1)C(O)=O Show InChI InChI=1S/C36H42O12S2/c1-33(2,29(37)38)21-49(43,44)27-17-13-25(14-18-27)47-31(41)35(5,6)23-9-11-24(12-10-23)36(7,8)32(42)48-26-15-19-28(20-16-26)50(45,46)22-34(3,4)30(39)40/h9-20H,21-22H2,1-8H3,(H,37,38)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Elastase. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50084686
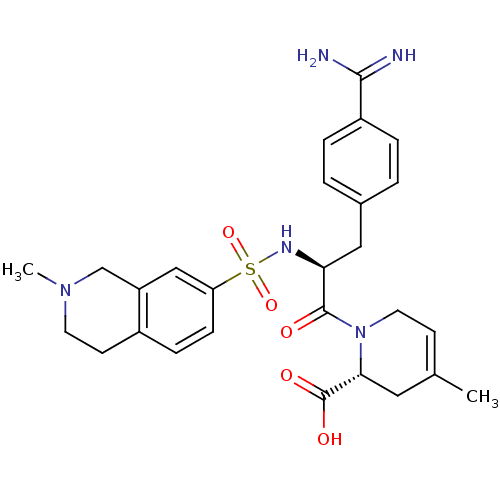
(1-[3-(4-Carbamimidoyl-phenyl)-2-(2-methyl-1,2,3,4-...)Show SMILES CN1CCc2ccc(cc2C1)S(=O)(=O)N[C@@H](Cc1ccc(cc1)C(N)=N)C(=O)N1CC=C(C)C[C@@H]1C(O)=O |t:33| Show InChI InChI=1S/C27H33N5O5S/c1-17-9-12-32(24(13-17)27(34)35)26(33)23(14-18-3-5-20(6-4-18)25(28)29)30-38(36,37)22-8-7-19-10-11-31(2)16-21(19)15-22/h3-9,15,23-24,30H,10-14,16H2,1-2H3,(H3,28,29)(H,34,35)/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Trypsin |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against HIV-1 protease enzyme. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM11863

(4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CCOCC1 Show InChI InChI=1S/C19H20ClNO6S/c20-14-1-3-15(4-2-14)27-16-5-7-17(8-6-16)28(24,25)13-19(18(22)21-23)9-11-26-12-10-19/h1-8,23H,9-13H2,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-13 (MMP-13). |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM11863

(4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CCOCC1 Show InChI InChI=1S/C19H20ClNO6S/c20-14-1-3-15(4-2-14)27-16-5-7-17(8-6-16)28(24,25)13-19(18(22)21-23)9-11-26-12-10-19/h1-8,23H,9-13H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-9 (MMP-9). |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM577

((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C25H35N3O6S/c1-18(2)15-28(35(31,32)22-10-8-20(26)9-11-22)16-24(29)23(14-19-6-4-3-5-7-19)27-25(30)34-21-12-13-33-17-21/h3-11,18,21,23-24,29H,12-17,26H2,1-2H3,(H,27,30)/t21-,23-,24+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against HIV-protease |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288192
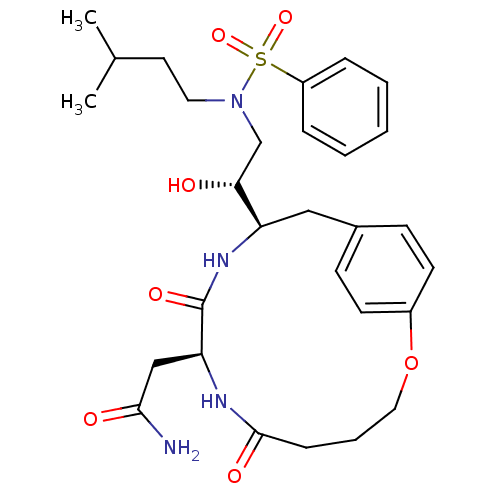
(CHEMBL83186 | N-[2-Hydroxy-2-((S)-8-isopropyl-6,9-...)Show SMILES CC(C)CCN(C[C@@H](O)[C@H]1Cc2ccc(OCCCC(=O)N[C@@H](CC(N)=O)C(=O)N1)cc2)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C29H40N4O7S/c1-20(2)14-15-33(41(38,39)23-7-4-3-5-8-23)19-26(34)24-17-21-10-12-22(13-11-21)40-16-6-9-28(36)31-25(18-27(30)35)29(37)32-24/h3-5,7-8,10-13,20,24-26,34H,6,9,14-19H2,1-2H3,(H2,30,35)(H,31,36)(H,32,37)/t24-,25+,26-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 6: 2531-2536 (1996)
Article DOI: 10.1016/0960-894X(96)00468-4
BindingDB Entry DOI: 10.7270/Q2S1830C |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM13928
