

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50339608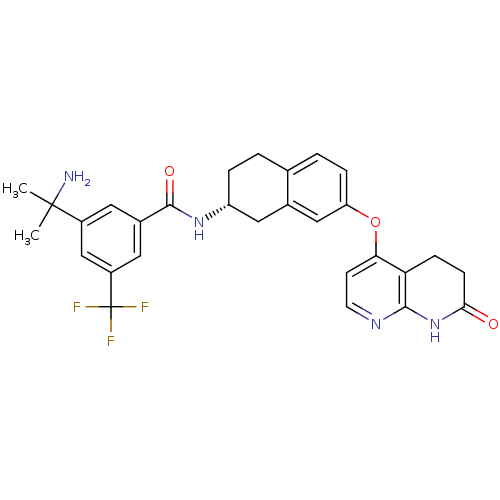 (3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RET | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50339609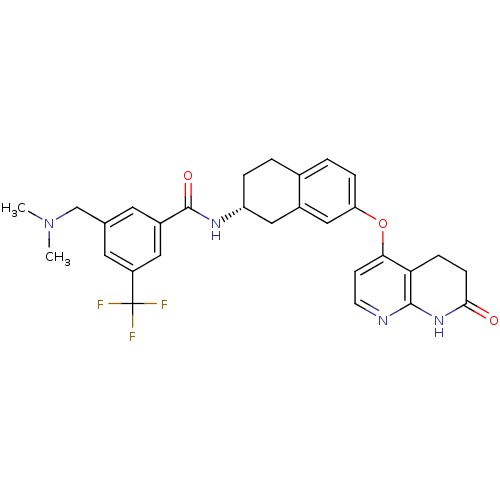 (3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Abl1 | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139157 (CHEMBL3764588) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50339609 (3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RET | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139150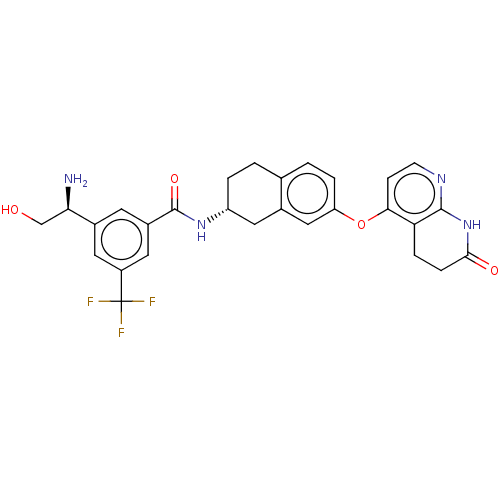 (CHEMBL3763999) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139149 (CHEMBL3765012) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139147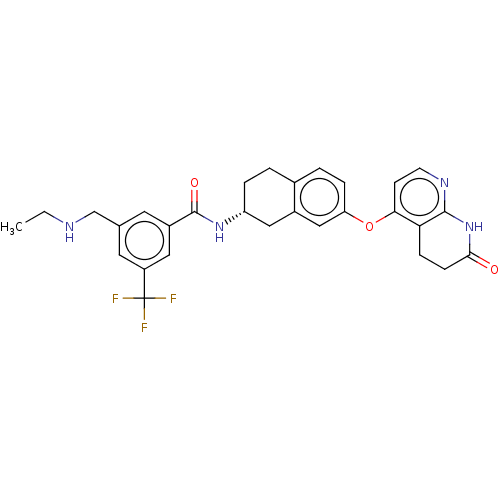 (CHEMBL3765509) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139153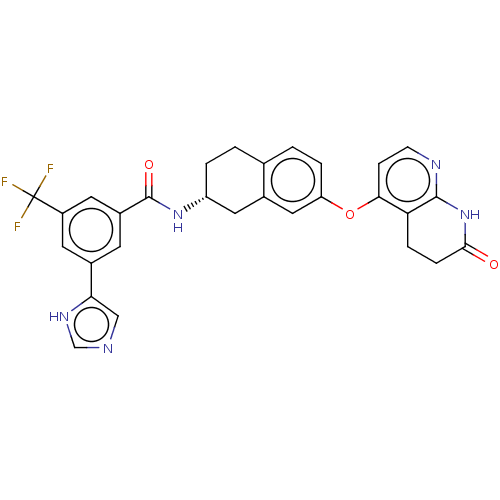 (CHEMBL3765355) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50339608 (3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139156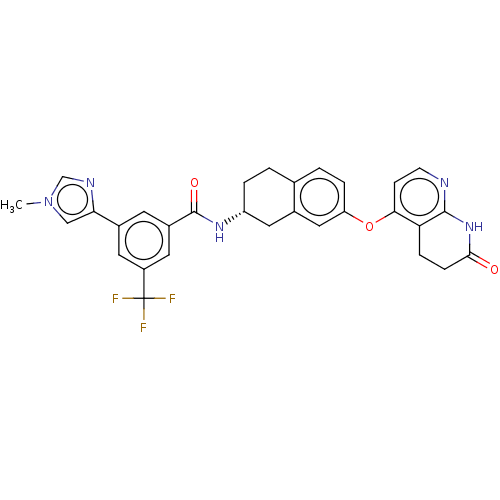 (CHEMBL3763694) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139158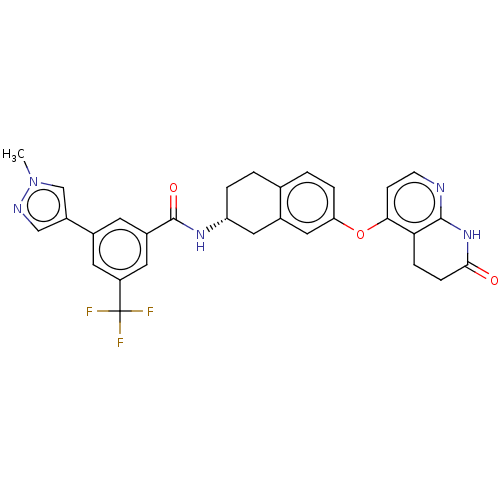 (CHEMBL3763646) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50339609 (3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50339621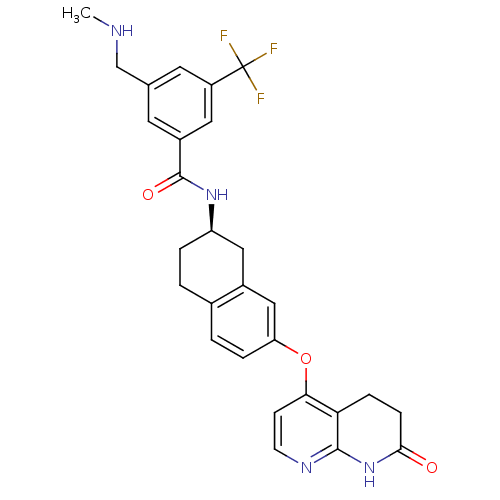 (3-[(methylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,8-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139151 (CHEMBL3764870) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50339608 (3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Discoidin domain-containing receptor 2 (Homo sapiens (Human)) | BDBM50339609 (3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of DDR2 | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50339609 (3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139155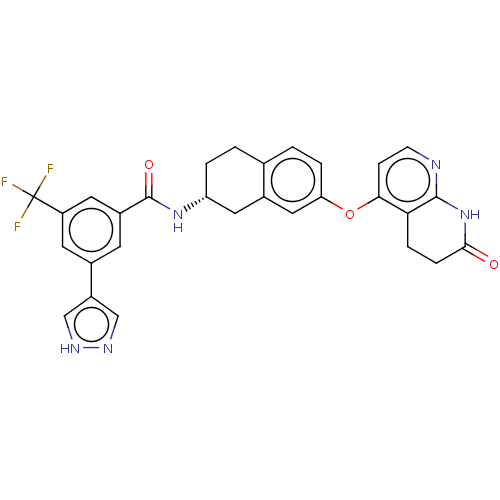 (CHEMBL3765708) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139148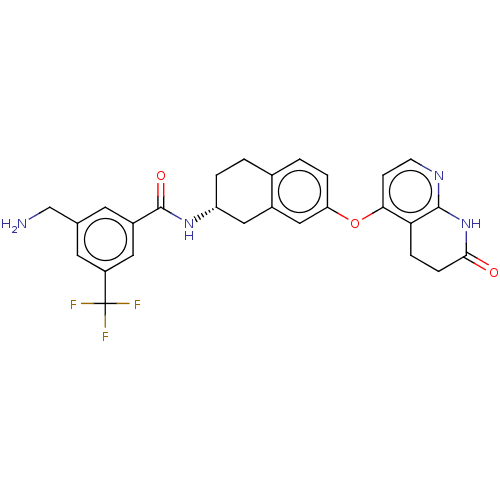 (CHEMBL3764101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50405402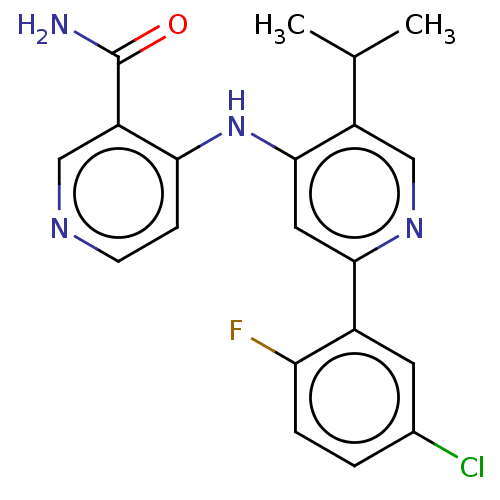 (CHEMBL5267945) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli leader peptidase using substrate A | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50405407 (CHEMBL5285361) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli leader peptidase using substrate A | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139159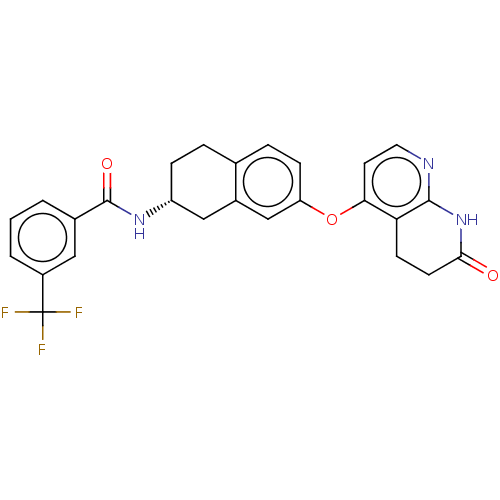 (CHEMBL3765596) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50339615 ((2S)-7-({2-[(Cyclopropylcarbonyl)amino]pyridin-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139152 (CHEMBL3764275) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50339608 (3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of wild-type B-Raf | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50339613 ((2S)-N-[3-(aminomethyl)-5-(trifluoromethyl)phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50339616 (7-({2-[(Cyclopropylcarbonyl)amino]pyridin-4-yl}oxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50339609 (3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of wild-type B-Raf | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50405385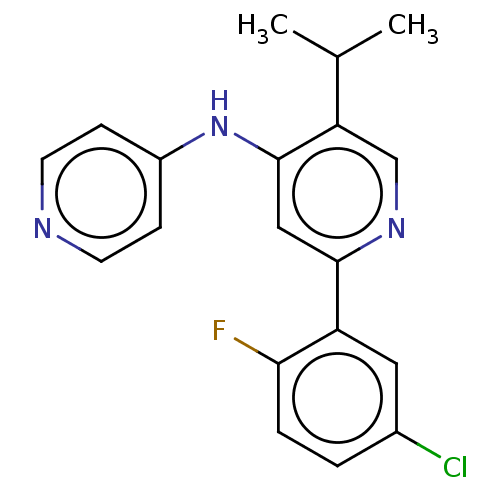 (CHEMBL5285746) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli leader peptidase using substrate A | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM16673 (4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50339611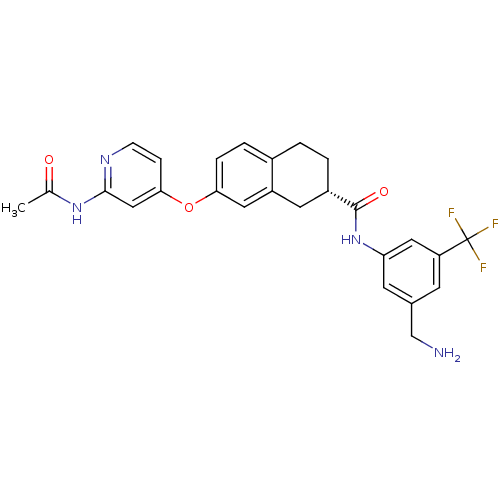 ((2S)-7-{[2-(acetylamino)pyridin-4-yl]oxy}-N-[3-(am...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50339614 ((2R)-N-[3-(aminomethyl)-5-(trifluoromethyl)phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50339612 (CHEMBL1688868 | N-[3-(Aminomethyl)-5-(trifluoromet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50339608 (3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of KDR | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM514529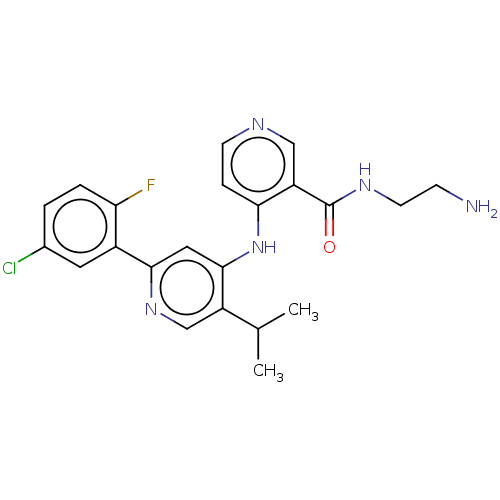 (N-(2-aminoethyl)-4-[[2-(5-chloro-2-fluoro-phenyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli leader peptidase using substrate A | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Discoidin domain-containing receptor 2 (Homo sapiens (Human)) | BDBM50339608 (3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of DDR2 | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50405403 (CHEMBL5274165) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli leader peptidase using substrate A | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50339623 (4-[3-(3-{[4-Chloro-3-(trifluoromethyl)phenyl]amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139154 (CHEMBL3765074) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50339622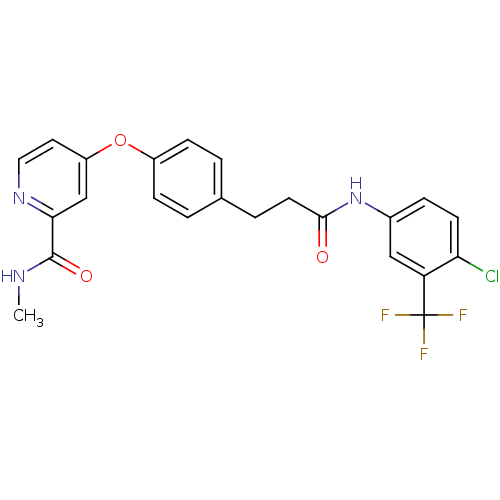 (4-[4-(3-{[4-Chloro-3-(trifluoromethyl)phenyl]amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50339610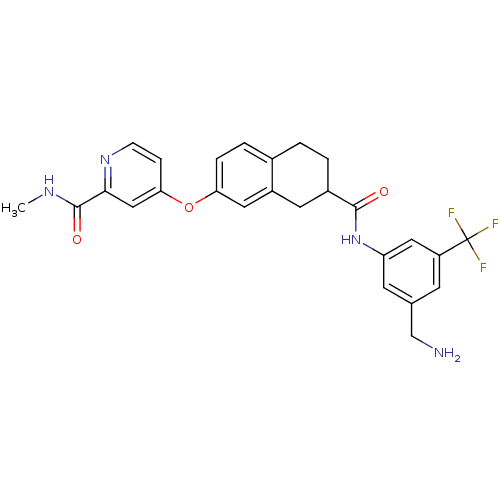 (4-{[7-({[3-(Aminomethyl)-5-(trifluoromethyl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 (Homo sapiens (Human)) | BDBM50339608 (3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of EPHA2 | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50405404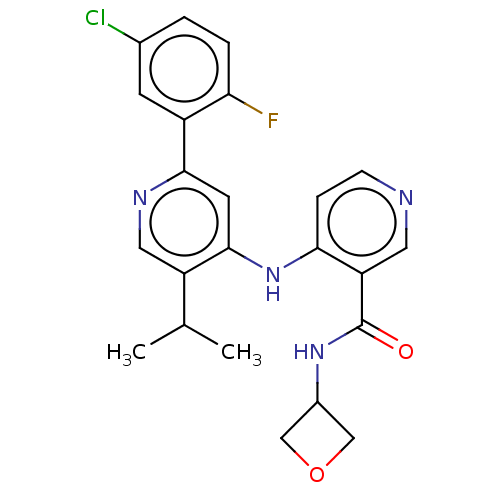 (CHEMBL5268400) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli leader peptidase using substrate A | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50405409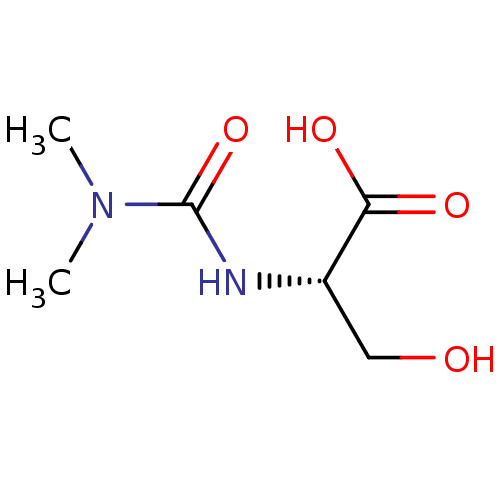 (CHEMBL5267264) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli leader peptidase using substrate A | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin-like modifier-activating enzyme ATG7 (Homo sapiens (Human)) | BDBM475984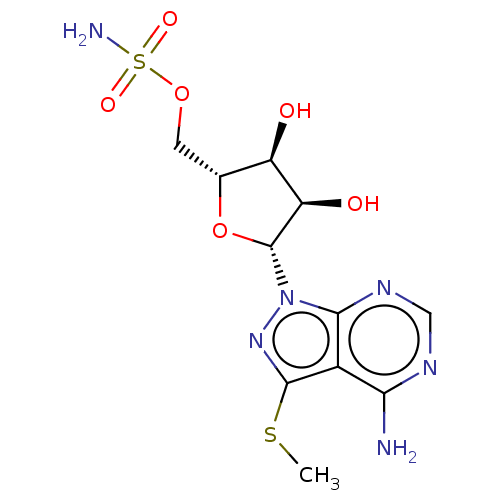 (US10865208, Compound I-42 | {(2R,3S,4R,5R)-5-[4-am...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant His-tagged human ATG7 incubated for 105 mins in presence of GST-tagged ATG3 and Flag-tagged Gabarap and measured after 2 hr... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115681 BindingDB Entry DOI: 10.7270/Q2M90DBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin-like modifier-activating enzyme ATG7 (Homo sapiens (Human)) | BDBM475978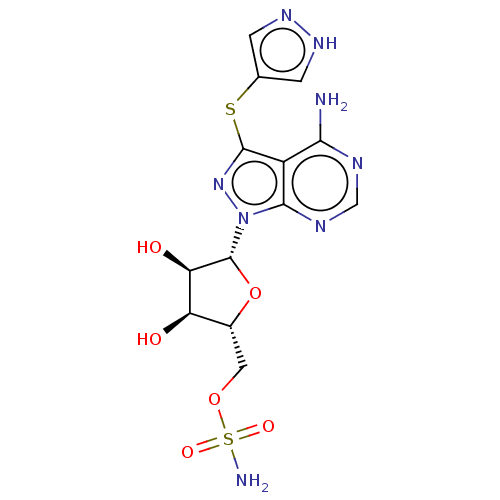 (US10865208, Compound I-36 | {(2R,3S,4R,5R)-5-[4-am...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant His-tagged human ATG7 incubated for 105 mins in presence of GST-tagged ATG3 and Flag-tagged Gabarap and measured after 2 hr... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115681 BindingDB Entry DOI: 10.7270/Q2M90DBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50339625 (4-{[7-({[4-(Aminomethyl)-3-(trifluoromethyl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM280348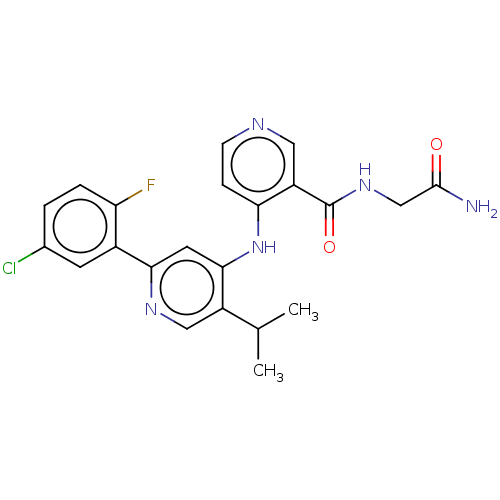 (N-(2-amino-2-oxo-ethyl)-4-[[2-(5-chloro-2-fluoro-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli leader peptidase using substrate A | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin-like modifier-activating enzyme ATG7 (Homo sapiens (Human)) | BDBM476001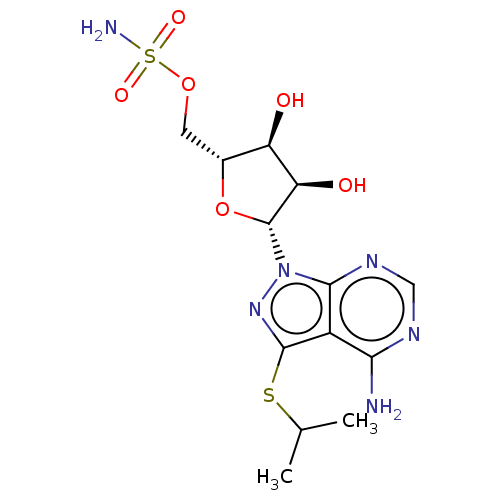 (US10865208, Compound I-58 | {(2R,3S,4R,5R)-5-[4-am...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant His-tagged human ATG7 incubated for 105 mins in presence of GST-tagged ATG3 and Flag-tagged Gabarap and measured after 2 hr... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115681 BindingDB Entry DOI: 10.7270/Q2M90DBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 (Homo sapiens (Human)) | BDBM50339609 (3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of EPHA2 | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 447 total ) | Next | Last >> |