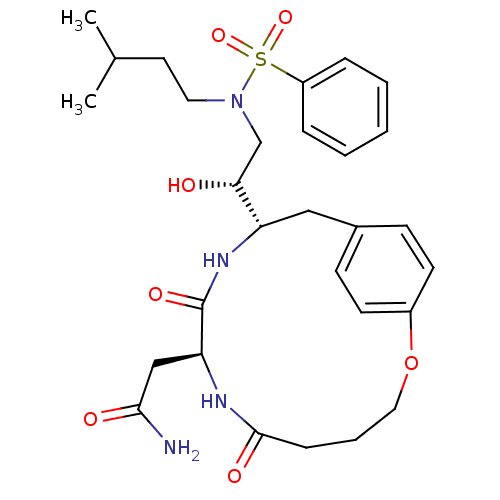
(2-[(8S,11S)-11-[(1R)-2-[benzene(3-methylbutyl)sulf...)Show SMILES CC(C)CCN(C[C@@H](O)[C@@H]1Cc2ccc(OCCCC(=O)N[C@@H](CC(N)=O)C(=O)N1)cc2)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C29H40N4O7S/c1-20(2)14-15-33(41(38,39)23-7-4-3-5-8-23)19-26(34)24-17-21-10-12-22(13-11-21)40-16-6-9-28(36)31-25(18-27(30)35)29(37)32-24/h3-5,7-8,10-13,20,24-26,34H,6,9,14-19H2,1-2H3,(H2,30,35)(H,31,36)(H,32,37)/t24-,25-,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.600 | -54.8 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland
| Assay Description
he Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in fl... |
Biochemistry 38: 7978-88 (1999)
Article DOI: 10.1021/bi990174x
BindingDB Entry DOI: 10.7270/Q20000B3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50077669

((S)-2-Benzyl-N-[(S)-1-((1S,2R,3S)-1-cyclohexylmeth...)Show SMILES CC(C)(C)S(=O)(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)C1CC1 Show InChI InChI=1S/C33H50N4O6S/c1-33(2,3)44(42,43)20-25(16-22-10-6-4-7-11-22)31(40)37-28(18-26-19-34-21-35-26)32(41)36-27(17-23-12-8-5-9-13-23)30(39)29(38)24-14-15-24/h4,6-7,10-11,19,21,23-25,27-30,38-39H,5,8-9,12-18,20H2,1-3H3,(H,34,35)(H,36,41)(H,37,40)/t25-,27+,28+,29+,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against aspartic protease renin. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine protease 1
(Homo sapiens (Human)) | BDBM50084682

(1-[3-(4-Carbamimidoyl-phenyl)-2-(2-methyl-1,2,3,4-...)Show SMILES Cc1cc(ccc1C(=O)N[C@@H](CCCNC(N)=N)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1)-c1ccccc1S(=O)(=O)NC(C)(C)C |TLB:30:28:25:23| Show InChI InChI=1S/C33H48BN5O5S/c1-20-17-21(24-11-8-9-12-25(24)45(41,42)39-31(2,3)4)14-15-23(20)29(40)38-28(13-10-16-37-30(35)36)34-43-27-19-22-18-26(32(22,5)6)33(27,7)44-34/h8-9,11-12,14-15,17,22,26-28,39H,10,13,16,18-19H2,1-7H3,(H,38,40)(H4,35,36,37)/t22?,26?,27-,28+,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Trypsin |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17941
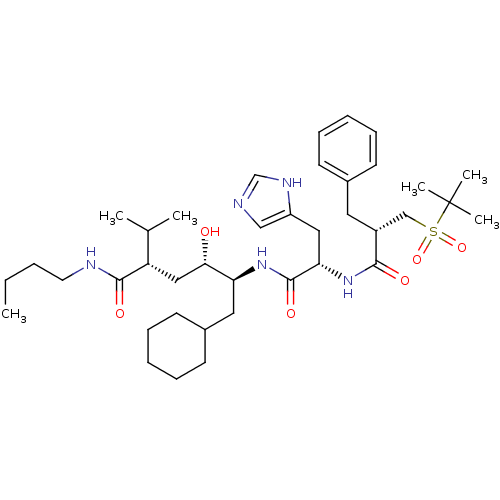
((2S,4S,5S)-5-[(2S)-2-[(2S)-2-benzyl-3-[(2-methylpr...)Show SMILES CCCCNC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)CS(=O)(=O)C(C)(C)C)C(C)C |r| Show InChI InChI=1S/C39H63N5O6S/c1-7-8-19-41-37(47)32(27(2)3)23-35(45)33(21-29-17-13-10-14-18-29)43-38(48)34(22-31-24-40-26-42-31)44-36(46)30(20-28-15-11-9-12-16-28)25-51(49,50)39(4,5)6/h9,11-12,15-16,24,26-27,29-30,32-35,45H,7-8,10,13-14,17-23,25H2,1-6H3,(H,40,42)(H,41,47)(H,43,48)(H,44,46)/t30-,32+,33+,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against aspartic protease renin. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin D
(Homo sapiens (Human)) | BDBM50084670
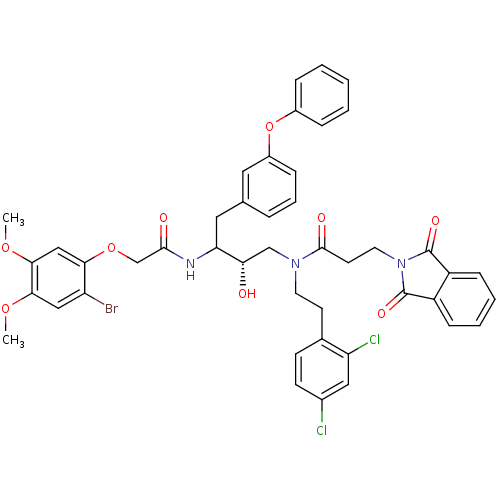
(CHEMBL323806 | N-[3-[2-(2-Bromo-4,5-dimethoxy-phen...)Show SMILES COc1cc(Br)c(OCC(=O)NC(Cc2cccc(Oc3ccccc3)c2)[C@@H](O)CN(CCc2ccc(Cl)cc2Cl)C(=O)CCN2C(=O)c3ccccc3C2=O)cc1OC Show InChI InChI=1S/C45H42BrCl2N3O9/c1-57-40-24-35(46)39(25-41(40)58-2)59-27-42(53)49-37(22-28-9-8-12-32(21-28)60-31-10-4-3-5-11-31)38(52)26-50(19-17-29-15-16-30(47)23-36(29)48)43(54)18-20-51-44(55)33-13-6-7-14-34(33)45(51)56/h3-16,21,23-25,37-38,52H,17-20,22,26-27H2,1-2H3,(H,49,53)/t37?,38-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against aspartic proteases |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
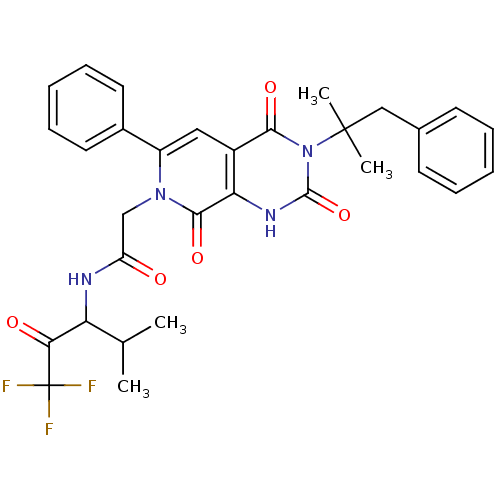
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50041291
(2-[(R)-3-(1,1-Dimethyl-2-phenyl-ethyl)-2,4,8-triox...)Show SMILES CC(C)C(NC(=O)Cn1c(cc2c([nH]c(=O)n(c2=O)C(C)(C)Cc2ccccc2)c1=O)-c1ccccc1)C(=O)C(F)(F)F Show InChI InChI=1S/C31H31F3N4O5/c1-18(2)24(26(40)31(32,33)34)35-23(39)17-37-22(20-13-9-6-10-14-20)15-21-25(28(37)42)36-29(43)38(27(21)41)30(3,4)16-19-11-7-5-8-12-19/h5-15,18,24H,16-17H2,1-4H3,(H,35,39)(H,36,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Elastase. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50086884
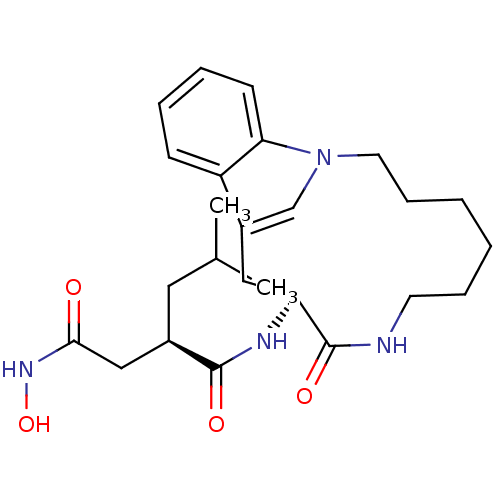
((R)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-9-oxo-1,8-di...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@H]1Cc2cn(CCCCCCNC1=O)c1ccccc21 Show InChI InChI=1S/C25H36N4O4/c1-17(2)13-18(15-23(30)28-33)24(31)27-21-14-19-16-29(22-10-6-5-9-20(19)22)12-8-4-3-7-11-26-25(21)32/h5-6,9-10,16-18,21,33H,3-4,7-8,11-15H2,1-2H3,(H,26,32)(H,27,31)(H,28,30)/t18-,21+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
binding affinity towards HIV-1 Protease enzyme |
J Med Chem 43: 1271-81 (2001)
BindingDB Entry DOI: 10.7270/Q23779F5 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50086880
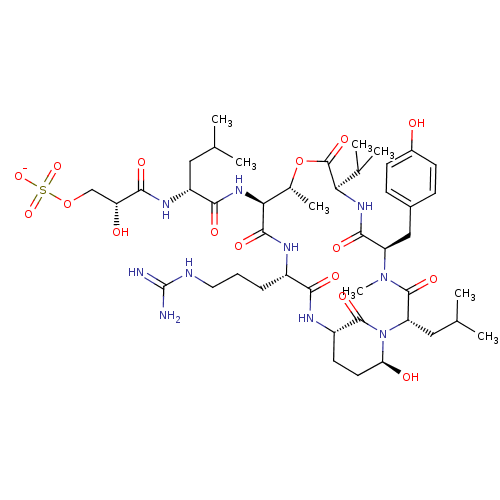
(Proteolytic Enzyme inhibitor)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](O)COS([O-])(=O)=O)C(=O)N[C@H]1[C@@H](C)OC(=O)[C@H](NC(=O)[C@@H](Cc2ccc(O)cc2)N(C)C(=O)[C@H](CC(C)C)N2[C@@H](O)CC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC1=O)C2=O)C(C)C Show InChI InChI=1S/C45H72N10O16S/c1-22(2)18-30(51-40(62)33(57)21-70-72(67,68)69)38(60)53-36-25(7)71-44(66)35(24(5)6)52-39(61)31(20-26-11-13-27(56)14-12-26)54(8)43(65)32(19-23(3)4)55-34(58)16-15-29(42(55)64)50-37(59)28(49-41(36)63)10-9-17-48-45(46)47/h11-14,22-25,28-36,56-58H,9-10,15-21H2,1-8H3,(H,49,63)(H,50,59)(H,51,62)(H,52,61)(H,53,60)(H4,46,47,48)(H,67,68,69)/p-1/t25-,28+,29+,30-,31-,32+,33-,34+,35-,36+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
binding affinity towards HIV-1 Protease enzyme |
J Med Chem 43: 1271-81 (2001)
BindingDB Entry DOI: 10.7270/Q23779F5 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50084633
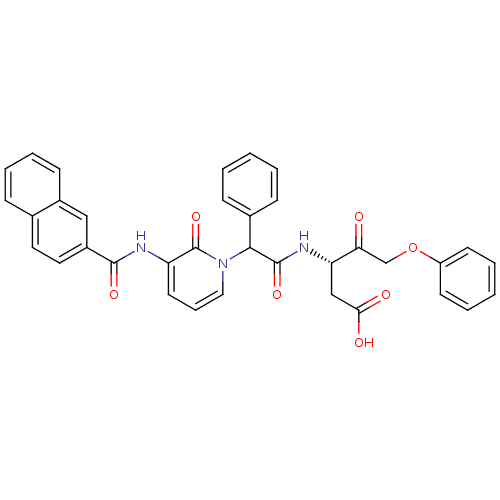
((S)-3-(2-{3-[(Naphthalene-2-carbonyl)-amino]-2-oxo...)Show SMILES OC(=O)C[C@H](NC(=O)C(c1ccccc1)n1cccc(NC(=O)c2ccc3ccccc3c2)c1=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C35H29N3O7/c39-30(22-45-27-14-5-2-6-15-27)29(21-31(40)41)37-34(43)32(24-11-3-1-4-12-24)38-19-9-16-28(35(38)44)36-33(42)26-18-17-23-10-7-8-13-25(23)20-26/h1-20,29,32H,21-22H2,(H,36,42)(H,37,43)(H,40,41)/t29-,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
The binding affinity against IL-1 beta converting enzyme |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50084617
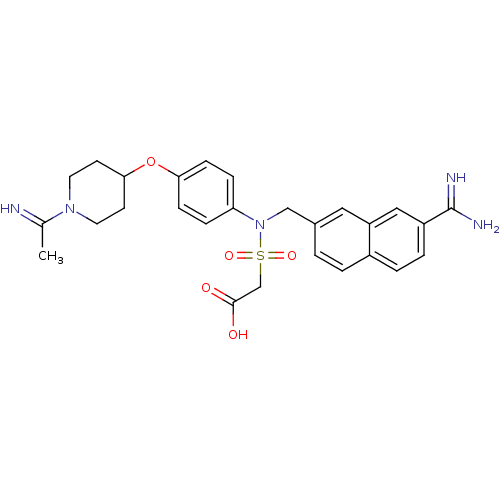
(((7-carbamimidoyl-naphthalen-2-ylmethyl)-{4-[1-(1-...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1)N(Cc1ccc2ccc(cc2c1)C(N)=N)S(=O)(=O)CC(O)=O Show InChI InChI=1S/C27H31N5O5S/c1-18(28)31-12-10-25(11-13-31)37-24-8-6-23(7-9-24)32(38(35,36)17-26(33)34)16-19-2-3-20-4-5-21(27(29)30)15-22(20)14-19/h2-9,14-15,25,28H,10-13,16-17H2,1H3,(H3,29,30)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Coagulation factor X |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50062632

((S)-1-(9-Hydroxy-9H-fluorene-9-carbonyl)-pyrrolidi...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)C2(O)c3ccccc3-c3ccccc23)CC1 |wU:9.8,(14.19,2.05,;14.19,.5,;12.85,-.27,;11.75,-1.35,;10.61,-.46,;9.27,-1.25,;7.92,-.46,;6.6,-1.25,;6.6,-2.79,;5.26,-.46,;5.74,1.01,;4.49,1.91,;3.25,1.01,;3.73,-.46,;2.38,-1.25,;2.38,-2.8,;1.06,-.48,;1.04,-2.03,;.88,1.06,;1.9,2.2,;1.42,3.67,;-.09,3.99,;-1.11,2.84,;-.63,1.38,;-1.4,.05,;-2.9,-.27,;-3.38,-1.73,;-2.34,-2.88,;-.85,-2.56,;-.37,-1.1,;12.11,-.06,;12.85,1.27,)| Show InChI InChI=1S/C26H31N3O3/c27-18-13-11-17(12-14-18)16-28-24(30)23-10-5-15-29(23)25(31)26(32)21-8-3-1-6-19(21)20-7-2-4-9-22(20)26/h1-4,6-9,17-18,23,32H,5,10-16,27H2,(H,28,30)/t17?,18?,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Thrombin. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM13931
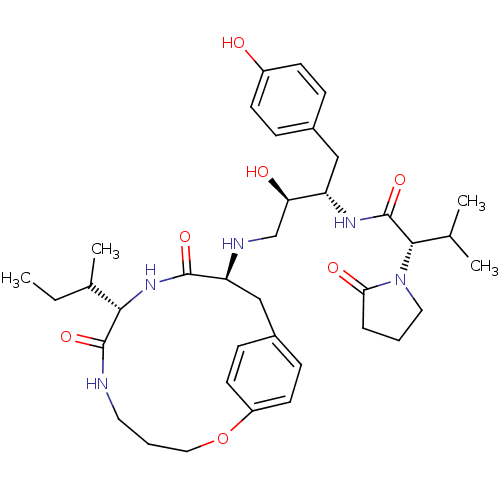
((2S)-N-[(2S,3R)-4-{[(8S,11S)-8-[(2R)-butan-2-yl]-7...)Show SMILES CCC(C)[C@@H]1NC(=O)[C@H](Cc2ccc(OCCCNC1=O)cc2)NC[C@@H](O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](C(C)C)N1CCCC1=O |r| Show InChI InChI=1S/C37H53N5O7/c1-5-24(4)33-36(47)38-17-7-19-49-28-15-11-26(12-16-28)21-30(35(46)41-33)39-22-31(44)29(20-25-9-13-27(43)14-10-25)40-37(48)34(23(2)3)42-18-6-8-32(42)45/h9-16,23-24,29-31,33-34,39,43-44H,5-8,17-22H2,1-4H3,(H,38,47)(H,40,48)(H,41,46)/t24?,29-,30-,31+,33-,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | -51.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland
| Assay Description
he Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in fl... |
Biochemistry 38: 7978-88 (1999)
Article DOI: 10.1021/bi990174x
BindingDB Entry DOI: 10.7270/Q20000B3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50366785
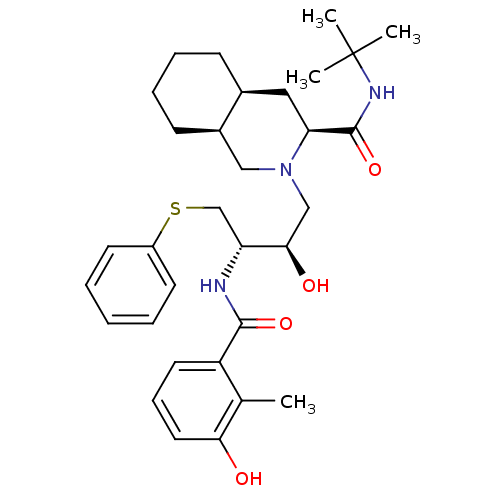
(NELFINAVIR)Show SMILES Cc1c(O)cccc1C(=O)N[C@H](CSc1ccccc1)[C@H](O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C32H45N3O4S/c1-21-25(15-10-16-28(21)36)30(38)33-26(20-40-24-13-6-5-7-14-24)29(37)19-35-18-23-12-9-8-11-22(23)17-27(35)31(39)34-32(2,3)4/h5-7,10,13-16,22-23,26-27,29,36-37H,8-9,11-12,17-20H2,1-4H3,(H,33,38)(H,34,39)/t22-,23+,26+,27-,29+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against HIV-protease inhibitor. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50084657
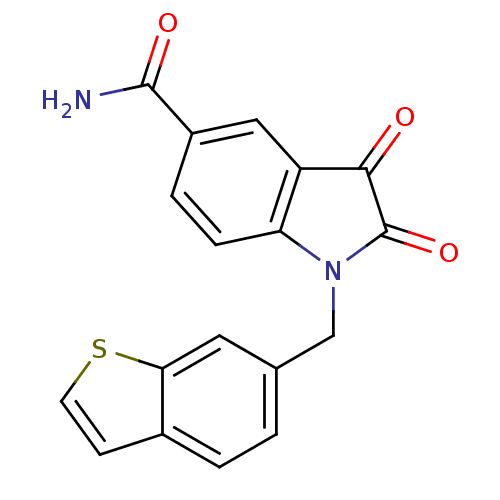
(1-Benzo[b]thiophen-6-ylmethyl-2,3-dioxo-2,3-dihydr...)Show SMILES NC(=O)c1ccc2N(Cc3ccc4ccsc4c3)C(=O)C(=O)c2c1 Show InChI InChI=1S/C18H12N2O3S/c19-17(22)12-3-4-14-13(8-12)16(21)18(23)20(14)9-10-1-2-11-5-6-24-15(11)7-10/h1-8H,9H2,(H2,19,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
The binding affinity against HRV-143C protease. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM13933
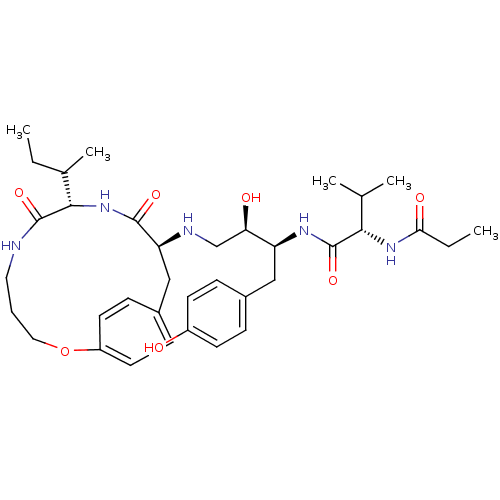
((2S)-N-[(2S,3R)-4-{[(8S,11S)-8-[(2S)-butan-2-yl]-7...)Show SMILES CCC(C)[C@@H]1NC(=O)[C@H](Cc2ccc(OCCCNC1=O)cc2)NC[C@@H](O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)CC)C(C)C |r| Show InChI InChI=1S/C36H53N5O7/c1-6-23(5)33-35(46)37-17-8-18-48-27-15-11-25(12-16-27)20-29(34(45)41-33)38-21-30(43)28(19-24-9-13-26(42)14-10-24)39-36(47)32(22(3)4)40-31(44)7-2/h9-16,22-23,28-30,32-33,38,42-43H,6-8,17-21H2,1-5H3,(H,37,46)(H,39,47)(H,40,44)(H,41,45)/t23?,28-,29-,30+,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3 | -50.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland
| Assay Description
he Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in fl... |
Biochemistry 38: 7978-88 (1999)
Article DOI: 10.1021/bi990174x
BindingDB Entry DOI: 10.7270/Q20000B3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50366461

(CHEMBL1790234)Show SMILES CC[C@@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(OCCCNC1=O)cc2)NC[C@@H](O)[C@@H]1Cc2ccc(OCCCCC(=O)N[C@@H](C(C)C)C(=O)N1)cc2 Show InChI InChI=1S/C38H55N5O7/c1-5-25(4)35-37(47)39-18-8-20-50-29-16-12-27(13-17-29)22-31(36(46)43-35)40-23-32(44)30-21-26-10-14-28(15-11-26)49-19-7-6-9-33(45)42-34(24(2)3)38(48)41-30/h10-17,24-25,30-32,34-35,40,44H,5-9,18-23H2,1-4H3,(H,39,47)(H,41,48)(H,42,45)(H,43,46)/t25-,30+,31+,32-,34+,35+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 6: 2531-2536 (1996)
Article DOI: 10.1016/0960-894X(96)00468-4
BindingDB Entry DOI: 10.7270/Q2S1830C |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM30344
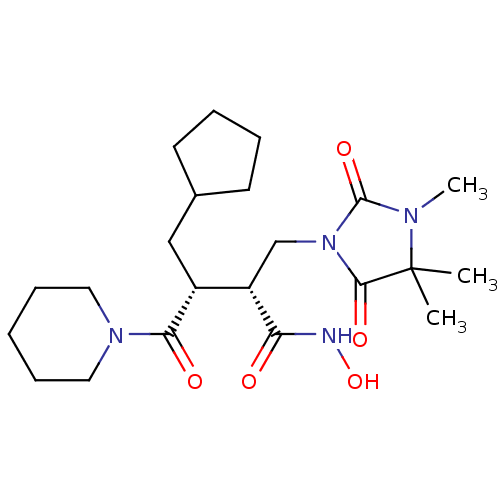
(Cipemastat | Trocade)Show SMILES CN1C(=O)N(C[C@@H]([C@@H](CC2CCCC2)C(=O)N2CCCCC2)C(=O)NO)C(=O)C1(C)C |r| Show InChI InChI=1S/C22H36N4O5/c1-22(2)20(29)26(21(30)24(22)3)14-17(18(27)23-31)16(13-15-9-5-6-10-15)19(28)25-11-7-4-8-12-25/h15-17,31H,4-14H2,1-3H3,(H,23,27)/t16-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-13 (MMP-13). |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50369797
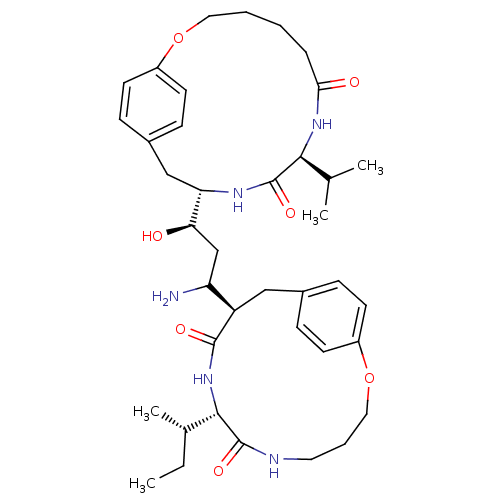
(CHEMBL1794029)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(OCCCNC1=O)cc2)C(N)C[C@@H](O)[C@@H]1Cc2ccc(OCCCCC(=O)N[C@@H](C(C)C)C(=O)N1)cc2 Show InChI InChI=1S/C39H57N5O7/c1-5-25(4)36-38(48)41-18-8-20-51-29-14-10-26(11-15-29)21-30(37(47)44-36)31(40)23-33(45)32-22-27-12-16-28(17-13-27)50-19-7-6-9-34(46)43-35(24(2)3)39(49)42-32/h10-17,24-25,30-33,35-36,45H,5-9,18-23,40H2,1-4H3,(H,41,48)(H,42,49)(H,43,46)(H,44,47)/t25-,30+,31?,32-,33+,35-,36-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
binding affinity towards HIV-1 Protease enzyme |
J Med Chem 43: 1271-81 (2001)
BindingDB Entry DOI: 10.7270/Q23779F5 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288203

(1-{3-[2-(2-Acetylamino-4-methyl-pentanoylamino)-1-...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(C)=O)C(=O)N[C@@H](C(C)C)C(N)=O Show InChI InChI=1S/C38H62N8O8/c1-8-23(6)33(38(54)44-32(22(4)5)34(40)50)45-37(53)29-15-12-16-46(29)20-30(48)26(18-25-13-10-9-11-14-25)42-36(52)28(19-31(39)49)43-35(51)27(17-21(2)3)41-24(7)47/h9-11,13-14,21-23,26-30,32-33,48H,8,12,15-20H2,1-7H3,(H2,39,49)(H2,40,50)(H,41,47)(H,42,52)(H,43,51)(H,44,54)(H,45,53)/t23-,26-,27-,28-,29-,30-,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 6: 2531-2536 (1996)
Article DOI: 10.1016/0960-894X(96)00468-4
BindingDB Entry DOI: 10.7270/Q2S1830C |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM30344

(Cipemastat | Trocade)Show SMILES CN1C(=O)N(C[C@@H]([C@@H](CC2CCCC2)C(=O)N2CCCCC2)C(=O)NO)C(=O)C1(C)C |r| Show InChI InChI=1S/C22H36N4O5/c1-22(2)20(29)26(21(30)24(22)3)14-17(18(27)23-31)16(13-15-9-5-6-10-15)19(28)25-11-7-4-8-12-25/h15-17,31H,4-14H2,1-3H3,(H,23,27)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-1 (MMP-1). |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against HIV-2 protease enzyme. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50366780
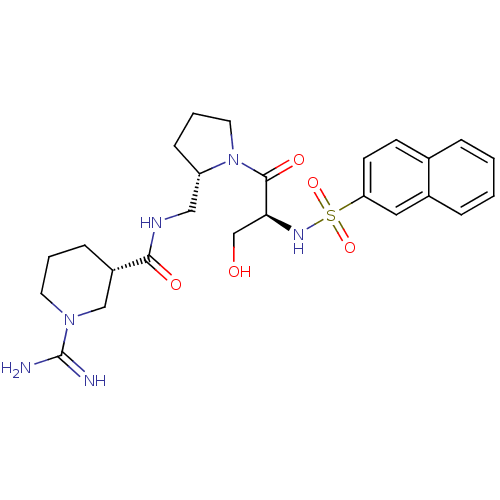
(BMS-189090 | CHEMBL138877)Show SMILES NC(=N)N1CCC[C@@H](C1)C(=O)NC[C@@H]1CCCN1C(=O)[C@H](CO)NS(=O)(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C25H34N6O5S/c26-25(27)30-11-3-7-19(15-30)23(33)28-14-20-8-4-12-31(20)24(34)22(16-32)29-37(35,36)21-10-9-17-5-1-2-6-18(17)13-21/h1-2,5-6,9-10,13,19-20,22,29,32H,3-4,7-8,11-12,14-16H2,(H3,26,27)(H,28,33)/t19-,20-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Thrombin. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM13929
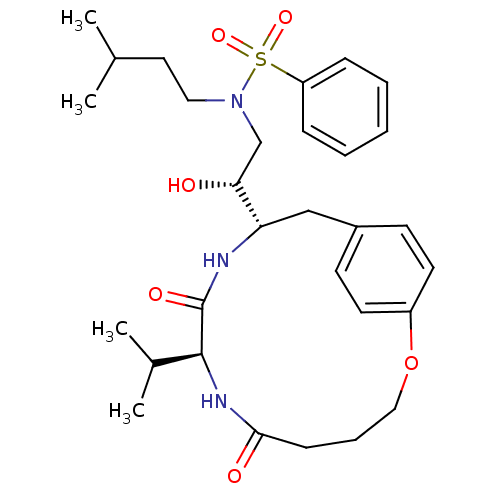
((2R)-2-[(8S,11S)-6,9-dioxo-8-(propan-2-yl)-2-oxa-7...)Show SMILES CC(C)CCN(C[C@@H](O)[C@@H]1Cc2ccc(OCCCC(=O)N[C@@H](C(C)C)C(=O)N1)cc2)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C30H43N3O6S/c1-21(2)16-17-33(40(37,38)25-9-6-5-7-10-25)20-27(34)26-19-23-12-14-24(15-13-23)39-18-8-11-28(35)32-29(22(3)4)30(36)31-26/h5-7,9-10,12-15,21-22,26-27,29,34H,8,11,16-20H2,1-4H3,(H,31,36)(H,32,35)/t26-,27+,29-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 4 | -49.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland
| Assay Description
he Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in fl... |
Biochemistry 38: 7978-88 (1999)
Article DOI: 10.1021/bi990174x
BindingDB Entry DOI: 10.7270/Q20000B3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM13932
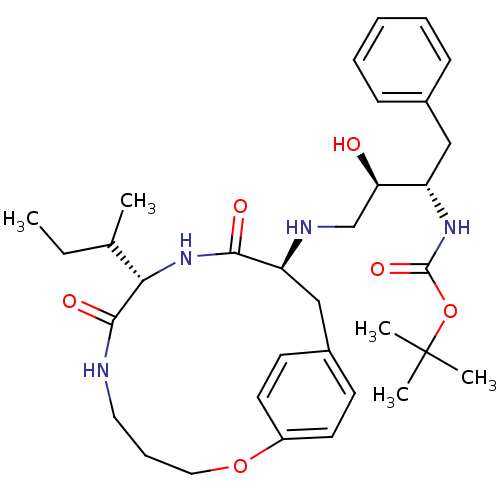
(Macrocyclic Peptidomimetic Inhibitor 6 | tert-buty...)Show SMILES CCC(C)[C@@H]1NC(=O)[C@H](Cc2ccc(OCCCNC1=O)cc2)NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C33H48N4O6/c1-6-22(2)29-31(40)34-17-10-18-42-25-15-13-24(14-16-25)20-27(30(39)37-29)35-21-28(38)26(19-23-11-8-7-9-12-23)36-32(41)43-33(3,4)5/h7-9,11-16,22,26-29,35,38H,6,10,17-21H2,1-5H3,(H,34,40)(H,36,41)(H,37,39)/t22?,26-,27-,28+,29-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4 | -49.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland
| Assay Description
he Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in fl... |
Biochemistry 38: 7978-88 (1999)
Article DOI: 10.1021/bi990174x
BindingDB Entry DOI: 10.7270/Q20000B3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
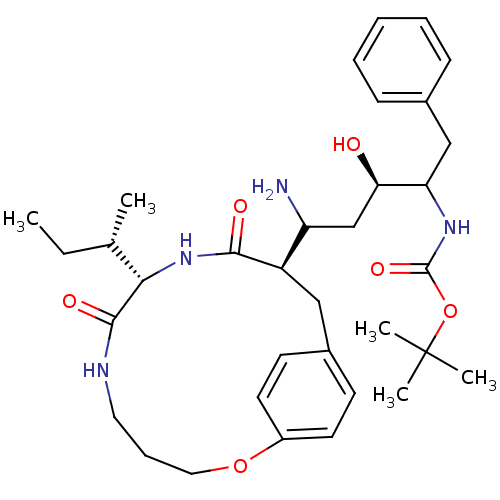
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50369799
(CHEMBL1794024)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(OCCCNC1=O)cc2)C(N)C[C@@H](O)C(Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C34H50N4O6/c1-6-22(2)30-32(41)36-17-10-18-43-25-15-13-24(14-16-25)19-26(31(40)38-30)27(35)21-29(39)28(20-23-11-8-7-9-12-23)37-33(42)44-34(3,4)5/h7-9,11-16,22,26-30,39H,6,10,17-21,35H2,1-5H3,(H,36,41)(H,37,42)(H,38,40)/t22-,26+,27?,28?,29+,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
binding affinity towards HIV-1 Protease enzyme |
J Med Chem 43: 1271-81 (2001)
BindingDB Entry DOI: 10.7270/Q23779F5 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288179
(1-{3-[2-(2-Acetylamino-4-methyl-pentanoylamino)-1-...)Show SMILES CC(C)CCN(C[C@@H](O)[C@H]1Cc2ccc(OCCCC(=O)N[C@@H](C(C)C)C(=O)N1)cc2)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C30H43N3O6S/c1-21(2)16-17-33(40(37,38)25-9-6-5-7-10-25)20-27(34)26-19-23-12-14-24(15-13-23)39-18-8-11-28(35)32-29(22(3)4)30(36)31-26/h5-7,9-10,12-15,21-22,26-27,29,34H,8,11,16-20H2,1-4H3,(H,31,36)(H,32,35)/t26-,27-,29+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 6: 2531-2536 (1996)
Article DOI: 10.1016/0960-894X(96)00468-4
BindingDB Entry DOI: 10.7270/Q2S1830C |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM30344

(Cipemastat | Trocade)Show SMILES CN1C(=O)N(C[C@@H]([C@@H](CC2CCCC2)C(=O)N2CCCCC2)C(=O)NO)C(=O)C1(C)C |r| Show InChI InChI=1S/C22H36N4O5/c1-22(2)20(29)26(21(30)24(22)3)14-17(18(27)23-31)16(13-15-9-5-6-10-15)19(28)25-11-7-4-8-12-25/h15-17,31H,4-14H2,1-3H3,(H,23,27)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-8 (MMP-8). |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50366456

(CHEMBL1790652)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1CC(O)[C@H]1Cc2ccc(OCC(=O)N[C@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1)cc2)C(=O)N[C@@H](C(C)C)C(N)=O Show InChI InChI=1S/C37H57N9O10/c1-5-20(4)32(37(55)44-31(19(2)3)33(40)51)45-36(54)26-7-6-14-46(26)17-27(47)24-15-21-8-10-22(11-9-21)56-18-30(50)41-23(12-13-28(38)48)34(52)43-25(16-29(39)49)35(53)42-24/h8-11,19-20,23-27,31-32,47H,5-7,12-18H2,1-4H3,(H2,38,48)(H2,39,49)(H2,40,51)(H,41,50)(H,42,53)(H,43,52)(H,44,55)(H,45,54)/t20-,23+,24+,25-,26-,27?,31-,32-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 6: 2531-2536 (1996)
Article DOI: 10.1016/0960-894X(96)00468-4
BindingDB Entry DOI: 10.7270/Q2S1830C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50084635

(3-[(9-Benzoylamino-6,10-dioxo-octahydro-pyridazino...)Show SMILES OC(=O)C[C@H](NC(=O)[C@@H]1CCCN2N1C(=O)C(CCC2=O)NC(=O)c1ccccc1)C=O Show InChI InChI=1S/C21H24N4O7/c26-12-14(11-18(28)29)22-20(31)16-7-4-10-24-17(27)9-8-15(21(32)25(16)24)23-19(30)13-5-2-1-3-6-13/h1-3,5-6,12,14-16H,4,7-11H2,(H,22,31)(H,23,30)(H,28,29)/t14-,15?,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
The binding affinity against IL-1 beta converting enzyme |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data